Immunotherapy is a tool used increasingly more in the field of oncology. Familiarity with it is advisable due to its growing uses, including the treatment of digestive system tumours (hepatocarcinoma,1 colorectal adenocarcinoma with high microsatellite instability2) and due to the adverse reactions that frequently affect the digestive tract.
We present the case of a 74-year-old man with a personal history of chronic obstructive pulmonary disease (COPD) and melanoma with lung metastases. Due to these pathologies, he regularly used a salbutamol inhaler and had been on treatment with nivolumab, which was suspended four months previously after he achieved a complete radiological response to the lung metastases.
The patient reported diarrhoeal symptoms coursing for one month, consisting of 3−4 stools (Bristol 5–6) without pathological products, affecting his night rest, and with associated intermittent central abdominal discomfort and weight loss of about 3−4 kg. He had not eaten raw foods or taken antibiotics or new medication. There were no cohabitants with the same symptoms and nor had the patient made any trips abroad. He denied having any other symptoms or relevant family history. The physical examination was unremarkable except for slight discomfort on deep palpation of the mesogastrium.
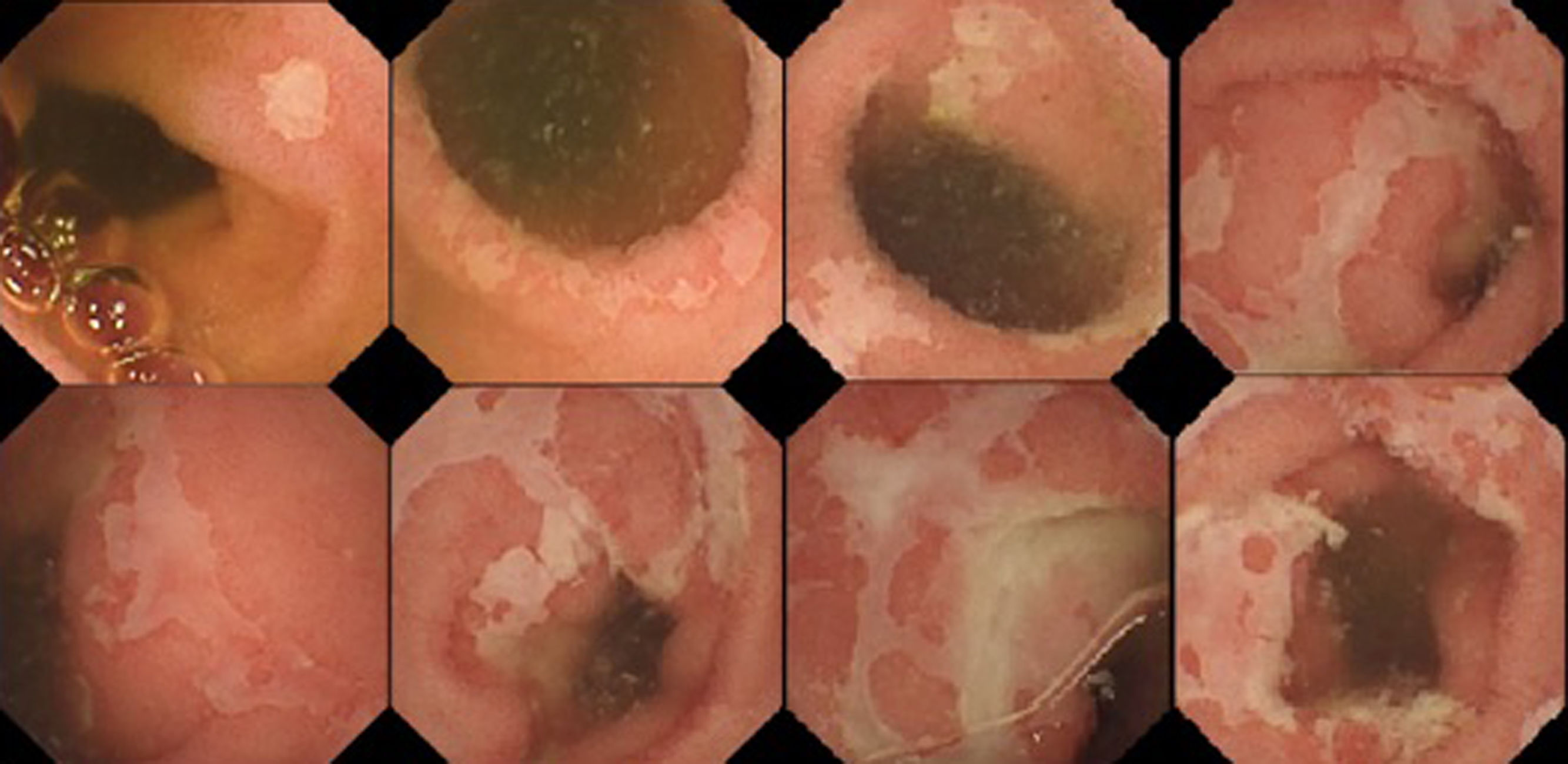
Initially, a blood test was requested that showed only a slight increase in acute phase reactants (CRP 7.6 mg/dl, ESR 22 mm) and mild malabsorption data (total protein 4.54 g/dl, albumin 2.31 g/dl and iron deficiency). Stool cultures with Clostridium difficile toxin were negative. In addition, as part of the oncology follow-up, a thoraco-abdominal CT scan had been performed two months previously in which the pulmonary metastases were not visualised and there were no abdominal pathological findings. The study was later expanded with an ileocolonoscopy that was normal and biopsies were taken that ruled out microscopic colitis. Coeliac disease serology and Mantoux tests requested were negative, as were thyroid hormones tests, which were normal. Five days after admission, the patient continued to present more or less fluctuating diarrhoeal symptoms. Finally, we obtained the faecal calprotectin result: 3351 ∝g/g, prompting an examination by endoscopic capsule (Fig. 1) which showed, after 1 h 31 min, the dispersed presence of ulcers, initially small in size, but in more extensive distal sections with geographic contours, while areas of significant stenosis or other pathological findings were not appreciated. With these findings and a relatively recent history of treatment with nivolumab, the patient was diagnosed, without any biopsies, with enteritis probably secondary to nivolumab. As part of the extension study, a gastroscopy was performed without pathological findings. Treatment with corticosteroids at 1 mg/kg/day was started, the patient experienced notable improvement in the first days and was discharged with a tapering-off regimen of corticosteroids. At the outpatient check-up six weeks after discharge, the patient was still being treated with 10 mg/day, was asymptomatic and presented normal laboratory tests. He is currently asymptomatic after seven months without corticosteroid treatment.
Nivolumab is an IgG4 antibody directed against PD1, a molecule responsible for inhibiting the lymphocyte response. By blocking it, the immune response is stimulated, with the consequent anti-tumour benefit, although autoimmune reactions can also occur. These vary in frequency from one study to another but may be estimated to occur in around 20% of patients, the majority being mild.3 Most commonly, they occur in the first weeks after the start of treatment, although it is not uncommon for them to present months after the cessation of treatment, which should be factored into the differential diagnosis of all patients who have undergone such treatment.4 In order of frequency, these reactions are diarrhoea, colitis and less frequently enteritis. However, any organ can be affected by an autoimmune mechanism, which can cause hepatitis, pancreatitis and mucositis.5 Treatment depends on the severity of the adverse effect and is based on assessing discontinuation of the drug, as well as combination with an immunosuppressant, with corticosteroids being the first line of treatment, as most patients respond quickly. Due to its effectiveness and growing use, in the near future in all probability we will have to contend with its adverse effects, so it is important to be aware of its existence and conduct clinical studies to improve its practical management.
Please cite this article as: Velamazan Sandalinas R, Laredo de la Torre V, García Mateo S, Abad Baroja D, Hijos Mallada G, Alfaro Almajano E, et al. Enteritis secundaria a nivolumab, una causa creciente de diarrea. Gastroenterol Hepatol. 2020. https://doi.org/10.1016/j.gastrohep.2020.03.016