Anti-tumour necrosis factor agents (anti-TNF) drugs are commonly used in patients with inflammatory bowel disease (IBD) and have proven effective in both induction and maintenance therapy in luminal Crohn's disease and ulcerative colitis. Their efficacy has also been proven in fistulising perianal Crohn's disease. However, the evidence in other scenarios, such as stricturing, penetrating and non-fistulising perianal Crohn's disease, extraintestinal IBD manifestations and ileoanal reservoir complications, is not as robust. The aim of this review was to perform an analysis of the available literature and to determine the role of anti-TNF drugs in common clinical practice in patients affected by these complications.
Los agentes anti-factor de necrosis tumoral (anti-TNF) son fármacos de uso común en los pacientes con enfermedad inflamatoria crónica intestinal (EICI) y han demostrado ser efectivos en inducción y mantenimiento en enfermedad de Crohn y colitis ulcerosa, así como en pacientes con afectación fistulizante perianal. Sin embargo, la evidencia relativa al uso de estos fármacos en otros escenarios dentro de EICI es menos sólida. Es el caso de la enfermedad de Crohn con afectación estenosante, penetrante o perianal no fistulizante, de las manifestaciones extraintestinales de la EICI y de las complicaciones del reservorio ileoanal. El objetivo de esta revisión fue realizar un análisis de la literatura disponible y determinar el papel de los anti-TNF en la práctica clínica en pacientes afectos por estas complicaciones.
The main anti-tumour necrosis factor agents (anti-TNF) available on the market are infliximab, adalimumab, golimumab, certolizumab pegol and etanercept. Despite having different molecular structures, there do not seem to be any differences in their ability to neutralise tumour necrosis factor or modulate lymphocyte apoptosis.1
The use of anti-TNF is approved in numerous autoimmune diseases. Etanercept has not been shown to be effective in chronic inflammatory bowel disease (IBD), but some authors associate the negative results obtained in certain studies with its use at insufficient doses.2 In other cases, the use of etanercept has even been implicated in the development of chronic IBI as a paradoxical effect, above all in patients treated for spondyloarthritis; this is more common with etanercept than with other anti-TNF agents.3 Infliximab, adalimumab and golimumab are approved by both the United States Food & Drug Administration and the European Medicines Agency (EMA) in ulcerative colitis (UC). In Crohn's disease (CD), use of infliximab and adalimumab is approved in both the United States and Europe; certolizumab pegol, only in the United States.4,5
These drugs were approved after demonstration of their effectiveness in different quality studies. Infliximab, adalimumab and golimumab were shown to be more effective than placebo in the ACT, ULTRA and PURSUIT studies, respectively, in the treatment of UC.6 In addition, in acute severe colitis secondary to UC, there seem to be no statistically significant differences between treatment with infliximab and ciclosporin.7 In CD, infliximab, adalimumab and certolizumab pegol have shown superiority to placebo,8 with infliximab also doing so in perianal fistulising disease.9 Some studies also have data showing efficacy of adalimumab in this context.10
However, IBD is a chronic disease and, given the early age of onset, it generally lasts for a long period of time. In the specific case of CD, although the disease does not tend to change location, a change in the pattern of behaviour is not so uncommon. It is estimated that up to 50% of affected patients will develop some type of stricturing or penetrating complication 20 years after diagnosis.11 Currently, the classification of CD behaviour into inflammatory, stricturing or penetrating is considered too static and the concept of cumulative chronic intestinal damage is becoming increasingly popular.12 Intestinal damage continues to be the main indication for surgery in patients with CD and, although the frequency of surgery seems to have been decreasing over recent decades,13 the risk is still estimated at 40–71% ten years after diagnosis. Surgery does not definitively cure the disease and recurrence is the norm, so further interventions are sometimes necessary. Other scenarios in IBD can also lead the patient to require surgical intervention, such as aggressive non-fistulising perianal involvement, which results in proctocolectomy in up to 43% of cases.14 Pouch failure in patients who have undergone colectomy is estimated to occur in 15% of cases at 10 years, and high morbidity rates have been associated with pouch excision.15
Moreover, chronic IBD can cause damage to other organs as a result of extraintestinal manifestations, which can also significantly reduce quality of life.
For all these reasons, it is important to find an effective medical treatment for the above scenarios in order to avoid surgery and improve quality of life in this population. As the process underlying these manifestations derives from local inflammation, it has been postulated that treatment with anti-TNF may be a useful option.
The aim of this review is to synthesise the results of the different published studies in which the role of anti-TNF in complicated chronic IBD has been analysed, excluding luminal disease and perianal fistulising disease, scenarios in which there is already quality supporting evidence.
Anti-TNF in stenosing diseaseApproximately 10% of patients already have CD complicated by strictures at diagnosis and, among those who exhibit non-stricturing, non-penetrating behaviour, up to 25% will develop some stricture after 5 years. Moreover, the resulting obstructive symptoms account for 40% of the indications for major surgery in this population.16
When considering stricturing disease, it is also important to mention the fibrogenesis process which, although not yet fully understood, is currently the subject of extensive research. Inflammation seems to be the trigger of the fibrosis, the main inflammatory mediator of which is tumour growth factor. However, it seems that fibrosis may subsequently progress independent of inflammation, due to the presence of fibroblasts permanently activated in response to previous tissue damage producing extracellular matrix in larger than physiological amounts, in conjunction with an imbalance in the regulatory proteins in the extracellular matrix, which would stop the matrix from being broken down.17 Although strictures have traditionally been classified as inflammatory or fibrotic, it seems that both components are present to varying degrees. The extent of the involvement of each component determines treatment decisions; for predominantly inflammatory strictures we will consider the use of anti-inflammatory medical treatment, while for predominantly fibrotic strictures we are more likely to opt for endoscopic or surgical treatment. The use of a medical antifibrotic treatment could be an option in this scenario in the future. However, these drugs are currently in the very early phases of research.
Considering the importance of the nature of the strictures, they need to be classified with imaging tests; there are no optimal techniques at present, but promising results have been published with some special magnetic resonance imaging (MRI) sequences.18
Despite the fact that anti-TNF used at an early stage of CD seem to prevent the development of strictures, in the 2000s an alarm sounded after the publication of series, most of them retrospective, of patients with CD treated with anti-TNF who developed stricture-related or obstructive complications.19 At that time, the hypothesis was raised that the rapid healing achieved with anti-TNF might result in the development of strictures.
However, subsequent analyses of large cohorts of patients treated with infliximab, such as the TREAT or ACCENT cohorts, showed that neither the use of infliximab nor the achievement of rapid mucosal healing with this drug were associated with an increased risk of strictures or obstructive symptoms.20
Once the initial doubts were resolved, study commenced of the effectiveness of anti-TNF in the treatment of patients with CD complicated by strictures. In the study by Pallotta et al.21 of 15 patients with stricture complications treated with infliximab for other indications, none experienced a worsening of the strictures and, even more interestingly, 60% achieved remission.
Adalimumab has also been studied in this context. The results have recently been published of the multicentre, prospective CREOLE study,22 in which the drug was administered to patients with stricturing ileal CD. After six months, two thirds of the cohort exhibited a response, defined as adalimumab maintenance without the need for steroids or change of anti-TNF agent, surgery or endoscopic dilation, and half of the responders continued to have sustained response after four years of follow-up. In addition, over 50% of the patients in the whole cohort had not required surgery four years after the start of the study. Despite these positive results, the high percentage of reported complications (72%) does need to be mentioned, although that figure includes the need for surgery, dilation or hospitalisation, which could be considered more as treatment failures than as true adverse events. It should also be noted that in the evaluation of the response to treatment, clinical criteria were not taken into account and it is not stated whether or not the patients had a significant improvement in the clinical obstruction score designed by the authors after the treatment.
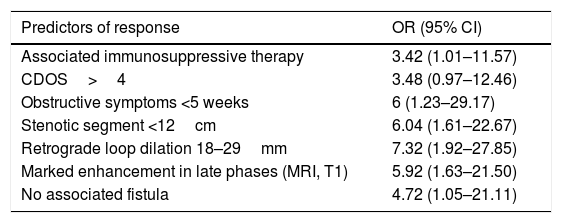
In our study, we also assessed predictors of good response to treatment (Table 1) and better outcomes were found in patients treated with adalimumab combined with an immunosuppressant, with short strictures, marked and short duration obstructive symptoms and moderate retrograde dilation of loops. Intense uptake on late T1-weighted sequences in MRI was also associated with good response. Although the significance of the intense uptake is not entirely clear, it may correspond to the presence of severe inflammation. Last of all, when stratifying the cohort according to the predictors of good response, the authors found that a much higher proportion of those with four or more responded compared to those with two or less (89% vs 6%, respectively).
Predictors of good response to anti-TNF treatment in patients with stenosing Crohn's disease (results of the CREOLE study).
| Predictors of response | OR (95% CI) |
|---|---|
| Associated immunosuppressive therapy | 3.42 (1.01–11.57) |
| CDOS>4 | 3.48 (0.97–12.46) |
| Obstructive symptoms <5 weeks | 6 (1.23–29.17) |
| Stenotic segment <12cm | 6.04 (1.61–22.67) |
| Retrograde loop dilation 18–29mm | 7.32 (1.92–27.85) |
| Marked enhancement in late phases (MRI, T1) | 5.92 (1.63–21.50) |
| No associated fistula | 4.72 (1.05–21.11) |
CDOS: Crohn's Disease Obstructive Score; MRI: magnetic resonance imaging.
Patients with CD have a 20–40% likelihood of developing some type of fistula.23 Fistulas are usually classified as internal, including enteroenteric, enterovesical, and rectovaginal; and external, which include perianal, enterocutaneous and peristomal.
The above classification seems to have therapeutic relevance, as external fistulas have been found to respond better to infliximab than internal fistulas. Of the external fistulas, the perianal in particular seem to show a better response than the enterocutaneous. Perianal fistulas have also been found to respond much better when they are isolated than when found in association with other types of fistula. This might suggest that non-perianal fistulising disease is a more aggressive disease phenotype.24
As far as the effectiveness of anti-TNF in non-perianal fistulising disease is concerned, it is important to point out first of all that the existing evidence is of poor quality and comes from retrospective studies, case series and subgroup analyses.
In the case of enterocutaneous fistulas, some studies have evaluated external fistulas as a whole, including perianal disease. These studies include several subgroup analyses from clinical trials of adalimumab versus placebo, only one of which shows favourable outcomes with adalimumab, with a response rate of 33% at one year of follow-up.25 There are few specific studies of enterocutaneous fistulas. The largest cohort is the one analysed in a French retrospective study that included 48 patients,26 where fistula closure was observed in a third of the patients after three months of treatment with anti-TNF, falling to 17% at three years, and patients with no associated strictures and those with a single fistulous tract responded better. One striking feature of that study was that almost a third of the patients had abscesses after starting treatment with anti-TNF, although patients with abscesses previously treated with antibiotics and drainage were initially included in the cohort. The results of a Spanish retrospective study of 24 patients with enterocutaneous fistulas treated with anti-TNF in whom 67% responded suggest that postoperative fistulas respond better than spontaneous fistulas and that the response correlates with that of perianal fistulas if associated.27
The effectiveness of anti-TNF in enterovesical fistulas has been analysed only retrospectively. One of the largest series is the Spanish one by Taxonera et al.,28 in which different therapeutic options were evaluated in 97 patients with enterovesical fistula. The only treatment that enabled patients to avoid surgery was anti-TNF (33 patients), with sustained response at three years in 45%. In the group of patients who finally required surgery, the previous use of anti-TNF was not associated with an increased risk of postoperative complications. Although they did not analyse factors associated with failure of anti-TNF treatment, it is worth noting that this subgroup of patients had coexisting complications (21.5% abscesses, 39.2% phlegmon and 30.4% other fistulas). It therefore seems important that patients be carefully selected.
The data on enterogenital fistulas come from subgroup analyses and retrospective series, with the response rates to anti-TNF ranging from 16% to 45%. The most-studied drug in this subgroup of patients was infliximab. The De la Poza series29 included 34 patients with enterogenital fistulas treated with anti-TNF (infliximab in 30/34), with response and remission rates of 30% and 16.7%, respectively.
An important issue with the use of anti-TNF in fistulising disease is safety. As mentioned above, some series have reported a high percentage of cases developing abscesses after starting biological treatment. A study published in 2003 which included patients with perianal disease detected that, despite closure of the fistula orifice, half of the patients had inflammatory fistulous tracts on MRI in the long term, which could result in fistula recurrence or abscess formation.30 These findings may also be applicable to fistulising disease in general. However, in the analysis of the ACCENT II cohort of patients with fistulising disease and treatment with anti-TNF, these patients developed no more abscesses than those treated with placebo, suggesting that the risk of abscesses may be more related to the actual fistulising behaviour of the disease than to the use of anti-TNF.31
In 2012 Cullen et al. published the results of the retrospective analysis of 13 patients with phlegmons,32 most of them (12) with associated abscesses, treated with anti-TNF. After control of the abscess with antibiotic in all cases plus drainage of those large in size, 80% were found to be in remission at two years after starting treatment with anti-TNF, with no recurrence of penetrating complications. The above results help demonstrate not only safety but also effectiveness in the use of these biological drugs in some patients with phlegmon and abscesses. The need when selecting these patients for prior control of any infection with antibiotics and drainage in the case of large abscesses has to be stressed. Once again, the factors associated with treatment response or failure in this scenario and which might direct patients towards medical or surgical treatment are not fully understood.
Anti-TNF in non-fistulising perianal diseaseIn addition to perianal fistulas, other lesions of the perianal region such as fissures, deep ulcers and anorectal strictures can also lead to tissue destruction and impair quality of life in patients with chronic IBD, especially in the case of painful deep ulcers or patients who develop faecal incontinence.33
The best evidence for the treatment of these non-fistulising perianal lesions with anti-TNF comes from the retrospective study by Bouguen et al.34; 72% of patients with perianal ulcers exhibited response to long-term infliximab, with a median follow-up of 3.2 years and independent of whether or not they had an associated fistula. It should be stressed that 94% of the initial responders had sustained response over the long term, and that there seemed to be a benefit of combined therapy with thiopurines in the case of deep ulcers (OR 0.13). Another important aspect was that the effectiveness of infliximab was associated with a rapid improvement in symptoms, also sustained over time.
However, with respect to anorectal strictures, the results of this study are more difficult to interpret. Although a long-term response was reported in approximately half of the cases, six of the 12 patients included had anal dilations during the anti-TNF treatment and that could have been a confounding factor. It was nevertheless concluded that the presence of this type of stricture does not contraindicate treatment with anti-TNF.
Anti-TNF in pouch complicationsAccording to the different series, up to 30% of patients with UC will require colectomy at some point in the course of their disease.35 Of those with ileal pouch, more than half will develop pouchitis, which will be refractory to the usual treatments in 10% of cases. Over the course of the follow-up after having had surgery, the diagnosis can change to CD or lesions suggestive of CD can develop in an appreciable proportion of these patients, ranging from 2.7% to 13% depending on the series.36
The best evidence on anti-TNF treatment in patients with pouch complications comes from the systematic review by Huguet et al.37 Very interesting is the authors’ classification of the patients into two subgroups: those with chronic refractory pouchitis, when inflammation is limited to the pouch; and those with “Crohn's-type” complications, including patients with fistulas or non-anastomotic strictures, as well as a significant pre-pouch ileitis. Of the 313 patients included in the full cohort, half responded at week eight and continued to have sustained response at one year, with no significant differences detected between infliximab and adalimumab. However, it is striking that, comparing the two subgroups of patients, a much higher percentage of patients with “Crohn's-type” complications responded at week eight than those with chronic refractory pouchitis (64% vs 10%), although these differences disappeared in the long term. It would therefore seem that patients with “Crohn's-type” complications may respond more rapidly, which could even be interpreted as the condition actually being CD, and the differentiation between these two disorders an aspect to take into account when assessing anti-TNF treatment.
Anti-TNF in extraintestinal manifestationsA high percentage of patients will develop extraintestinal manifestations over the course of their disease. In chronic IBD, according to the results of a Swiss cohort of 1249 patients,38 patients with extraintestinal manifestations are more likely to receive anti-TNF treatment than those without (58% vs 21%). At times, the extraintestinal manifestations are themselves the indication for treatment (43%). The lack of studies which have analysed the effectiveness of anti-TNF drugs in this indication is therefore quite striking, especially in UC.
The results of the CARE study39 showed a reduction in the prevalence of extraintestinal manifestations as a whole after 20 weeks of treatment with adalimumab, decreasing from 53% to 30%, with the remission of extraintestinal manifestations being associated with disease remission in terms of activity according to the Harvey-Bradshaw index. However, in this analysis the combined use of other treatments was allowed, such as immunosuppressants and corticosteroids, which may also have affected the response. Moreover, the assessment of response only took into account clinical criteria, when in some manifestations, such as joint symptoms, information provided by imaging tests is also important.
The systematic review by Peyrin-Biroulet et al.40 shows that anti-TNF agents could be effective in the treatment of joint symptoms, with reported response rates generally higher than 60%, both with infliximab and adalimumab and in both peripheral and axial arthritis. However, there are no clinical trials comparing the results of these drugs with placebo.
For the whole area of extraintestinal manifestations, there has only been one clinical trial that compared anti-TNF with placebo,41 and it only included patients with pyoderma gangrenosum. The response to infliximab was significantly greater than to placebo (46% vs 6%; p<0.05). Other studies of lower quality have also shown good outcomes in pyoderma gangrenosum, both with infliximab and adalimumab.42–44 There have been few analyses of anti-TNF in other types of cutaneous manifestations.
The few studies that have analysed the response of eye manifestations to anti-TNF have also shown good outcomes, with remarkably high response rates above 88%, although the small number of patients included prevents definitive conclusions from being drawn.45
It seems that anti-TNF agents may also have a beneficial effect on the bone disease associated with chronic IBD, although this effect has generally been studied in bone turnover markers, in some cases detecting an increase in these markers.46 It seems that the anaemia associated with chronic IBD may also improve with anti-TNF treatment. However, it has been pointed out that this effect could be related to the control of intestinal inflammation.47
Conclusions about the use of anti-TNF in non-luminal disease: place in clinical practiceAnti-TNF agents have been shown to improve quality of life and reduce hospital admissions and the need for surgery in patients with so-called “uncomplicated” disease. However, the utility of these drugs in other scenarios under the umbrella of chronic IBD is more subject to debate and, as discussed above, available evidence is scant and of low level.
Surgery is still necessary and may be the first therapeutic step in many patients with “complicated” disease. However, anti-TNF agents may prevent or delay surgery in selected cases. Patient selection must be made with the risks associated with the treatment and the predictors of response in mind. We must not forget that it is essential to reach agreement on the treatment with the different specialists involved and the patients themselves. We would state that treatment with anti-TNF:
- 1)
In stenosing disease: may avoid surgery in a considerable percentage of patients, particularly in those with short strictures, with an inflammatory component, recent-onset obstructive symptoms and without associated fistula.
- 2)
In fistulising disease: in enterocutaneous fistulas, the European Crohn's and Colitis Organisation (ECCO) guidelines48 state that the use of anti-TNF may be considered in those which are superficial and not associated with strictures or abscesses. In enterovesical fistulas, treatment with anti-TNF is an option that can prevent surgery in the medium/long term. The absence of associated complications (abscesses, stenosis, etc.) is most likely a better scenario for the efficacy and safety of biological treatment; combination with antibiotic therapy49 and close monitoring due to the risk of infectious complications is recommended. Infliximab and adalimumab may have a role in enterogenital fistulas, although the need for surgical treatment is high.
- 3)
In perforating disease, phlegmons and abscesses: treatment with anti-TNF can be an option in selected cases and always with prior control of the infection with concomitant antibiotic and drainage, if necessary. Studies are needed to support the use of biological therapy in this scenario and to determine which factors may affect its efficacy and safety.
- 4)
In extraintestinal manifestations: the European guidelines50 recommend that anti-TNF agents should be considered in patients with spondyloarthritis who do not respond or are intolerant to NSAIDs, and in those with persistent peripheral arthritis which affects their quality of life. In pyoderma gangrenosum, infliximab can be considered when there is no rapid response to corticosteroids or if it is located in areas with aesthetic implications. Adalimumab is an alternative. Anti-TNF agents can also be useful in cases of recurrent or persistent erythema nodosum. Use of anti-TNF can be considered for eye manifestations, particularly posterior uveitis and scleritis.
- 5)
In pouchitis: anti-TNF agents would be indicated in chronic pouchitis refractory to the usual treatment (antibiotic, budesonide, etc.).51 In the case of the complications defined by the authors as “Crohn's type”, anti-TNF agents are placed in the first therapeutic steps, and the response seems to be faster than in pouchitis.
- 6)
In anal disease: infliximab is indicated in severe perianal ulcers, due to the risk of incontinence and ileostomy. If response is obtained, it brings rapid symptom relief. The effectiveness of anti-TNF agents in anorectal stricture is unclear, but the presence of such strictures does not contraindicate treatment when indicated for other reasons.
Although the lack of evidence does not enable solid recommendations to be made, the use of anti-TNF in selected patients with complicated chronic IBD could avoid the need for more aggressive treatments such as surgery and improve patient quality of life. Once again, a multidisciplinary approach in these scenarios is paramount. The risks and benefits of anti-TNF treatment have to be weighed up together and anti-TNF use should not delay a surgical intervention when the indication is clear.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Núñez-Gómez L, Mesonero-Gismero F, Albillos-Martínez A, López-Sanromán A. Agentes anti-factor de necrosis tumoral en enfermedad de Crohn y colitis ulcerosa: más allá de la enfermedad luminal. Gastroenterol Hepatol. 2018;41:576–582.