The COVID-19 pandemic has led to the suspension of programmed activity in most of the Endoscopy Units in our environment. The aim of this document is to facilitate the resumption of elective endoscopic activity in an efficient and safe manner.
Material and methodsA series of questions considered to be of clinical and logistical relevance were formulated. In order to elaborate the answers, a structured bibliographic search was carried out in the main databases and the recommendations of the main Public Health and Digestive Endoscopy institutions were reviewed. The final recommendations were agreed upon through telematic means.
ResultsA total of 33 recommendations were made. The main aspects discussed are: 1) Reassessment and prioritization of the indication, 2) Restructuring of spaces, schedules and health personnel, 3) Screening for infection, 4) Hygiene measures and personal protective equipment.
ConclusionThe AEG and SEED recommend restarting endoscopic activity in a phased, safe manner, adapted to local resources and the epidemiological situation of SARS-CoV-2 infection.
La pandemia por COVID-19 ha conllevado la suspensión de la actividad programada en la mayoría de las Unidades de Endoscopia de nuestro medio. El objetivo del presente documento es facilitar el reinicio de la actividad endoscópica electiva de forma eficiente y segura.
Material y métodosSe formuló una serie de preguntas consideradas de relevancia clínica y logística. Para la elaboración de las respuestas, se realizó una búsqueda bibliográfica estructurada en las principales bases de datos y se revisaron las recomendaciones de las principales instituciones de Salud Pública y de endoscopia digestiva. Las recomendaciones finales se consensuaron por vía telemática.
ResultadosSe han elaborado un total de 33 recomendaciones. Los principales aspectos que se discuten son: 1) la reevaluación y priorización de la indicación; 2) la restructuración de espacios, agendas y del personal sanitario; 3) el cribado de la infección, y 4) las medidas de higiene y los equipos de protección individual.
ConclusiónLa AEG y la SEED recomiendan reiniciar la actividad endoscópica de forma escalonada, segura, adaptada a los recursos locales y a la situación epidemiológica de la infección por SARS-CoV-2.
The World Health Organization (WHO) declared the SARS-CoV-2 coronavirus as a pandemic on 11 March 2020. By mid-April, the total number of confirmed cases around the world had exceeded 2 million people, affecting more than 200 countries and causing more than 139,000 deaths.1 In Spain, by the same point, a total number of 188,068 confirmed cases and 19,478 deaths had been reported.2
For several weeks, the vast majority of the country's Endoscopy Units (EU) decided to suspend their scheduled activity in order to reduce the risk of contracting the SARS-CoV-2 infection and help to reduce its spread. Considering the possibility of causing harm to patients, and taking into account the principle of primum non nocere (first, do no harm), only urgent procedures, or those whose delay would entail significant clinical worsening, were carried out. As a result of this, a significant number of patients could not undergo their scheduled procedures.
In light of this exceptional situation brought about by the pandemic, the Spanish Gastroenterology Association (AEG) and the Spanish Society of Gastrointestinal Endoscopy (SEED) commissioned the drafting of this document on the resumption of endoscopic activity. This document provides evidence-based recommendations for the rescheduling of cancelled procedures at the sites where the patients were originally due to attend. A commitment is therefore required from gastroenterology specialists and the directors of the aforementioned institutions to resolve the situation brought about by the pandemic at the affected sites.
The expected scope of application covers those Endoscopy Units that may have been affected by the epidemic and the time until the normal resumption of endoscopic activity under the terms in which they were carried out prior to the pandemic.
This consensus of experts seeks to facilitate the work of heads and other professionals in Spanish Endoscopy Units. It seeks to support decision-making in a new and particularly complicated context, allowing for the rescheduling of cancelled procedures during the most acute phase of the pandemic, and the safe performance thereof.
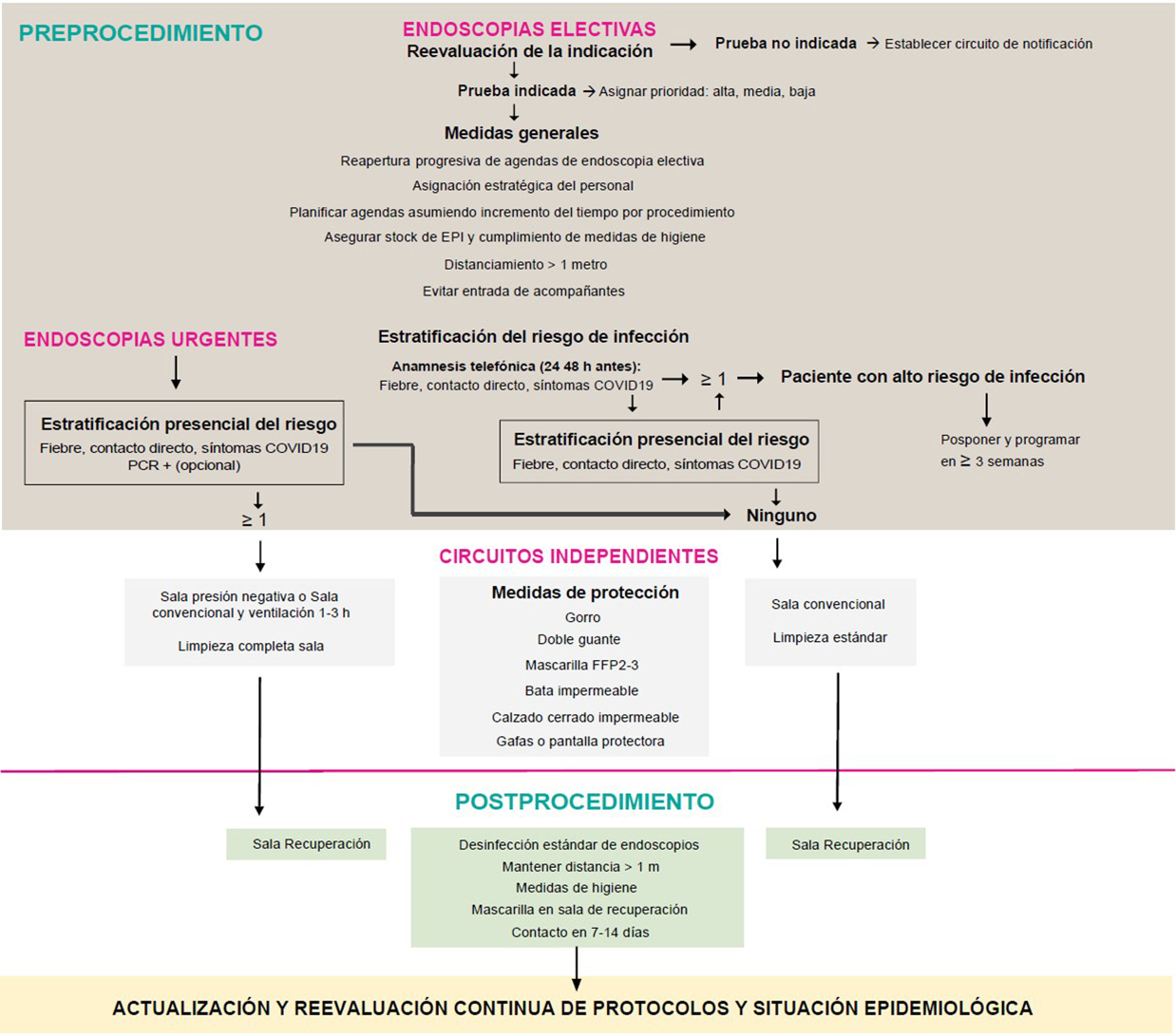
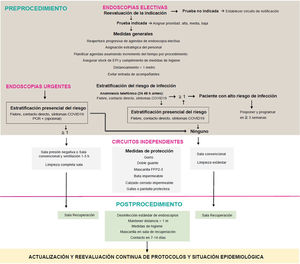
Material and methodsFirstly, this set of authors formulated a series of clinically and logistically-relevant questions for the resumption of endoscopic activity. A literature search was then carried out in Embase, PubMed and the Cochrane Database of Systematic Reviews using keywords, following the strategy detailed in Appendix, Supplementary Table 1, with no restriction on language, date or design. Articles cited in the references of the reviewed papers, and others considered to be of interest, were also consulted during the elaboration of the answers by means of non-systematised searches. The current recommendations of national and international institutions and the main scientific societies of Gastroenterology and Digestive Endoscopy, dated 17 April 2020 (Appendix, Supplementary Table 1), were reviewed. All of the authors reached an agreement on the final recommendations online, with these recommendations being summarised in Table 1. The action proposal of this document is presented in Fig. 1.
Summary of recommendations.
| The AEG and the SEED recommend the following: |
|---|
| Pre-procedure |
| A phased resumption of endoscopic activity that is adapted to local resources and the epidemiological situation of the SARS-CoV-2 infection |
| Reassessing the indication for endoscopy and prioritising procedures with the greatest expected benefit |
| Prioritising endoscopic procedures based on the probability of finding clinically-relevant lesions |
| Monitoring the results of the prioritisation plan |
| Establishing a record of the requests evaluated on the endoscopic procedure waiting list, their priority level and the reasons for cancellation, if applicable |
| Recording, in the patient's medical record, the reason for which a scheduled procedure was suspended when said procedure was cancelled because it was not deemed appropriate |
| Ensuring proper communication between the various individuals involved in the process: assessment specialists, requesting physicians and patients |
| Screening for SARS-CoV-2 infection via directed medical history in all patients |
| As things stand, no reliable scientific evidence supports screening for SARS-CoV-2 infection by PCR or antibody detection prior to the performance of an endoscopic procedure. For the moment, its diagnostic performance in contexts other than that of symptomatic patients is not well known, and entails considerable interpretation difficulties |
| Delaying elective cases that are suspected of having COVID-19. The other cases will be managed as if all patients were potentially infected, while the area remains at a high risk |
| Holding meetings with all members of the EU working team in order to raise awareness of the action protocols and ensure compliance with them |
| The wearing of surgical masks, as well as adhering to the social distancing measures proposed by the WHO, during working team meetings |
| Not allowing the patient's accompanying parties to enter the Endoscopic Unit, apart from in selected cases |
| For patients to maintain a minimum distance between people of 1−2 m during their stay in the EU. |
| Providing patients with protective masks and hospital clothing while ensuring that they perform correct hand hygiene practices |
| Modifying the time allocated to each endoscopy and the volume of procedures, due to the need to implement additional health and safety measures |
| Procedure |
| Establishing a separate patient circuit for patients with highly-suspected or confirmed SARS-CoV-2 infection. |
| Encouraging the application of basic hygiene measures in order to prevent staff members from becoming infected |
| The wearing of PPE by all healthcare staff involved in the performance of an endoscopic procedure |
| Not performing the endoscopic procedure if the PPE required to ensure that said procedure is carried out in a safe manner is not available |
| Not systemically using additional barriers over the patient’s naso-buccal area The usefulness and efficacy of these measures, as well as the application of other barriers over the endoscope valves, should be evaluated within research protocols |
| Supplementing oxygen therapy with exhalation filter masks in patients with highly-suspected or confirmed SARS-CoV-2 infection and who are undergoing a colonoscopy. If these devices are not available, it is recommended for them to wear a surgical mask above the nasal tubes, or a Venturi mask |
| Procedures on patients with highly-suspected or confirmed SARS-CoV-2 infection should be carried out by strategically assigned experienced personnel |
| Promoting the participation of Gastroenterology residents in endoscopic procedures carried out on patients with a low risk of having SARS-CoV-2 infection, provided that the necessary resources for ensuring the safety of the procedure are available |
| Avoiding the taking of biological samples during the endoscopy when the clinical impact of the result is expected to be marginal |
| Carrying out the processing of biological samples in line with the standardised biosecurity protocols for substances with a high infectivity capacity |
| Post-procedure |
| Disinfecting and re-processing of endoscopes in line with the usual protocols |
| Not using single-use devices more than once |
| Assigning dedicated cleaning staff to the Endoscopic Unit |
| Applying protocols for the cleaning and disinfection of endoscopy rooms and materials that have come into contact with the patient or his/her secretions |
| Managing waste in line with the local protocols of each centre for category B waste with high infectivity capacity (UN3291). |
| Ensuring a distance between people of 1−2 m, maintaining basic hygiene practices and establishing a separate patient circuit in the recovery rooms. |
| Considering the implementation of follow-up programmes for patients 7−15 days after the procedure to assess the onset of symptoms that are compatible with SARS-CoV-2 infection. |
PCR: detection of viral RNA by polymerase chain reaction.
The AEG and the SEED recommend a phased resumption of endoscopic activity that is adapted to local resources and the epidemiological situation of the SARS-CoV-2 infection.
While it is not the only factor for evaluating the potential spread of an infectious disease, the basic reproduction number (R0) defines the average number of secondary infections caused per case in the population. When R0 is <1, each infected case does not cause an additional infection, meaning the population would be protected. The WHO’s first estimations of R0 placed it between 1.4 and 2.5. Since then, more recently, slightly higher values have been reported (an average of 3.28).3
In Spain, the R0 value is 0.91 as of 16 April 2020.4 At the beginning, this was above 7. The current R0 value suggests that the spread of the virus is slowing down in the population. This indicator, however, is constantly changing, and depends on the infection control measures that are being implemented.
The SARS-CoV-2 infection presents various challenges: there are no vaccines or effective treatments, while we still do not know the prevalence of the coronavirus in the Spanish population or the duration of immunity. This makes it impossible to predict whether or not this pathogen will continue to circulate in our population, or if it will gradually disappear. Furthermore, recent mathematical models have suggested that some type of social distancing may need to remain in place until 2022.5 It is therefore difficult to predict when the scheduled activity can be resumed. If we look at what happened in China, the number of cases appeared to stabilise at two-digit figures three months after the appearance of the first cases. According to the recent Asia-Pacific Consensus, endoscopic activity at full capacity should only be considered when no cases have been detected for at least two weeks and when stocks of personal protective equipment (PPE) are optimal.6 In Spain, while this document was being written, there were delays in the arrival of appropriate PPE, while other protective equipment of little or no quality7 was also being received. Some experts estimate that healthcare activity, with rooms at full capacity, could be postponed for at least two to three months. However, new requests and the incidence of gastrointestinal diseases will remain unchanged.8 While gastrointestinal endoscopy was not expressly mentioned, the Spanish Society of Preventive Medicine, Public Health and Hygiene expressly positioned itself in favour of not establishing stages in the transition phase of the pandemic; rather, it supports a staged adaptive response.9 The most reasonable solution, therefore, is to reach a consensus on the procedures that need to be carried out with greatest urgency, as well as those procedures that can be postponed. This decision needs to be taken on a case-by-case basis in accordance with the local epidemiological situation. If possible, a consensus should also be reached with the Preventive Medicine Department.
Does the indication for endoscopy need to be reassessed prior to the re-scheduling of activities following the peak of the SARS-CoV-2 pandemic?It is recommended to reassess the indication for endoscopy and to prioritise procedures with the greatest expected benefit.
Open-access Endoscopy Units have been suffering, for a long time, with a healthcare overload, which has been exacerbated with the arrival of colorectal cancer (CRC) screening programmes. Assessing the correct indication is one of the options that will help to reduce said overload. The objective of the guidelines evaluating the suitability of endoscopic requests, such as the European Panel on the Appropriateness of Gastrointestinal Endoscopy (EPAGE), is to assist professionals with decision-making by increasing the probability of detecting relevant lesions.10
The percentage of unsuitability is roughly 30%.11 This implies that, by reviewing the unsuitability of three endoscopy rooms, a full schedule can be freed-up to schedule procedures which have been properly requested. The potential financial savings and reduced waiting times are clear benefits of this. The stoppage of scheduled activity during the peak of the coronavirus pandemic makes it necessary for these measures to be taken.
Which criteria should be employed to prioritise endoscopic procedures in our setting?It is recommended to prioritise endoscopic procedures in accordance with the probability of finding clinically relevant lesions, based on symptoms and complementary tests: mainly positive FOBT in colonoscopies and the presence of anaemia in gastroscopies.
Some of the requests for procedures that are awaiting rescheduling, which are applicable to the most common requests, gastroscopies and colonoscopies, include: 1) therapeutic examinations; 2) colonoscopy as part of a CRC screening following a positive iFOBT test; 3) endoscopy due to new-onset symptoms; and 4) follow-up or vigilance of previous diseases.
ColonoscopyFor both colonoscopies and gastroscopies, therapeutic examinations are the cases that should be resolved most quickly in order to avoid, insofar as possible, a loss of opportunity.
With regards to the priority assigned to the population screening, the QUALISCOPIA study demonstrated a higher overall adenoma detection rate (ADR) (46.4% vs 28.1%) and advanced adenoma detection rate (AADR) (26.3% vs 10.5%) in this population compared to symptomatic subjects.12 The percentage of CRC cases, however, was similar (5.1% vs 4.5%). Some authors have recommended the use of faecal haemoglobin quantification to assign different priority levels.13 However, a recent study has shown that there are no significant differences in the number of CRCs detected or the number of advanced-stage cancers when comparing waiting times of less than 30 days with waiting times of up to nine months.14 Other studies evaluating the waiting list time of CRC patients have also observed no difference in the proportion of more advanced cases when comparing delays of more and less than two months.15
In the third situation, when the examinations are requested due to new-onset symptoms, it seems reasonable to assign a prioritisation system based on the indications, available clinical data and the potential risk of diagnosing relevant diseases. When, in addition to relevant symptoms, there is a positive iFOBT test, it seems reasonable to prioritise these cases. This is because the clinic, in isolation, does not adequately predict the presence of CRC. In fact, according to the COLONPREDICT study, faecal haemoglobin values – either alone or in combination with predictive models – demonstrated greater accuracy than symptoms in detecting CRC.16 The combination of iFOBT and faecal calprotectin have not been shown to increase diagnostic accuracy in detecting CRC.17
Lastly, for patients already included in vigilance programmes, it seems reasonable to allow greater flexibility. Insofar as colonoscopies are concerned, at least, the study by Mangas-Sanjuan et al. observed a cancer risk in polyp follow-up indications of 0.8%, which is much lower than the risk of symptomatic patients or those with a positive iFOBT test result, with an AADR slightly higher than that of patients referred to a clinic due to symptoms.12
Unfortunately, for procedures that were requested due to new-onset symptoms, scientific evidence on waiting list times considered to be acceptable is limited, and the suggested intervals are often the result of expert consensus.18
GastroscopyIn an Italian study, inadequate requests were estimated at 27%, rising to 50% for patients who had previously undergone a gastroscopy. There was a lack of relevant lesions in 82.5% of the cases. The factors that correlated with a higher proportion of clinically relevant lesions (cancer, peptic disease, oesophageal varices, coeliac disease, Barrett’s oesophagus or dysplasia in atrophic gastritis) were the preferential request, adequate follow-up of guidelines and agreement on the level of priority between primary and specialist care.19
On the other hand, referral circuits such as that proposed by the National Institute for Health and Care Excellence guidelines for patients with dyspepsia or gastroesophageal reflux, who are over 55 years old and refractory to treatment, have not shown that more malignant lesions are detected in that group of patients.20 However, some indications showed a higher positive predictive value for the diagnosis of cancer. In order of highest to lowest relevance: dysphagia; weight loss (with or without anaemia); dyspepsia with anaemia or weight loss as alarm signs; the isolated presence of anaemia; and, finally, dyspepsia in isolation.21
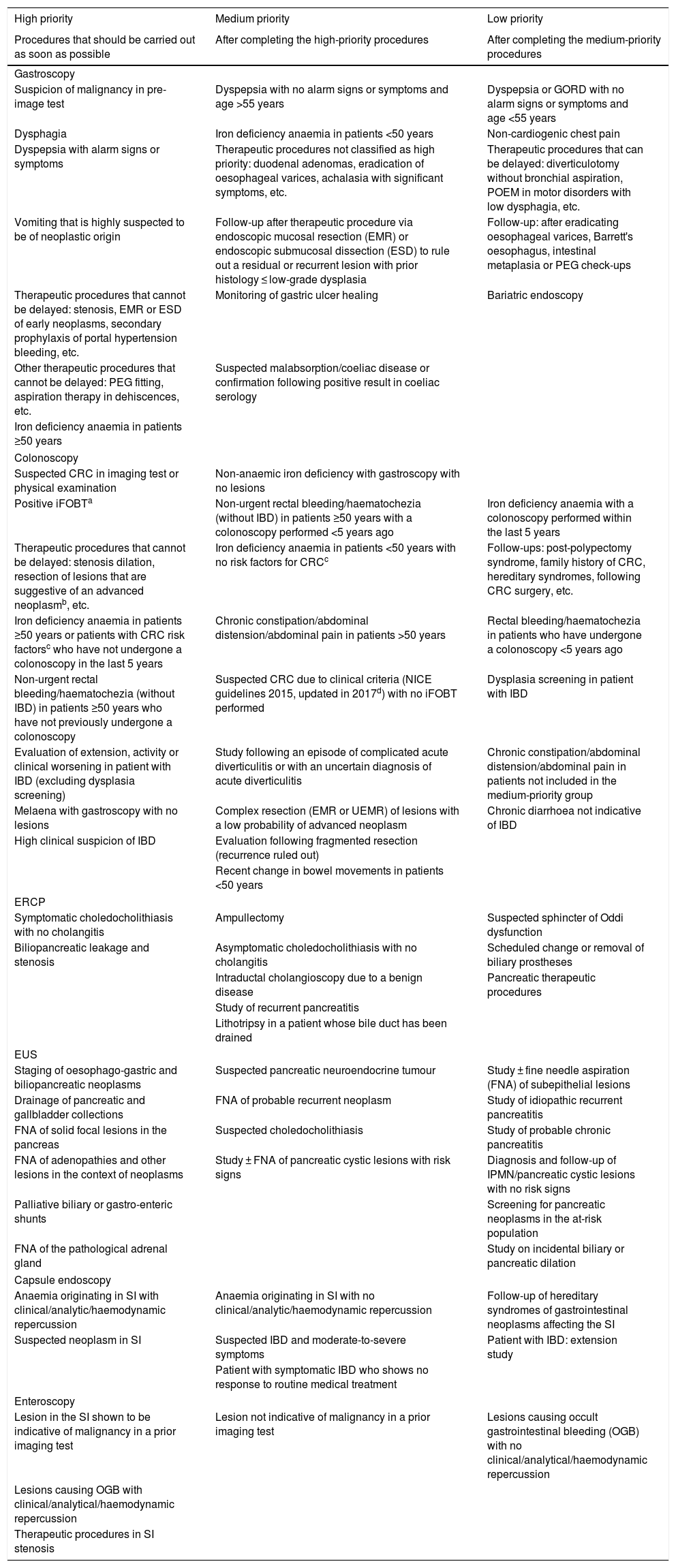
This document includes a table of priorities (Table 2, with a more detailed table being provided in the Appendix: supplementary Table 2) stratified by the most common procedures. For each of these procedures, a series of grounds for request have been defined, which are not intended to be exhaustive. Rather, they are simply conceived to provide guidance for the managers of Endoscopy Units. The recommendations described can be adapted in accordance with availability and the local situation.
Table summarising the priority groups in accordance with the indication.
| High priority | Medium priority | Low priority |
|---|---|---|
| Procedures that should be carried out as soon as possible | After completing the high-priority procedures | After completing the medium-priority procedures |
| Gastroscopy | ||
| Suspicion of malignancy in pre-image test | Dyspepsia with no alarm signs or symptoms and age >55 years | Dyspepsia or GORD with no alarm signs or symptoms and age <55 years |
| Dysphagia | Iron deficiency anaemia in patients <50 years | Non-cardiogenic chest pain |
| Dyspepsia with alarm signs or symptoms | Therapeutic procedures not classified as high priority: duodenal adenomas, eradication of oesophageal varices, achalasia with significant symptoms, etc. | Therapeutic procedures that can be delayed: diverticulotomy without bronchial aspiration, POEM in motor disorders with low dysphagia, etc. |
| Vomiting that is highly suspected to be of neoplastic origin | Follow-up after therapeutic procedure via endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD) to rule out a residual or recurrent lesion with prior histology ≤ low-grade dysplasia | Follow-up: after eradicating oesophageal varices, Barrett's oesophagus, intestinal metaplasia or PEG check-ups |
| Therapeutic procedures that cannot be delayed: stenosis, EMR or ESD of early neoplasms, secondary prophylaxis of portal hypertension bleeding, etc. | Monitoring of gastric ulcer healing | Bariatric endoscopy |
| Other therapeutic procedures that cannot be delayed: PEG fitting, aspiration therapy in dehiscences, etc. | Suspected malabsorption/coeliac disease or confirmation following positive result in coeliac serology | |
| Iron deficiency anaemia in patients ≥50 years | ||
| Colonoscopy | ||
| Suspected CRC in imaging test or physical examination | Non-anaemic iron deficiency with gastroscopy with no lesions | |
| Positive iFOBTa | Non-urgent rectal bleeding/haematochezia (without IBD) in patients ≥50 years with a colonoscopy performed <5 years ago | Iron deficiency anaemia with a colonoscopy performed within the last 5 years |
| Therapeutic procedures that cannot be delayed: stenosis dilation, resection of lesions that are suggestive of an advanced neoplasmb, etc. | Iron deficiency anaemia in patients <50 years with no risk factors for CRCc | Follow-ups: post-polypectomy syndrome, family history of CRC, hereditary syndromes, following CRC surgery, etc. |
| Iron deficiency anaemia in patients ≥50 years or patients with CRC risk factorsc who have not undergone a colonoscopy in the last 5 years | Chronic constipation/abdominal distension/abdominal pain in patients >50 years | Rectal bleeding/haematochezia in patients who have undergone a colonoscopy <5 years ago |
| Non-urgent rectal bleeding/haematochezia (without IBD) in patients ≥50 years who have not previously undergone a colonoscopy | Suspected CRC due to clinical criteria (NICE guidelines 2015, updated in 2017d) with no iFOBT performed | Dysplasia screening in patient with IBD |
| Evaluation of extension, activity or clinical worsening in patient with IBD (excluding dysplasia screening) | Study following an episode of complicated acute diverticulitis or with an uncertain diagnosis of acute diverticulitis | Chronic constipation/abdominal distension/abdominal pain in patients not included in the medium-priority group |
| Melaena with gastroscopy with no lesions | Complex resection (EMR or UEMR) of lesions with a low probability of advanced neoplasm | Chronic diarrhoea not indicative of IBD |
| High clinical suspicion of IBD | Evaluation following fragmented resection (recurrence ruled out) | |
| Recent change in bowel movements in patients <50 years | ||
| ERCP | ||
| Symptomatic choledocholithiasis with no cholangitis | Ampullectomy | Suspected sphincter of Oddi dysfunction |
| Biliopancreatic leakage and stenosis | Asymptomatic choledocholithiasis with no cholangitis | Scheduled change or removal of biliary prostheses |
| Intraductal cholangioscopy due to a benign disease | Pancreatic therapeutic procedures | |
| Study of recurrent pancreatitis | ||
| Lithotripsy in a patient whose bile duct has been drained | ||
| EUS | ||
| Staging of oesophago-gastric and biliopancreatic neoplasms | Suspected pancreatic neuroendocrine tumour | Study ± fine needle aspiration (FNA) of subepithelial lesions |
| Drainage of pancreatic and gallbladder collections | FNA of probable recurrent neoplasm | Study of idiopathic recurrent pancreatitis |
| FNA of solid focal lesions in the pancreas | Suspected choledocholithiasis | Study of probable chronic pancreatitis |
| FNA of adenopathies and other lesions in the context of neoplasms | Study ± FNA of pancreatic cystic lesions with risk signs | Diagnosis and follow-up of IPMN/pancreatic cystic lesions with no risk signs |
| Palliative biliary or gastro-enteric shunts | Screening for pancreatic neoplasms in the at-risk population | |
| FNA of the pathological adrenal gland | Study on incidental biliary or pancreatic dilation | |
| Capsule endoscopy | ||
| Anaemia originating in SI with clinical/analytic/haemodynamic repercussion | Anaemia originating in SI with no clinical/analytic/haemodynamic repercussion | Follow-up of hereditary syndromes of gastrointestinal neoplasms affecting the SI |
| Suspected neoplasm in SI | Suspected IBD and moderate-to-severe symptoms | Patient with IBD: extension study |
| Patient with symptomatic IBD who shows no response to routine medical treatment | ||
| Enteroscopy | ||
| Lesion in the SI shown to be indicative of malignancy in a prior imaging test | Lesion not indicative of malignancy in a prior imaging test | Lesions causing occult gastrointestinal bleeding (OGB) with no clinical/analytical/haemodynamic repercussion |
| Lesions causing OGB with clinical/analytical/haemodynamic repercussion | ||
| Therapeutic procedures in SI stenosis | ||
This priority table is to be used as guidance only, and should not replace proper clinical judgement Urgent or highly preferable procedures performed during the pandemic phase are not included.
CRC: colorectal cancer; EMR: endoscopic mucosal resection; ERCP: endoscopic retrograde cholangiopancreatography; ESD: endoscopic submucosal dissection; EUS: endoscopic ultrasound; FNA: fine needle aspiration; GORD: gastro-oesophageal reflux disease; iFOBT: immunological faecal occult blood testing; IPMN: intraductal papillary mucinous neoplasm; IBD: inflammatory bowel disease; OGB: occult gastrointestinal bleeding; PEG: percutaneous endoscopic gastrostomy; POEM: per-oral endoscopic myotomy; SI: small intestine; UEMR: underwater endoscopic musical resection.
It is advised to adjust the priority level based on the patient's age, gender and the quantitative result of the iFOBT.
Advanced lesion: adenoma ≥10 mm, villous component or high-grade dysplasia. Serrated lesion ≥10 mm or lesion with dysplasia.
Three prioritisation strata are established: 1) high: the procedures to be rescheduled first; 2) medium: procedures to be carried out after 'high priority' procedures; and 3) low: endoscopic techniques to be rescheduled after the ‘medium priority’ procedures. An additional stratum corresponding to ‘low priority’ or vigilance procedures has also been considered, in which the procedure is considered susceptible to an additional delay of 6–12 months. On the other hand, some situations have been included in which it is felt that the procedure can be suspended, based on the information provided in the reason for the request. For other situations, meanwhile, the requesting physician should re-evaluate the need for the procedure (Appendix, Supplementary Table 2). We recommend taking into account other circumstances, in addition to the reasons for the request. These include access to the medical history and a more precise assessment of the request, the time spent on the waiting list, the acceptable delay based on the various priority types and psychosocial factors affecting certain patients.
It was preferred not to establish pre-defined time intervals for the following reasons: 1) it is difficult to predict how the pandemic will evolve; 2) it is not known when the EUs can resume their routine scheduled activity; 3) the scarce and heterogeneous evidence available regarding waiting times; and 4) the different local situations for each endoscopy unit.
It is recommended to monitor the results of the prioritisation plan.
Finally, whenever feasible, and depending on the resources of the institution in question, it is recommended for quantifiable indicators to be collected that allow for an evaluation of the results and the impact of the strategy set forth in this document, in order to assess the achievement of the proposed objectives. These indicators must be frequently re-evaluated in order to minimise the risk for patients on the waiting list. If necessary, the priority assignment plan should also be modified.
Should specific management circuits be put together in order to establish the different priority levels?It is recommended to establish a record of the requests evaluated on the endoscopic procedure waiting list, their priority level and the reasons for cancellation, if applicable.
There is little available evidence on specific management systems regarding the prioritisation of endoscopic activity. In an Italian study, the working teams comprising GPs, gastroenterology specialists and the head of department for the given speciality initially showed a low level of agreement in the assignment of priority levels22 when the so-called homogeneous delay groups (HDG) were created, but this improved over time19. These HDGs assign a priority level and a maximum waiting time per category to each request, regardless of whether the request is for a gastroscopy or a colonoscopy.
In the sites that already have systems for debating the suitability of procedures, similar to the one mentioned above, which functions appropriately, it seems reasonable to keep them in place. Where this is not the case, it is recommended to establish a record of the evaluated requests that are awaiting rescheduling, reflecting the reason for the request, the assigned priority level and the reason for which the procedure was cancelled, if applicable.6
When a procedure is cancelled because it is not deemed appropriate, the reason for this decision should be recorded in the patient's medical record.
With regard to requests that are considered unsuitable and for which rescheduling is not considered to be indicated, it is recommended that the reason for cancellation be reflected in the patient’s medical record, as well as a descriptive commentary justifying the decision, based on clinical practice guidelines (CPGs) or positioning documents. It would also be advisable to propose alternative courses for action based on CPGs.
Should communication channels be established between health service providers in order to report on modifications to the schedule of pending procedures?It is recommended to ensure proper communication between the various individuals involved in the process: assessment specialists, requesting physicians and patients.
As in the previous case, in the sites where the aforementioned multidisciplinary working teams already exist, the communication channel and the continuous improvement cycle will be established and will remain as the fundamental communication tool.
When this tool is not available, it is recommended for each site to make a local decision on the best way to establish communication between users of the healthcare system, requesting physicians and assessment specialists regarding suitability and prioritisation. It is recommended for the cancelled procedures to be reported, at least. This must be communicated to both the requesting physician and the patient.
Should all patients be screened for the SARS-CoV-2 infection prior to undergoing an endoscopic procedure?It is recommended that all patients be screened for the SARS-CoV-2 infection by means of a medical history, aimed at detecting symptoms or signs of COVID-19.
By preventing and controlling the infection, it is possible to ensure the safety of both the healthcare staff and the patients. Many publications support screening for detecting the SARS-CoV-2 infection, although no consensus currently exists on the best strategy.6,23–26 No comparative studies are available that allow us to establish the most cost-effective screening method.
It is recommended to ask the patient about respiratory symptoms, the presence of fever, occupational hazards or recent contact with patients who have been diagnosed with COVID-19, take his/her temperature and consider including questions aimed at detecting gastrointestinal manifestations, anosmia and ageusia.6,23,24 It is recommended for this targeted medical history to be carried out, if possible, on the day prior to the procedure and before the patient enters the EU.25,27 There are no studies evaluating the diagnostic validity of this medical history and symptom-based screening, but the cost of the strategy is small. It could be argued that the gastrointestinal manifestations of COVID-19 may overlap with the indication for the test. According to a recent meta-analysis, the most common gastrointestinal symptoms are anorexia (26.8%), diarrhoea (12.5%), nausea and vomiting (10.2%) and abdominal pain (9.2%), with these being most common in patients with a severe illness.28 In a recent Western series detailing the chronic nature of the symptoms, none of the patients developed gastrointestinal symptoms in isolation or as an expression of COVID-19.29 As such, when gastrointestinal symptoms are found in the screening medical history, attention should be paid to their temporary nature (acute in COVID-19 vs chronic in most scheduled procedures), in addition to the presence of concomitant symptoms. This will allow for a personalised decision to be made.
Different laboratory tests can be employed to diagnose SARS-CoV-2 infection: PCR tests, rapid antigen tests and serological studies.27 Rapid antigen tests have the advantage of yielding immediate results, but their sensitivity is insufficient.30
In China, a thoracic CT scan and an analysis are requested in addition to this triage. This is because radiological findings may precede positive results from the PCR test.26 This strategy, however, is difficult to generalise in Western countries. Furthermore, on the procedure day, a rapid PCR test will be performed if the procedure is urgent (3 h delay). In elective procedures, a PCR will be performed three days prior to the procedure. If the result is negative, the endoscopy is performed. If the result is positive, the procedure is postponed.31,32
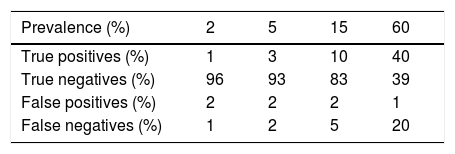
In any case, for these results to be accurate, in addition to requiring sufficient sensitivity to the diagnostic tests, the prevalence of the disease in the local population must be known. Only with this information, as well as the validity parameters of the test, is it feasible to obtain the post-test probability that a specific patient is a carrier of the disease.31 These calculations can be performed intuitively using free online tools (https://calculator.testingwisely.com/playground). Table 3 displays the variation in case identification, maintaining the sensitivity and specificity of the test, while only the prevalence varies between 2% and 60%.
Accuracy of a diagnostic test according to prevalence.
| Prevalence (%) | 2 | 5 | 15 | 60 |
|---|---|---|---|---|
| True positives (%) | 1 | 3 | 10 | 40 |
| True negatives (%) | 96 | 93 | 83 | 39 |
| False positives (%) | 2 | 2 | 2 | 1 |
| False negatives (%) | 1 | 2 | 5 | 20 |
Examples taking into account a sensitivity of 67% and specificity of 98% (positive likelihood ratio = 33; negative likelihood ratio = 0.34) corresponding to those described for PCR in the detection of COVID-19 in the days following the onset of symptoms.
As things stand, no reliable scientific evidence supports screening for SARS-CoV-2 infection by PCR or antibody detection prior to the performance of an endoscopic procedure. For the moment, its diagnostic performance in contexts other than that of symptomatic patients is not well known, and entails considerable interpretation difficulties.
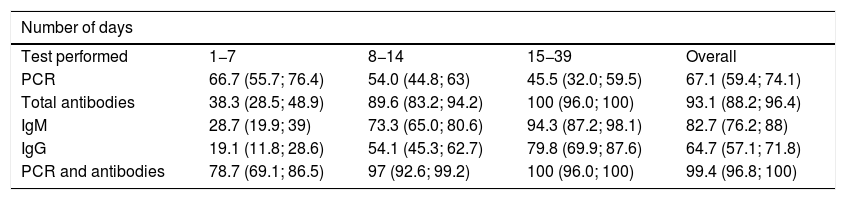
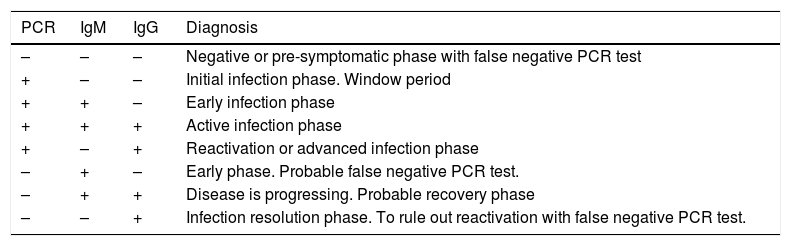
It should also be stressed that sensitivity varies depending on the period of infection, as shown in Table 4.33 The situation that maximises the probability of correct case classification, assuming adequate resource availability, is the combination of PCR and antibody levels during the symptomatic or resolution phase of the infection. However, even in combination, sensitivity remains below 80% during the first week of infection and in subjects with incipient symptoms. In fact, although antibody levels have high sensitivity for detecting previous contagion from the third and fourth week onwards, PCR has low sensitivity for confirming active infection. In addition, existing data on diagnostic accuracy in the pre-symptomatic phase or in individuals with asymptomatic infection are scarce. An overview of the interpretation of these test results is presented in Table 5.34 In addition to the risk posed by a false negative result for healthcare staff and other patients, the disadvantages associated with false positives should be considered. The implications for the latter may be mild (isolation for at least 14 days for the subject and people with whom he/she has come into contact with, as well as absences from work due to temporary disability) or severe (increased likelihood of contagion when passing through circuits established for patients with COVID-19, or increased diagnostic-therapeutic delay by several additional weeks). The expected and desirable reduction in the prevalence of infection in the population will be associated with a decrease in the positive predictive value of the tests. Therefore, there will be an increase in false positives when they are used as universal screening techniques.
Sensitivity (%) and 95% confidence interval of detection tests for SARS-CoV-2 infection stratified by the number of days since the onset of symptoms.
| Number of days | ||||
|---|---|---|---|---|
| Test performed | 1−7 | 8−14 | 15−39 | Overall |
| PCR | 66.7 (55.7; 76.4) | 54.0 (44.8; 63) | 45.5 (32.0; 59.5) | 67.1 (59.4; 74.1) |
| Total antibodies | 38.3 (28.5; 48.9) | 89.6 (83.2; 94.2) | 100 (96.0; 100) | 93.1 (88.2; 96.4) |
| IgM | 28.7 (19.9; 39) | 73.3 (65.0; 80.6) | 94.3 (87.2; 98.1) | 82.7 (76.2; 88) |
| IgG | 19.1 (11.8; 28.6) | 54.1 (45.3; 62.7) | 79.8 (69.9; 87.6) | 64.7 (57.1; 71.8) |
| PCR and antibodies | 78.7 (69.1; 86.5) | 97 (92.6; 99.2) | 100 (96.0; 100) | 99.4 (96.8; 100) |
PCR: detection of viral RNA by polymerase chain reaction.
Modified by Caraguel and Vanderstichel.33
Simplified interpretation of the diagnostic tests for SARS-CoV-2 infection.
| PCR | IgM | IgG | Diagnosis |
|---|---|---|---|
| – | – | – | Negative or pre-symptomatic phase with false negative PCR test |
| + | – | – | Initial infection phase. Window period |
| + | + | – | Early infection phase |
| + | + | + | Active infection phase |
| + | – | + | Reactivation or advanced infection phase |
| – | + | – | Early phase. Probable false negative PCR test. |
| – | + | + | Disease is progressing. Probable recovery phase |
| – | – | + | Infection resolution phase. To rule out reactivation with false negative PCR test. |
PCR: detection of viral RNA by polymerase chain reaction.
For further details, it is recommended to consult the official document of the Spanish Ministry of Health in collaboration with the Spanish Society of Infectious Diseases and Clinical Microbiology.35
It is recommended to delay elective cases that are suspected of having COVID-19. As long as the epidemiological situation in that geographical area entails a high risk of transmission, the rest will be carried out as if all patients were potentially infected.
On the other hand, the availability of PCR and serology tests does not enable us to completely rule out whether or not an individual can transmit the disease. This is because several cases have been reported that had overcome the acute process and subsequently yielded a positive PCR result, either by reactivation or by the presence of traces of viral RNA. In these cases, the PCR yielded a positive result 4–17 days after the previous negative result.36 Nor are there data on whether or not an individual who has overcome the disease can become re-infected and start carrying the virus asymptomatically. Whether or not the antibodies are protective, and if so, for how long they will remain so, is still unknown. In this complex situation it is not feasible, even when combining PCR and serology tests, to make a decision on whether or not to employ additional protective measures. In addition, these imperfect tests are likely to be unavailable. Furthermore, for the time being, there are no clinical data on the cost-effectiveness of said measures. Owing to the above, the use of personal protective equipment (PPE) for all procedures seems reasonable while there is a high prevalence of the disease in the population, in addition to medical history studies and temperature readings. The procedure will be delayed for patients who are suspected of having COVID-19. With the decreased incidence of infection in the population, it is expected that the number of false negative results decline. However, it is also expected that the number of false positive results increase. Finally, these assumptions may change over time: substantial reductions in the prevalence of the infection and the emergence of new tests, that offer greater sensitivity and diagnostic accuracy and which are more independent of the stage of the virus, can dramatically reduce false negative results.37
Is it advisable to establish daily meetings of EU staff in order to specifically discuss the working method?It is recommended to hold meetings with all members of EU staff at the start of each working day. This will allow all parties to be fully aware of the action protocols, and will ensure compliance with them.
Decision-making capacity, communication between the different strata of healthcare staff and with the patient, anticipation of complex situations and leadership skills are all crucial in forming a successful team.36 With the current high risk of transmission of the SARS-CoV-2 infection in the hospital setting, it is crucial to review EU protocols regarding the circulation of patients and their accompanying parties, screening strategies for COVID-19, the availability of PPE and disinfection measures for endoscopy rooms and equipment. Each member of the team must be familiar with the work flow, and each of the responsibilities need to be clearly defined.
It is recommended to wear a surgical mask and to adhere to the social distancing measures proposed by the WHO during working team meetings.
Given that the action guidelines are constantly evolving, it seems reasonable for meetings to be held on a daily basis.25 It is recommended for representatives of each working group to meet in a large, well-ventilated area while following the recommendations established on social distancing and the use of surgical masks.27,38
Should the entry of accompanying parties to the EU be restricted?Except for in selected cases, it is advisable for patients' accompanying parties not to enter the EU. If they need to enter, they should be screened for SARS-CoV-2.
As per the recommendations of the European Society of Gastrointestinal Endoscopy (ESGE), family members and carers should not enter the EU.27 If they need to enter under exceptional circumstances, they should be screened for SARS-CoV-2 in the same way that the patients are.30 These recommendations are also considered in other publications.6,24,38
Should any protective measures be recommended for patients entering the endoscopy unit?It is recommended for patients to maintain a minimum distance between people of 1−2 metres during their time in the EU.
It is recommended that patients wear surgical masks, hospital clothing and take appropriate steps to ensure their hand hygiene.
The general measure recommended by the WHO for social distancing also applies to the EU setting. The minimum recommended distance between people is 1 m.27,38 Prior to entering the EU, patients will be provided with surgical masks and, if available, hospital clothing.24,39 It is also recommended for pertinent measures to be taken in order to ensure good hand hygiene (such as washing hands with running water and soap, or using a hydro-alcoholic solution), at least before entering and leaving the EU. These measures shall be maintained or gradually withdrawn in accordance with the current epidemiological situation.
Is it necessary to modify the usual time recommended for each endoscopic procedure?Due to the need to implement additional health and safety measures, it is recommended to modify the time assigned to each endoscopy and the volume of procedures.
The circulation of patients in the EU is expected to be slower as a result of SARS-CoV-2 infection screening and hygiene measures. With regards to healthcare staff, the need to put on and take off PPE could slightly reduce the amount of time that they are available to work on procedures. Each EU should also establish with the cleaning staff the times at which the rooms will be disinfected. If, as is recommended, cleaning takes place between each procedure, this will entail an additional delay.
Furthermore, until the pandemic is successfully contained, the reduced exposure of the population to the hospital setting remains a priority. This involves reducing the amount of people in waiting rooms, in order to ensure appropriate social distancing. The most reasonable way of achieving this is to reduce the elective endoscopic activities that take place in each examination room.24 If possible, it is recommended to schedule morning and afternoon shifts. This allows the procedures to be spaced out, and the endoscopic activity to be maintained.
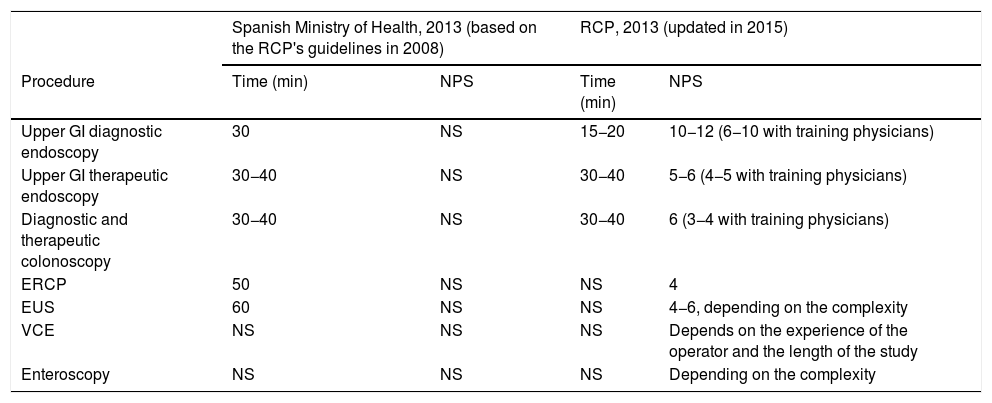
The Spanish Ministry of Health has published the times to be allocated to endoscopic procedures based on the British CPGs for 2008. However, these only reflect the time to be spent on each procedure, and not the total amount of time per working day. Neither do they consider the times for particularly complex procedures such as the mucosal resection of large lesions, endoscopic dissection or enteroscopy procedures. Table 6 contains the current recommendations in Spain and the latest edition of the British CPGs.
Comparison of the recommendations for time and the number of procedures to assign per endoscopy schedule between the standards of the Spanish Ministry of Health and the Royal College of Physicians (RCP).
| Spanish Ministry of Health, 2013 (based on the RCP's guidelines in 2008) | RCP, 2013 (updated in 2015) | |||
|---|---|---|---|---|
| Procedure | Time (min) | NPS | Time (min) | NPS |
| Upper GI diagnostic endoscopy | 30 | NS | 15−20 | 10−12 (6−10 with training physicians) |
| Upper GI therapeutic endoscopy | 30−40 | NS | 30−40 | 5−6 (4−5 with training physicians) |
| Diagnostic and therapeutic colonoscopy | 30−40 | NS | 30−40 | 6 (3−4 with training physicians) |
| ERCP | 50 | NS | NS | 4 |
| EUS | 60 | NS | NS | 4−6, depending on the complexity |
| VCE | NS | NS | NS | Depends on the experience of the operator and the length of the study |
| Enteroscopy | NS | NS | NS | Depending on the complexity |
ERCP: endoscopic retrograde cholangiopancreatography; NPS: number of procedures per schedule; NS: not specified; EUS: endoscopic ultrasound; VCE: video capsule endoscopy.
*Includes the entire process: sedation, procedure and report.
In short, while the time spent on the procedure itself may not vary substantially, it is foreseen that periprocedural times will be significantly prolonged. The percentage of endoscopic cabinet occupation will depend on the reduced incidence of infected people and the availability of physical and material resources, including waiting room capacity, in order to ensure a minimum distance between people of one metre.
ProcedureIs it necessary to establish a separate circuit for patients with highly-suspected or confirmed SARS-CoV-2 infection?It is recommended to establish a separate circuit for patients with highly-suspected or confirmed SARS-CoV-2 infection.
The SARS-CoV-2 virus can remain on surfaces for more than three days, and can be suspended in aerosols for three hours or more.41 Considering its mechanism of transmission, it is recommended to establish a separate circuit for patients with highly-suspected or confirmed infection.6,23,27,42,43 Both circuits must be kept separate from one another before, during and after the endoscopy. This circuit must include a toilet and sink for the patient, since the aspiration current produced during the evacuation of faecal waste generates aerosols that may have an infectivity capacity.44
It is recommended that endoscopic procedures on patients with highly-suspected or confirmed infection, which cannot be delayed, be performed in rooms with negative pressure. It is also recommended to introduce a gap of at least 30 min between each procedure.6,45 If the EU does not have a room with these characteristics, as is frequently the case in our setting, it is recommended to perform the procedure in a negative-pressure room that is located outside of the unit. If the site does not have a negative pressure room, it is recommended to set up a room that is well ventilated and which has separate environments for this group of patients. The installation of a negative-pressure system should also be promoted. Lastly, it is recommended to schedule these procedures at the end of each shift, if the clinical condition of the patient so allows.
Which hygiene and protection measures should be implemented for healthcare staff? If personal protective equipment is not available, should the endoscopy go ahead? Is it recommended to disinfect and re-use FFP2-3/N95 masks?It is recommended to encourage the application of basic hygiene measures in order to prevent staff members from becoming infected.
Hand hygiene is a crucial measure that has proven to be effective in various clinical trials and meta-analyses.46,47 The implementation of programmes that ensure correct hand hygiene practices improves the efficacy of these measures, and should therefore be guaranteed in all EUs.48 Changing out of work uniform at the end of each shift and showering prior to leaving the hospital are basic hygiene measures that are recommended by the European Centre for Disease Prevention and Control (ECDC).49
It is recommended for all healthcare staff involved in the performance of endoscopic procedures to wear PPE.
No studies have been found that compare different PPE during the SARS-CoV-2 epidemic. The Spanish Ministry of Health indicates that PPE must be certified in accordance with Regulation (EU) 2016/425, which is evidenced by the CE marking.50
The ESGE and Asia-Pacific Consensus guidelines suggest that PPE can be decided on the basis of the patient's risk of infection, with the main difference being the type of mask (surgical masks for low-risk patients vs. FFP2-3 masks for high-risk patients) and the hospital gown (minimal or moderate protection). It is recommended for all procedures to be undertaken using the equipment listed in Table 7, regardless of the a priori risk of infection to the patient. The decision to not stratify PPE is due to multiple reasons: 1) the level of the epidemic in Spain; 2) the possibility of aerosol generation; 3) the lack of validated infection screening strategies; and 4) to guarantee the maximum safety of healthcare staff. It is recommended to establish a clean zone, which is physically separated from the endoscopy room, in which staff can put on their PPE. Our recommendations on PPE are based on the guidelines of the WHO, the ECDC, the Spanish Government and other scientific societies.6,27,45,49,50,53,54
Recommended personal protective equipment (PPE) in the Endoscopy Unit.
| Location | Personal protective equipment |
|---|---|
| Endoscopy room | FFP2-FFP3/N95-N99/PAPR maska |
| Disposable cap | |
| Long-sleeve disposable gown that is resistant to penetration by microorganisms | |
| Wearing two pairs of gloves | |
| Air-tight eye protection or protective visor | |
| Disposable shoe covers or easy-to-wash closed waterproof shoes | |
| Cleaning room | Surgical/FFP2/N95 maskb |
| Biopsy processing room | Long-sleeve disposable gown that is resistant to penetration by microorganisms |
| Gloves | |
| Eye protection or protective visor | |
| Disposable shoe covers or easy-to-wash closed waterproof shoes | |
| Reception or triage area | Surgical mask |
| Distance of 1−2 m | |
| Gloves (optional) | |
| Physical barrier or protective screen | |
| Administration area | Surgical mask (optional) |
| Distance of 1−2 m | |
| Physical barrier (if available) | |
| Waiting room | Surgical mask |
| Distance of 1−2 m |
PAPR: powered air-purifying respirator.
In line with the American Gastroenterological Association, we recommend the use of FFP2-3/N95-N99 masks in patients who are classified as being at a low risk of infection.45 The use of FFP3 masks is preferred in patients with highly-suspected or confirmed infection. The masks must comply with the UNE-EN 149:2001 + A1:2009 standard.50 If available, it is recommended to use one FFP2-3/N95-99 mask per patient, as the endoscopy generates aerosols that may contaminate the surface of the mask. There is no evidence to support the safety of re-use (>5 uses) or prolonged use (>8−12 h) of masks in the SARS-CoV-2 pandemic.45,55 The WHO and the Spanish Ministry of Health suggest that the useful life of the mask could be extended in situations in which resources are scarce.50,54 Wearing a disposable surgical mask on top of these masks is another measure that reduces the risk of FFP2-3/N95-99 mask contamination, and may serve to prolong their useful life.55 The disinfection and re-use of masks should only be considered as a last resort.55 In this case, said actions must be undertaken in accordance with a protocol endorsed at the institutional level.
Protective gownThe member of staff's uniform must be protected from possible splashes of biological fluids or secretions. While the CPGs do not specify the preferred gown type for the endoscopy, this material must comply with the UNE-EN 14126:2004 standard which includes specific tests for resistance to penetration by microorganisms.50 It is recommended for these to be single-use gowns, thus avoiding potential contagion between patients.50 The Centers for Disease Control and Prevention (CDC) and the ECDC recommend using a single waterproof gown per aerosol-generating procedure in patients with highly-suspected or confirmed infection (it does not necessarily need to be classified as a surgical gown).49,56 If these resources are not available, the possibility of wearing reusable gowns (treated by standardised washing protocols) or disposable plastic gowns on top of more waterproof gowns will be considered.56
All healthcare staff in Endoscopy Units should receive training on how to use PPE. PPE training has been shown to reduce the risk of inadvertent contamination, and is a prerequisite to using this equipment.57–59
Eye or face protectionAppropriate eye and face protection must be ensured during the endoscopy. A recent study showed that unnoticed splashes onto the face of the endoscopist are relatively common. The rate of exposure to microorganisms with infectivity capacity was 5.6 per every 100 days of endoscopy.60 The type of exposure that exists during the digestive endoscopy means that protective goggles (compliant with the UNE-EN 166:2002 standard) and face visors are recommended.50
GlovesGloves must be disposable, and they must comply with the UNE-EN ISO 374.5:2016 standard. Wearing two pairs of gloves at the same time has been shown to reduce the risk of contamination when taking off PPE, compared to wearing one pair of gloves.45,61 Administrative staff who do not come into contact with the patient are not required to wear gloves.50
If the PPE required to ensure the safety of the endoscopy is not available, this procedure is not advised.
Healthcare staff involved in a digestive endoscopy are at a high risk of infection.62,63 The use of appropriate PPE is crucial, not only to safeguard the health of healthcare staff, but also to stop them becoming carriers of the virus. The competent authorities should be required to ensure the availability of appropriate PPE. In line with the recommendations put forward by the ECDC, it is recommended to ensure the availability of 3–6 items of full PPE per procedure in order to ensure the protection of all healthcare staff.49
In procedures with a high probability of aerosol generation, is it advisable to use additional barrier methods over the patient's naso-buccal area or the valves of the endoscope?The systemic use of additional barriers over the patient's naso-buccal area, or the valves of the endoscope, is not recommended. The usefulness and efficacy of these measures should be evaluated within research protocols.
No recommendations or studies have been found which demonstrate the benefit of using additional devices over the naso-buccal area. Case series and case reports of urgent endoscopic procedures performed during the coronavirus epidemic, in which additional barrier devices were used, have been published.64,65 However, the benefit and efficacy of these measures remains unknown.
Should any specific precautions be taken for oxygen therapy during sedation?In patients with highly-suspected or confirmed SARS-CoV-2 infection and who are undergoing a colonoscopy, it is advisable for oxygen therapy to be supplemented with exhalation filter masks. If these devices are not available, it is recommended to place a surgical mask above the nasal tubes or Venturi mask.
The supplementary oxygen therapy during sedation generates aerosols. When nasal tubes are fitted, the distance that the exhaled particles can reach is directly proportional to the flow of oxygen. It is therefore important to adjust the flow to the physiological requirements of the patient, regardless of the risk of infection.60,61
For high-risk patients or those who are already confirmed as having SARS-CoV-2 infection, the Spanish Ministry of Health recommends the use of masks with exhalation filters.66 These devices can be worn during a colonoscopy, so their use is recommended during these procedures. If these devices are not available, it is recommended to place a surgical mask above the nasal tubes or Venturi mask.67
Who should carry out the endoscopic procedures?It is recommended that procedures on patients with highly-suspected or confirmed SARS-CoV-2 infection be carried out by strategically assigned experienced personnel.
The number of staff in the endoscopy room should be reduced to the minimum in order to reduce the risk of exposure and transmission. It is recommended for procedures to be carried out by experienced and independent endoscopists. This recommendation is unanimously endorsed by scientific societies.6,27,45 It is also advisable for nursing staff and auxiliary technicians to be trained and suitably experienced in the procedure that they are going to carry out. Members of staff currently undergoing training are advised not to intervene in these procedures, in order to reduce the risk of infection and the procedure time.
It is recommended for Gastroenterology residents to participate in endoscopic procedures on patients with a low risk of SARS-CoV-2 infection, provided that the necessary resources for ensuring the safety of the procedure are available.
No specific recommendations have been found on how endoscopic training should be carried out in the current epidemiological context. It is essential to promote and guarantee the continuity of academic training after the peak of the coronavirus pandemic. The undersigned scientific societies are openly in favour of Gastroenterology residents continuing to perform procedures, under direct supervision, on patients who are deemed to have a low risk of having SARS-CoV-2 infection. The return of residents to the endoscopy room should be done in a phased manner, based on their level of previously-acquired skills and the foreseen complexity of the procedure. This recommendation is subject to the availability of human and material resources, as well as the local epidemiological situation.
The promotion of theoretical training, the use of e-learning tools and training in endoscopy simulators (when available) are also recommended.
How should endoscopic biopsies be processed?It is recommended for biological samples to be processed in line with the standardised biosecurity protocols for substances with a high infectivity capacity.
In our search, no specific recommendations were found regarding the processing of endoscopic biopsies. The WHO suggests that the faecal-oral route does not play a significant role at the community level. However, it states that faecal-oral transmission is possible, based on indirect data.28,43,68 In this regard, culture of live virus in faecal samples has been reported.69 In a recent meta-analysis, 48.1% of patients with SARS-CoV-2 infection presented with viral RNA in their stool sample. The presence of faecal RNA remained in 70.3% of the patients, despite the absence of RNA in the nasopharyngeal exudate, including patients in whom symptoms had first appeared 33 days earlier.28
Until further information is available on the subject, we consider that all samples obtained during the endoscopy should be processed as potentially infective of SARS-CoV-2. The processing and transportation of these samples should be carried out according to the WHO's recommendations for biological material with infectivity capacity, or local standardised protocols that have been agreed with other services.70 It is advised for all EU staff involved in the handling or transportation of samples to wear the PPE mentioned in this document. It is also recommended to list, in the request form sent to the receiving service, whether the sample comes from a patient who is suspected of or confirmed as having SARS-CoV-2 infection.71
We recommend against taking biological samples during the endoscopy when the clinical impact of the result is expected to be marginal.
The taking of unnecessary biopsies during the endoscopy is a practice that is documented in previous studies, leading to greater cost and risk of bleeding events.72,73 It is recommended to optimise the taking of biopsies by following the indications established by the CPGs. This premise becomes particularly relevant by considering the infectious potential of biological samples and the need to optimise material resources.
Post-procedureHow should devices, equipment, surfaces and endoscopy rooms be disinfected? How should waste be managed?It is advisable for the disinfection and re-processing of endoscopes to be carried out according to the usual protocols.
SARS-CoV-2 is an enveloped virus, which makes it sensitive to commonly-used disinfectants.74 There is no evidence to support the need for differential disinfection and re-processing in patients with SARS-CoV-2 infection. All scientific societies agree that pre-pandemic disinfection guidelines remain valid in the current context.6,27,45,51,74,75 It is advisable for all centres to review and ensure the correct implementation of these recommendations.
It is not recommended for single-use devices to be used more than once.
The ESGE's CPGs from 2018 advise against the re-use of single-use endoscopic accessories.75 This recommendation is particularly relevant in the current context. If resources are available, it is advised to prioritise the use of single-use endoscopic accessories over ones that can be used multiple times.6,75
It is recommended to apply protocols for the cleaning and disinfection of endoscopy rooms and materials that have come into contact with the patient or his/her secretions.
Commonly-used cleaning agents that comply with the European standard EN 14885 are valid in the current context, and can be consulted in other sources.74,75 For patients with highly-suspected or confirmed SARS-CoV-2 infection, we recommend a thorough disinfection of the room after each endoscopy.6,27 If a negative-pressure system is not available, the room should be ventilated for at least 1−3 h between procedures.74 If said room does not have exterior windows, the use of high-efficiency particulate air (HEPA) filters or alternative methods of disinfection, such as ultraviolet rays or ozone, is recommended.74,76
For patients who are considered to be at low risk of infection, standard cleaning protocols are recommended.6,27 All surfaces must be disinfected after each procedure, including bedding and railings, the floor, the endoscopy tower, vital signs monitoring equipment and any other devices that may have come into contact with the patient. At the end of each shift, it is recommended to carry out a thorough cleaning of the walls, furniture and all equipment present in the room.6,74
The assignment of dedicated EU cleaning staff is recommended.
In light of the expected increased requirements for disinfection tasks, the AEG and the SEED recommend assigning dedicated EU cleaning staff. This facilitates the correct training of cleaning staff and reduces room cleaning delays.
It is recommended for waste management to be carried in accordance with the local protocols of each centre for category B waste with high infectivity capacity (UN3291).
The waste management of the gastrointestinal EU should not be managed on an individual basis; rather, it should be managed within the framework of hospital protocols that comply with current regulations. The ECDC recommends that the waste be managed as category B highly infectious waste material (UN3291).74 Disposable PPE should be placed in the corresponding bins and managed as class iii bio-sanitary waste.50
Should any additional measures be taken in the recovery rooms following the performance of an endoscopy?It is advised to maintain a distance between people of 1−2 metres, implement basic hygiene measures and establish a separate patient circuit in the recovery rooms.
Protective measures and basic hygiene practices must be maintained after the procedure. The recovery room for patients at high risk of infection must be physically separated from the recovery room for patients at low risk of infection.6,22,26,38 It is recommended to instruct patients on how to safely take off their gloves and surgical mask, and to encourage them to adopt hand hygiene practices when they leave the EU.
Should healthcare staff take off their PPE in the same room in which the procedure was performed, or should they take it off in a room designated for this purpose?It is recommended for healthcare staff to remove their PPE in a specific room or hallway that is designated for this purpose. If a room of this description is not available, we recommend taking off the PPE outside the endoscopy room; ideally, in an area of transition between the clean area and the contaminated area.
The removal of PPE is a fundamental step in ensuring the safety of the procedure. The PPE should be taken off following a pre-defined sequence in an area that is neither in the EU's clean area or exposure area. The ECDC and the CDC agree that PPE should not be taken off in the same room in which the procedure was performed.77,78 The protective equipment can be taken off in a room or a hallway designated for this purpose. If a room of this description is not available, staff are advised to take off the PPE outside the endoscopy room; ideally, in an area of transition between the clean area and the contaminated area.77 If this is not feasible, staff are advised to take off the PPE by the door to the endoscopy room. The face mask should be taken off last of all. This should always be taken off outside the part of the EU that is potentially contaminated. It is crucial to perform suitable hand hygiene practices immediately after removing PPE.50
Is it necessary to evaluate the onset of SARS-CoV-2 infection after the endoscopic procedure?It is recommended to consider the implementation of follow-up programmes for patients 7−15 days after the procedure to assess the onset of symptoms that are compatible with COVID-19.
The principle of traceability and post-intervention infection control is a maxim of the quality of the endoscopic procedure.79 Most authors and societies agree that the incidence of SARS-CoV-2 infection should be evaluated following an endoscopic procedure.6,23,25,27 The aim is to detect possible sources of transmission in the EU at an early stage. This measure also serves to quickly identify the EU staff and patients who came into contact with a person confirmed as having COVID-19 detected after the procedure. The contact should be established via telephone or online, and should not be face-to-face.
On the other hand, the implementation of this measure poses logistical problems, and there is no direct evidence supporting its benefits. In a recent study covering 851 procedures carried out between 27 January and 13 March 2020 in hospitals in northern Italy, the response rate was 94.1%. The contact was established over the phone by a nurse and study coordinators. Eight patients (1%) developed symptoms compatible with COVID-19. Its retrospective nature, the lack of evaluation of the infection by means of laboratory tests, and the fact that the study was not conducted during the peak of the coronavirus pandemic are major limitations, which mean we need to interpret these results with caution.80
Finally, patients who develop COVID-19 may mistakenly assume that the contagion took place in the EU, so it is crucial to inform the patient of the purpose of this contact. As such, the decision to establish this circuit should be made locally depending on the available resources involving other hospital services responsible for infection control, and preferably within a research protocol to evaluate its efficacy.
FundingNone.
Conflicts of interestNone.
Review committee:
AEG: Francesc Balaguer, Luis Bujanda, Gloria Fernández-Esparrach, Begoña González Suárez and Angels Ginés.
SEED: Carlos Dolz Abadía, Maite Herráiz Bayod, Vicente Lorenzo-Zúñiga, Manuel Pérez-Miranda, Vicente Pons and Juan J. Vila.
We would also like to thank the following representatives of scientific societies for endorsing this document:
Please cite this article as: Marín-Gabriel JC, Rodríguez de Santiago E, en representación de la Asociación Española de Gastroenterología y la Sociedad Española de Endoscopia Digestiva. Documento de posicionamiento AEG-SEED para el reinicio de la actividad endoscópica tras la fase pico de la pandemia de COVID-19. Gastroenterol Hepatol. 2020;43:389–407.