Opportunistic infections by atypical mycobacteria are rare complications of anti-tumor necrosis factor (TNF)-α therapy. We report the case of a patient with ulcerative colitis and metabolic syndrome on infliximab who developed a cutaneous infection caused by Mycobacterium marinum.
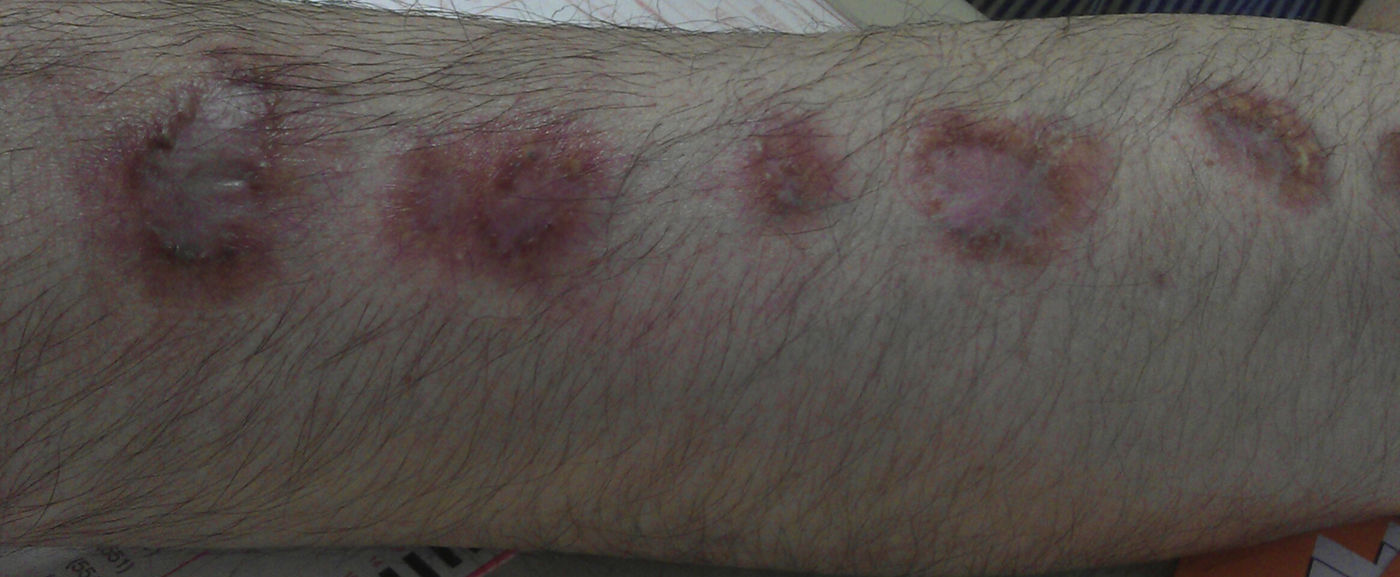
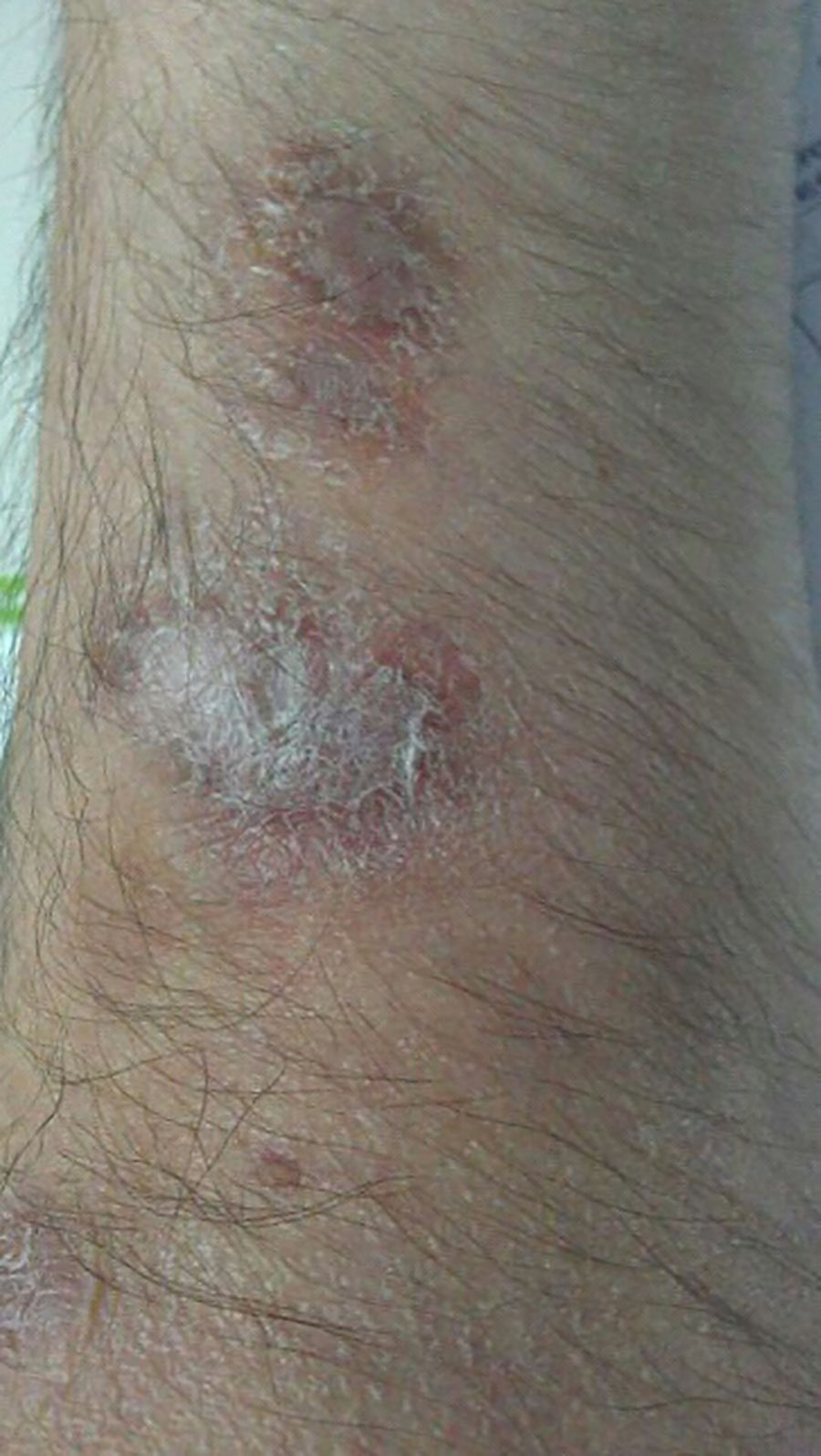
A 54-year-old man was diagnosed with ulcerative rectosigmoiditis in 1999 and treated with topical and oral mesalazine with poor therapeutic adherence. There was a history of obesity, type 2 diabetes mellitus, depressive syndrome and chronic heavy alcohol intake of 120g ethanol/day over the last 35 years. In November 2011, he was admitted to the hospital because of a severe flare of ulcerative colitis (UC). Contrast-enhanced ultrasound showed extension of inflammation up to the splenic flexure and severe hepatic steatosis. High-dose intravenous corticosteroids were ineffective. After screening for latent infections, an excellent clinical response was obtained with infliximab at standard induction doses. Before the second infusion dose of infliximab, the patient presented with history of fever (39°C) and appearance of a painful indurated erythematous nodule on the back of the left hand with subsequent sporotrichoid spread to the arm (Figures 1 and 2). He was not aware of any trauma but reported to take care of a fish tank at home. A skin biopsy showed positivity for non-tuberculous mycobacteria and culture yielded growth of M. marinum. Treatment with ethambutol (1200mg/day) and rifampin was prescribed, but after identification of the causative organism, rifampin was substituted by clarithromycin (500mg twice daily), and infliximab was stopped. During the next 4 years, the clinical course was characterized by intermittent clearance and reappearance of the cutaneous infection with negative and positive cultures, thus requiring multiple antimicrobial combinations. In April 2014, quadruple treatment with clarithromycin, trimethoprim–sulfamethoxazole, ethambutol and rifampin (600mg/day) was given. Definitive clearance of the lesions and negative cultures for M. marinum were achieved in February 2016. During this 4-year period, remission of the UC was maintained with mesalazine, although in May 2016, the patient presented a mild flare, which was successfully treated with beclomethasone dipropionate. Treatment with azathioprine was started. At present, the UC is in remission, and there are no signs of cutaneous infection.
M. marinum has been rarely described as the causative organism of non-tuberculous cutaneous granulomas in patients Crohn's disease treated with anti-TNF-α drugs,1–4 with only a single previous case of a patient with UC treated with infliximab.5 Therefore, with the patient here described there are apparently only two case reports of M. marinum cutaneous infections in infliximab-treated patients with UC. Most M. marinum infections in infliximab-treated patients stem from aquarium exposure, cleaning fish tanks,1–4 although Fallon et al.2 reported an infection in the leg after swimming on holiday in the Canary Islands. Definitive diagnosis, however, is established by identification of the microorganism in cultures of skin biopsies, which is positive in 70–80% of cases. In our patient, cultures grew M. marinum but PRC amplification and DNA sequencing of the hsp65 gene fragment was not performed.
The rapid onset of infection, only 10 days after starting treatment with infliximab and the torpid clinical course, with intermittent phases of clearance and reappearance of lesions despite early and aggressive therapy, may be explained by associated obesity, metabolic syndrome, and chronic liver disease present in our patient combined with anti-TNF-α therapy.
Optimal treatment for skin lesions has not yet been established nor the duration of treatment. In our patient, different antimicrobial combinations were administered but clearance of lesions was finally achieved with clarithromycin, trimethoprim-sulfamethoxazole, ethambutol and rifampin quadruple regimen. It seems advisable to prolong treatment at least between 2 and 6 months after resolution of the lesions. Given the protracted clinical course, antimicrobial treatment was exceptionally maintained for 48 months. The decision to discontinue treatment with infliximab should be individualized but in our case biological therapy was withdrawn.
Infection caused by M. marinum in infliximab-treated patients with inflammatory bowel disease should be suspected in the presence of skin lesions with a sporotrichoid pattern of spread, especially when minor trauma and history of injury from fish fins or exposure to contaminated water such as home fish tanks are recalled by the patients.