To analyse laboratory parameters, clinical and fibrosis evolution in F3-F4 patients cured with direct-acting antivirals (DAA).
MethodsUnicentric, observational and prospective study. All F3-F4 hepatitis C patients cured with DDA from 01/11/2014 to 31/08/2019 were included. A basal visit (BV) was performed and 12 weeks (12 w), 1, 2, 3 and 4 years after treatment.
Demographic and laboratory variables, fibrosis measured by non-invasive tests, indirect markers of portal hypertension, the presence of esophageal varices, cirrhosis decompensation and hepatoceullar carcinoma were collected.
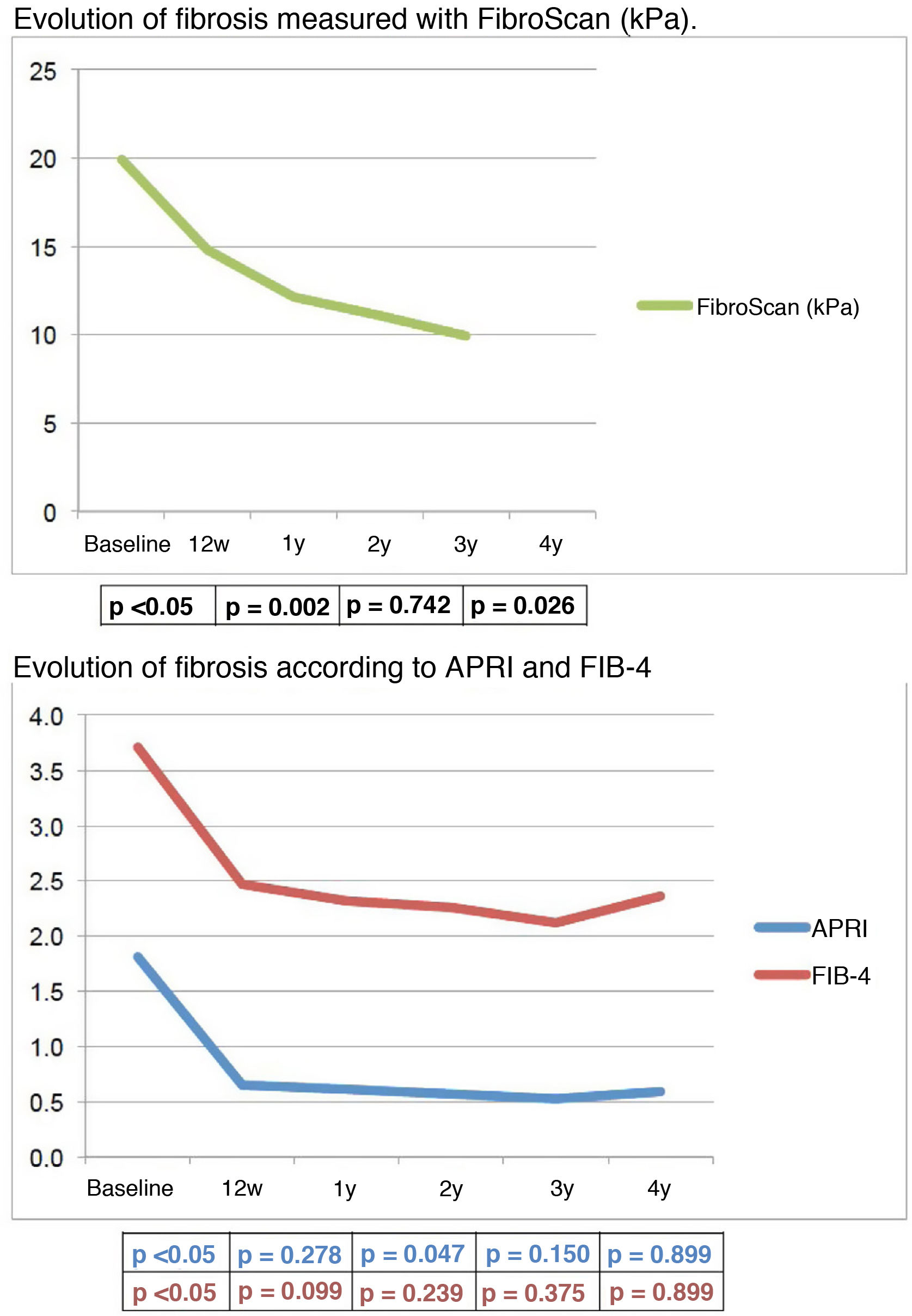
Results169 patients were treated: 123(72,8%) men, age 57,5 ± 12 years; 117(69,2%) with cirrhosis, 99(84,6%) Child A. 96,4% achieved SVR. The study was conducted for a median follow-up of 46,14 (2,89–62,55) months. It was observed a significant increase in platelets [155 × 10³/µl (BV);163 × 10³/µl (12 w)], cholesterol [158 mg/dl (BV);179 mg/dl (12 w)] and albumin [4,16 g/dl (BV); 4,34 g/dl (12 w)] and a significant decrease in ALT [82 UI/l (BV);23 UI/l (12 w], AST [69UI/L(BV);26UI/l (12 w)], GGT [118 UI/l (BV);48UI/l (12 w)] and bilirrubin [0,9 mg/dl (BV);0,7 mg/dl (12 w)]. Fibrosis also improved early in follow-up, both by serological methods and Fibroscan [19,9 KPa (BV); 14,8 KPa (12 w;p < 0.05].
8,1% of compensated cirrhosis patients had some decompensation. 4,5% developed esophageal varices. Nine patients (5,52%) had “de novo” hepatocellular carcinoma; 6 (3,68%) had hepatoceullar carcinoma in BV and 40% had a recurrence. During follow-up mortality was 9,2%.
ConclusionsThere is an improvement in laboratory parameters and fibrosis measured by non-invasive methods in F3–F4 patients cured with DAA. However, the risk of decompensation and the incidence/recurrence of hepatocellular carcinoma still remain, so there is a need to follow these patients.
Analizar la evolución analítica, clínica y de la fibrosis en pacientes F3-F4 curados con antivirales de acción directa (AAD).
Pacientes y métodosEstudio unicéntrico, observacional y prospectivo. Se incluyeron todos los pacientes con hepatitis C F3-F4 curados con AAD del 01/11/2014 al 31/08/2019. Se realizó una visita basal (VB) y a las 12 semanas (12 s), 1, 2, 3 y 4 años tras finalizar el tratamiento.
Se recogieron variables demográficas, analíticas, medición no invasiva de la fibrosis, marcadores indirectos de hipertensión portal, presencia de varices esofágicas, descompensaciones de la cirrosis y hepatocarcinoma.
ResultadosSe trataron 169 pacientes: 123 (72,8%) hombres, edad 57,5 ± 12 años; 117 (69,2%) presentaban cirrosis, 99 (84,6%) Child A. El 96,4% consiguió RVS.
La mediana de seguimiento fue de 46,14 (2,89–62,55) meses. Durante el seguimiento se observó precozmente un aumento significativo de plaquetas [155 × 10³/µL(VB);163 × 10³/µL(12 s)], colesterol [158 mg/dL(VB);179 mg/dL(12 s)] y albúmina [4,16 g/dL(VB);4,34 g/dL (12 s)] y un descenso significativo de GPT [82UI/L(VB);23UI/L(12 s)], GOT [69UI/L(VB);26UI/L(12 s)], GGT [118UI/L(VB);48UI/L(12 s)], y bilirrubina [0,9 mg/dL(VB);0,7 mg/dL(12 s)]. La fibrosis disminuyó, también inicialmente, tanto con métodos serológicos como Fibroscan [19,9KPa(VB); 14,8 KPa(12 s)]; p < 0.05].
El 8,1% de los pacientes con cirrosis compensada presentó alguna descompensación. Un 4,5% desarrolló varices esofágicas.
Nueve (5,52%) pacientes presentaron hepatocarcinoma “de novo”; seis (3,68%) lo presentaban basalmente y el 40% sufrió recidiva.
Durante el seguimiento la mortalidad fue del 9,2%.
ConclusionesExiste mejoría de los parámetros analíticos y de la fibrosis hepática medida por métodos no invasivos en los pacientes F3-F4 curados con AAD. Sin embargo, el riesgo de descompensación y de hepatocarcinoma persiste, por lo que se debe mantener el seguimiento.
Hepatitis C virus (HCV) infection affects approximately 170 million people worldwide1 and can lead to complications such as cirrhosis of the liver and hepatocellular carcinoma (HCC), which can ultimately require a liver transplantation or even be fatal.2 Interferon (IFN)-based treatments were the only therapeutic option for patients with HCV infection for a long time. Not only did these therapies have relatively low sustained virologic response (SVR) rates.3 they were also associated with significant toxicity and poor tolerance, so many patients could not be treated. In addition, given the high risk of decompensation, treatment with IFN was contraindicated in patients with decompensated liver disease.4 Taking these selection biases into account, studies have shown that an SVR obtained after IFN-based therapies was associated with a clinical improvement in the patient.5–7
With the advent of direct-acting antivirals (DAA), the range of patients who can be treated has increased significantly and SVR rates have also increased significantly in all stages of fibrosis,8 for which reason it is regarded as one of the most important therapeutic achievements of the last 20 years.9 What remains to be determined is whether an SVR combined with DAA treatment is also related to short- and long-term clinical benefits for the patient. These benefits may be directly related to liver disease (improved fibrosis and regression of cirrhosis, improved liver function, decreased risk of HCC and liver disease-related mortality) or overall patient survival. Documenting these potential benefits related to the elimination of the virus with DAA is fundamental to establishing the true usefulness of these treatments in HCV patients.9
The objective of our study was to analyse the evolution of laboratory test variables, that of fibrosis and the onset of clinical events (onset of oesophageal varices, decompensations of cirrhosis, HCC) in patients with advanced fibrosis or cirrhosis treated and cured with DAA throughout follow-up.
Patients and methodsStudy designIt was an observational, prospective study conducted in a Spanish tertiary hospital (Hospital Universitario de Burgos [Burgos University Hospital]). All patients with chronic hepatitis C virus (HCV) infection with advanced fibrosis (F3) or cirrhosis (F4) treated with direct-acting antiviral drugs (DAA) from November 2014 to August 2019 and with an SVR were consecutively enrolled. Patients with a transient elastography (TE) value between 9.5 and 12.5 kPa were classified as F3, and those with a TE value of >12.5 kPa, biopsy, or clinical, laboratory and/or ultrasound data consistent with cirrhosis were classified as F4. Treatment was freely prescribed by each physician in accordance with the Summary of Product Characteristics and the Spanish and European guidelines for the management of HCV infection in force when treatment was initiated. Following the antiviral treatment, the patients were followed up according to these guidelines, with the cut-off date set at 16 June 2020. The study protocol included a baseline visit (BV) at which the patient was evaluated and treatment with DAA was instituted. Follow-up visits were performed at 12 weeks (12 w) after the end of treatment and at one year (1y), two years (2y), three years (3y), and four years (4y) after the end of treatment.
VariablesAt the baseline visit, demographic and epidemiological variables related to liver disease were collected (age, sex, height, weight, body mass index [BMI], presence of diabetes mellitus [DM], coinfection with hepatitis B virus [HBV], parenteral drug use, alcohol consumption, previous treatment history and type, virus genotype and current treatment). Laboratory variables (haemoglobin, platelets, ALT, AST, GGT, ALP, cholesterol, triglycerides, albumin, bilirubin, creatinine, INR), Child–Pugh score and MELD score were recorded at the baseline and follow-up visits. Liver fibrosis was measured with non-invasive markers: serological markers (APRI, FIB-4) and by TE (FibroScan®, Echosens, Paris). SVR was defined as undetectable HCV RNA at week 12 post-treatment. HCV RNA levels were determined with the COBAS® AmpliPrep/COBAS TaqMan® Analyzer (Roche Molecular Systems, Pleasanton, CA, United States), with a detection limit of 15 IU/ml.
Similarly, the presence of indirect portal hypertension markers (spleen volume, portal vein diameter, collateral circulation) was assessed at baseline and during follow-up. Baseline presence and post-treatment onset of oesophageal varices (OV), decompensated cirrhosis (ascites, gastrointestinal bleeding secondary to OV and hepatic encephalopathy) and HCC were also recorded, as was undergoing a liver transplantation or death during follow-up.
The post-treatment evolution of patients who had baseline OV, decompensations or HCC was studied.
Ethical considerationsThe study was approved by the Ethics Committee of our centre and was carried out in accordance with the Declaration of Helsinki. The participants signed an informed consent form in order to take part in the study.
Statistical analysisThe data were analysed using the IBM® SPSS® 20.0 statistical program. The descriptive analysis was expressed in means (standard deviation [SD]), medians (interquartile range) and frequencies (percentages) depending on the characteristics of the variables and the type of distribution.
To assess the evolution of the variables, repeated test measurements were used according to the type of variable and distribution: Student's t (continuous, normal distribution or large samples), McNemar (dichotomous) and Wilcoxon (samples without normal distribution).
A p-value of less than 0.05 was considered to be statistically significant.
ResultsDuring the study period, 169 patients with F3-F4 chronic HCV infection were treated with DAA. Of these, 123 (72.8%) were men, with a mean age of 57.5 (±12) years, and 117 (69.2%) had cirrhosis, 99 (84.6%) Child–Pugh class A. Of the patients with cirrhosis, 27 patients (23.1%) had baseline OV. No patient had had upper gastrointestinal bleeding (UGB) due to OV, 10 (8.5%) had ascites, and 1 patient (5.1%) had presented hepatic encephalopathy. After the first treatment with DAA, 160 patients (94.7%) achieved an SVR. After a second treatment with DAA, another 3 patients (1.8%) were cured. Overall, the SVR in our cohort was 96.4 %.
Median follow-up was 46.14 (2.89–62.55) months. Fifty patients (29.6% of the cohort) completed four years of follow-up.
Table 1 shows the baseline characteristics of patients in the cohort as a whole and of patients with an SVR.
Baseline characteristics of the entire cohort and of patients with an SVR after DAA.
| Total | n = 169 | SVR YES | n = 163 | |
|---|---|---|---|---|
| n/mean | %/SD | n/mean | %/SD | |
| Age | ||||
| Years | 57.56 | ± 12.03 | 57.60 | ± 12.16 |
| Sex | ||||
| Female | 46 | 27.2% | 45 | 27.6% |
| Male | 123 | 72.8% | 118 | 72.4% |
| Height | ||||
| Metres | 1.68 | ± 0.10 | 1.69 | ± 0.10 |
| Weight | ||||
| kg | 76.05 | ± 16.98 | 75.70 | ± 16.99 |
| BMI | 26.98 | ± 5.38 | 26.90 | ± 5.37 |
| DM | ||||
| No | 139 | 82.2% | 133 | 81.6% |
| Yes | 30 | 17.8% | 30 | 18.4% |
| HBV | ||||
| No | 167 | 98.8% | 161 | 98.80% |
| Yes | 2 | 1.2% | 2 | 1.20% |
| HPDA | ||||
| Current | 0 | 0.0% | 0 | 0% |
| Never | 117 | 69.2% | 114 | 69.9% |
| ExHPDA | 48 | 28.4% | 45 | 27.6 % |
| Methadone | 4 | 2.4% | 4 | 2.5% |
| Alcohol | ||||
| Current | 8 | 4.7% | 8 | 4.9% |
| Never | 121 | 71.6% | 118 | 72.4% |
| Former drinker | 40 | 23.7% | 37 | 22.7% |
| Genotype | ||||
| 1 | 101 | 59.8% | 99 | 60.7% |
| 2 | 2 | 1.2% | 2 | 1.2% |
| 3 | 42 | 24.9% | 40 | 24.5% |
| 4 | 22 | 13.0% | 20 | 12.3% |
| 5 | 2 | 1.2% | 2 | 1.2% |
| Previous treatment | ||||
| No | 94 | 55.6% | 92 | 56.4% |
| Yes | 75 | 44.4% | 71 | 43.6% |
| Type previous treatment | ||||
| IFN | 9 | 12.0% | 9 | 12.7% |
| IFN + RBV | 48 | 64.0% | 47 | 66.2% |
| DAA | 18 | 24.0% | 15 | 21.1% |
| Liver transplant | ||||
| No | 165 | 97.6% | 159 | 97.5% |
| Yes | 4 | 2.4% | 4 | 2.5% |
| Cirrhosis | ||||
| No | 52 | 30.8% | 52 | 31.9% |
| Yes | 117 | 69.2% | 111 | 68.1% |
| CHILD | 5.62 | ± 1.23 | 5.57 | ± 1.21 |
| CHILD | ||||
| A | 100 | 85.4% | 97 | 86.6% |
| B | 15 | 12.8% | 12 | 10.7% |
| C | 2 | 1.7% | 2 | 2.7% |
| MELD | 8.51 | ± 3.52 | 8.44 | ± 3.51 |
| Fibrosis | ||||
| kPa | 20.11 | ± 12.91 | 19.90 | ± 13.00 |
| APRI | 1.96 | ± 2.03 | 1.81 | ± 1.77 |
| FIB-4 | 3.94 | ± 3.49 | 3.68 | ± 2.91 |
| Spleen volume | ||||
| Normal | 87 | 57.6% | 86 | 58.9% |
| Increased | 64 | 42.4% | 60 | 41.4% |
| Portal vein calibre | ||||
| Normal | 124 | 81.0% | 121 | 82.3% |
| Increased | 29 | 19.0% | 26 | 17.7% |
| Collateral circulation | ||||
| Absent | 148 | 96.1% | 145 | 98.0% |
| Present | 6 | 3.9% | 3 | 2.0% |
With regard to baseline levels, a statistically significant increase in platelet counts was observed after treatment (155⋅103/μl [BV]; 163⋅103/μl [12 w]), cholesterol (158 mg/dl [BV]; 179 mg/dl [12 w]) and albumin (4.16 g/dl [BV]; 4.34 g/dl [12 w]), and a significant decrease in ALT (82 IU/l [BV]; 23 IU/l [12 w]), AST (69 IU/l [BV]; 26 IU/l [12 w]), GGT (118 IU/l [BV]; 48 IU/l [12 w]) and bilirubin (0.9 mg/dl [BV]; 0.7 mg/dl [12 w]). These laboratory test changes occurred early, between the baseline and 12-week visits, and subsequently remained stable throughout the entire follow-up period.
Table 2 summarises the evolution of the different laboratory test parameters over time.
Evolution of laboratory test values in patients with an SVR.
| Baseline | p ΔB-12 w | 12 w | p Δ12 w-1y | 1y | p Δ1y-2y | 2y | p Δ2y-3y | 3y | p Δ3y-4y | 4y | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | ||||||
| Platelets (×109/l) | 163 | 154.63 | ±62.62 | 0.000 | 160 | 163.36 | ± 63.17 | 0.003 | 132 | 169.34 | 62.66 | 0.219 | 110 | 173.94 | ± 70.63 | 0.811 | 94 | 173.86 | ± 63.28 | 0.431 | 56 | 164.79 | ± 61.66 |
| Haemoglobin (g/dl) | 163 | 14.85 | ± 2.03 | 0.886 | 160 | 14.85 | ± 1.98 | 0.169 | 131 | 15.11 | ± 1.70 | 0.000 | 110 | 14.90 | ± 1.70 | 0.134 | 94 | 14.83 | ± 1.68 | 0.836 | 56 | 14.75 | ± 2.00 |
| ALT (U/l) | 163 | 81.78 | ± 57.64 | 0.000 | 157 | 23.24 | ± 15.62 | 0.364 | 132 | 24.21 | ± 17.44 | 0.691 | 110 | 24.07 | ± 17.96 | 0.314 | 93 | 23.49 | ± 15.86 | 0.165 | 56 | 26.05 | ± 25.16 |
| AST (U/l) | 162 | 68.83 | ± 44.98 | 0.000 | 150 | 26.47 | ± 17.81 | 0.983 | 123 | 26.33 | ± 19.55 | 0.067 | 97 | 24.86 | ± 17.14 | 0.915 | 82 | 25.31 | ± 20.48 | 0.653 | 49 | 24.31 | ± 9.07 |
| GGT (U/l) | 162 | 118.22 | ± 148.96 | 0.000 | 155 | 48.03 | ± 78.93 | 0.097 | 132 | 59.20 | ± 119.06 | 0.371 | 108 | 57.90 | ± 96.22 | 0.340 | 89 | 58.54 | ± 103.70 | 0.972 | 55 | 69.80 | ± 142.18 |
| ALP (UI/l) | 155 | 90.86 | ± 57.56 | 0.652 | 151 | 88.70 | ± 71.16 | 0.109 | 128 | 81.15 | ± 40.24 | 0.841 | 105 | 81.51 | ± 49.65 | 0.924 | 89 | 76.03 | ± 40.19 | 0.229 | 54 | 76.87 | ± 44.15 |
| Cholesterol (mg/dl) | 153 | 158.09 | ± 34.49 | 0.000 | 145 | 179.47 | ± 36.81 | 0.235 | 128 | 178.05 | ± 34.25 | 0.097 | 109 | 177.06 | ± 33.91 | 0.436 | 89 | 180.45 | ± 37.36 | 0.596 | 53 | 183.55 | ± 31.85 |
| Triglycerides (mg/dl) | 153 | 115.98 | ± 66.44 | 0.748 | 143 | 114.01 | ± 73.15 | 0.454 | 127 | 122.38 | ± 72.99 | 0.675 | 108 | 123.93 | ± 71.20 | 0.635 | 89 | 131.44 | ± 83.10 | 0.303 | 54 | 123.26 | ± 74.66 |
| Albumin (g/dl) | 146 | 4,164.72 | ± 509.50 | 0.000 | 147 | 4,335.78 | ± 467.62 | 0.133 | 126 | 4,420.10 | ± 416.73 | 0.230 | 97 | 4,342.12 | ± 471.21 | 0.478 | 82 | 4,349.90 | ± 424.69 | 0.071 | 48 | 4,452.88 | ± 813.88 |
| Bilirubin (mg/dl) | 163 | 0.87 | ± 0.68 | 0.000 | 156 | 0.71 | ± 0.51 | 0.990 | 130 | 0.72 | ± 0.42 | 0.877 | 106 | 0.71 | ± 0.39 | 0.924 | 92 | 0.76 | ± 0.56 | 0.264 | 55 | 0.84 | ± 1.07 |
| Creatinine (mg/dl) | 160 | 0.99 | ± 1.16 | 0.452 | 160 | 1.01 | ± 1.00 | 0.047 | 131 | 1.03 | ± 0.94 | 0.130 | 111 | 1.04 | ± 0.92 | 0.110 | 94 | 1.03 | ± 0.93 | 0.426 | 55 | 1.03 | ± 0.97 |
| INR | 139 | 1.10 | ± 0.30 | 0.677 | 88 | 1.09 | ± 0.21 | 0.068 | 98 | 1.11 | ± 0.38 | 0.621 | 81 | 1.07 | ± 0.16 | 0.049 | 62 | 1.10 | ± 0.24 | 0.823 | 38 | 1.07 | ± 0.11 |
Regarding the liver function indicators, no significant differences were observed in the mean of the Child–Pugh numerical value (BV: 5.57; 12 w: 5.28; 1y: 5.13; 2y: 5.15; 3y: 5.23; 4y: 5.41) or the MELD (BV: 8.44; 12 w: 8.32; 1y: 8.15; 2y: 8.16; 3y: 8.69; 4y: 8.69) throughout the study follow-up period in patients who had an SVR.
Regarding the indirect markers of portal hypertension, with the exception of platelet count, no significant changes were observed in spleen volume, portal vein diameter or collateral circulation throughout follow-up (data not shown).
Stratifying the results into two groups based on baseline fibrosis (F3 and F4) revealed that there was a statistically significant initial improvement in ALT, AST, GGT and an increase in cholesterol in both groups. In contrast, the improvement in platelet count, albumin and bilirubin was only significant in the F4 patients, who had more altered baseline values (Table 3).
Evolution of fibrosis measured by TE and laboratory values according to baseline fibrosis (F3-F4) in the first year after treatment.
| Baseline | 12 w | 1y | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | p ΔB-12 w | n | Mean | SD | p Δ12 w-1y | n | Mean | SD | |
| F3 | |||||||||||
| Fibrosis (kPa) | 52 | 11.07 | ± 0.94 | 0.000 | 37 | 8.62 | ± 3.16 | 0.010 | 41 | 7.48 | ± 1.85 |
| Platelets (×109/l) | 52 | 186.17 | ± 67.40 | 0.218 | 50 | 191.08 | ± 67.76 | 0.381 | 44 | 192.93 | ± 58.60 |
| Haemoglobin (g/dl) | 52 | 15.36 | ± 1.77 | 0.174 | 50 | 15.15 | ± 1.71 | 0.430 | 44 | 15.30 | ± 1.61 |
| ALT (U/l) | 52 | 72.77 | ± 45.49 | 0.000 | 50 | 20.12 | ± 9.88 | 0.330 | 44 | 21.55 | ± 11.93 |
| AST (U/l) | 52 | 56.13 | ± 30.80 | 0.000 | 46 | 22.24 | ± 12.31 | 0.957 | 39 | 22.36 | ± 7.63 |
| GGT (U/l) | 52 | 81.69 | ± 56.22 | 0.000 | 50 | 30.36 | ± 32.44 | 0.372 | 44 | 28.52 | ± 25.23 |
| ALP (UI/l) | 50 | 74.86 | ± 23.50 | 0.464 | 50 | 84.32 | ± 88.10 | 0.244 | 42 | 67.12 | ± 19.60 |
| Cholesterol (mg/dl) | 48 | 164.08 | ± 37.15 | 0.000 | 45 | 188.80 | ± 40.38 | 0.861 | 43 | 186.26 | ± 34.64 |
| Triglycerides (mg/dl) | 48 | 120.31 | ± 66.14 | 0.886 | 44 | 119.41 | ± 101.25 | 0.648 | 43 | 128.95 | ± 67.89 |
| Albumin (g/dl) | 45 | 4,289.56 | ± 434.04 | 0.605 | 47 | 4,320.06 | ± 454.19 | 0.238 | 41 | 4,431.95 | ± 460.10 |
| Bilirubin (mg/dl) | 52 | 0.66 | ± 0.34 | 0.099 | 50 | 0.59 | ± 0.32 | 0.566 | 44 | 0.58 | ± 0.31 |
| Creatinine (mg/dl) | 52 | 0.98 | ± 0.93 | 0.088 | 50 | 1.01 | ± 1.00 | 0.008 | 44 | 1.10 | ± 1.18 |
| INR | 38 | 1.09 | ± 0.49 | 0.435 | 19 | 1.02 | ± 0.09 | 1.000 | 26 | 1.17 | ± 0.63 |
| F4 | |||||||||||
| Fibrosis (kPa) | 92 | 26.33 | ± 13.47 | 0.000 | 70 | 18.11 | ± 10.03 | 0.020 | 59 | 15.59 | ± 10.38 |
| Platelets (×109/l) | 92 | 138.93 | ± 53.60 | 0.000 | 92 | 150.60 | ± 56.55 | 0.008 | 74 | 159.66 | ± 64.14 |
| Haemoglobin (g/dl) | 92 | 14.66 | ± 2.07 | 0.299 | 92 | 14.80 | ± 2.00 | 0.359 | 73 | 15.01 | ± 1.68 |
| ALT (U/l) | 92 | 92.95 | ± 64.56 | 0.000 | 90 | 25.16 | ± 18.75 | 0.622 | 74 | 25.91 | ± 21.09 |
| AST (U/l) | 91 | 81.73 | ± 50.67 | 0.000 | 88 | 29.25 | ± 20.83 | 0.875 | 73 | 28.67 | ± 24.32 |
| GGT (U/l) | 91 | 144.78 | ± 186.95 | 0.000 | 88 | 57.89 | ± 96.52 | 0.172 | 74 | 76.68 | ± 150.03 |
| ALP (UI/l) | 87 | 94.87 | ± 36.00 | 0.003 | 86 | 86.27 | ± 30.07 | 0.224 | 73 | 83.07 | ± 25.90 |
| Cholesterol (mg/dl) | 86 | 155.30 | ± 33.84 | 0.000 | 85 | 177.02 | ± 35.35 | 0.018 | 72 | 172.29 | ± 34.52 |
| Triglycerides (mg/dl) | 86 | 111.28 | ± 67.26 | 0.708 | 84 | 111.27 | ± 57.57 | 0.917 | 71 | 116.15 | ± 73.13 |
| Albumin (g/dl) | 83 | 4,123.75 | ± 534.32 | 0.000 | 84 | 4,375.88 | ± 430.01 | 0.395 | 71 | 4,417.90 | ± 369.31 |
| Bilirubin (mg/dl) | 92 | 0.96 | ± 0.74 | 0.002 | 89 | 0.76 | ± 0.55 | 0.623 | 73 | 0.78 | ± 0.46 |
| Creatinine (mg/dl) | 89 | 0.86 | ± 0.26 | 0.073 | 92 | 0.89 | ± 0.32 | 0.143 | 73 | 1.00 | ± 0.87 |
| INR | 87 | 1.11 | ± 0.18 | 0.968 | 58 | 1.12 | ± 0.23 | 0.135 | 62 | 1.10 | ± 0.24 |
The mean fibrosis values measured by non-invasive methods, either serologically or by TE, generally decreased throughout follow-up, albeit initially very significantly, once SVR was achieved. Specifically, the mean TE value improved by 5.5 kPa (from 19.90 to 14.78 kPa) between baseline and 12 w.
Progressive improvement in TE values was significant in patients with baseline fibrosis F3 and in those with F4 (Table 3).
The mean values of APRI and FIB-4 over the course of follow-up were as follows: BV: 1.81; 12 w: 0.65; 1y: 0.61; 2y: 0.57; 3y: 0.52; 4y: 0.59, and BV: 3.71; 12 w: 2.47; 1y: 2.32; 2y: 2.26; 3y: 2.12; 4y: 2.36, respectively. The reduction in fibrosis values in these patients is represented graphically in Fig. 1.
Clinical evolution. Onset of decompensationsOf the 117 patients with cirrhosis, 111 (94.9%) had an SVR. Nine (8.1%) of these patients had some form of de novo decompensation of their liver disease.
Ascites was the most frequent in 8 cases (7.2%), followed by UGB due to OV in 3 patients (2.7%) and the onset of hepatic encephalopathy in 3 patients (2.7%). The onset of OV was identified in 5 patients (4.5%).
Of these 9 patients, 3 (33.3%) had genotype 1, one patient had genotype 2 (11.1%), and 5 patients had genotype 3 (55.6%). Patients with genotype 1 were treated with sofosbuvir + simeprevir ± ribavirin. The remaining patients (genotypes 2 and 3) received sofosbuvir + daclatasvir ± ribavirin.
At baseline, 24 patients (21.6%) had OV. Ten patients (9%) were decompensated before starting treatment (all of them had ascites and one had hepatic encephalopathy prior to treatment).
During the follow-up of patients with baseline OV, 15 patients (62.5%) had stable OV, in 1 (4.2%) there was OV progression, 3 patients (12.5%) had UGB secondary to OV, and in 5 (20.8%) the OV disappeared.
Of the patients with baseline decompensation, during follow-up 3 (30%) had a similar degree of ascites, 3 (30%) had a worsening of ascites, 2 (20%) had better control of ascites with reduced need for diuretics, and the ascites disappeared in 2 patients (20%). The patient with baseline hepatic encephalopathy had no new episodes of encephalopathy after treatment.
Regarding the evolution of Child–Pugh, of the 97 patients who initially had Child–Pugh A, 96 (99%) maintained Child–Pugh A during follow-up and 1 (1%) progressed to Child–Pugh C at 12 w due to the onset of HCC. Of the 12 patients with Child–Pugh B at baseline, 3 patients (25%) remained in the same Child–Pugh class, 1 (8.3%) underwent a transplant at 12 w, 5 (41.7%) progressed to Child–Pugh C over time (one of them decompensated during treatment and the others progressed as of 2y). The remaining 3 patients (25%) improved to Child–Pugh A, 2 at 12 w and the third at 1y.
Finally, of the 2 Child–Pugh C patients, 1 (50%) remained in the same class and the other (50%) improved progressively to Child–Pugh class A at the 1y visit.
Onset of HCC. Incidence and recurrenceNine patients (5.52%) presented de novo HCC after treatment with DAA in a median time of 14 (6–37) months following completion of antiviral therapy; 2 of them (22.22%) had FibroScan stage 3 fibrosis, the rest were cirrhotic. Two of the 9 patients who developed HCC had received two treatments with DAA due to lack of response to the first treatment. The first of these patients presented with cirrhosis of the liver and received the second treatment 9 months after the first, being diagnosed with HCC 12 months after the start of the second treatment. The other patient had FibroScan F3 fibrosis, received both treatments in an interval of 15 months and was diagnosed with HCC 9 months after the start of the second treatment.
All patients who developed de novo HCC had at least one of the following comorbidities before starting the ADD. The most frequent was being overweight, present in 6 of the patients. In addition, 4 patients had a history of risky alcohol consumption, 3 had a history of parenteral drug use and 1 patient was diabetic. The two F3 patients who developed HCC were overweight and one of them also had a history of parenteral drug use.
Regarding the recurrence of HCC, 6 patients (3.68%) had HCC at baseline (prior to starting on DAA). Of them, 5 patients (83.33%) were treated for HCC before antiviral therapy was started and were in radiological remission; the median time between HCC treatment and starting antiviral therapy was 49 months (10–132). Of these, 2 patients (40%) had a recurrence of HCC within 4 and 10 months of antiviral treatment.
The 3 patients who did not have a recurrence of HCC had a single SOL (BCLC stages 0 and A). Two of them had undergone surgical resection 14 and 132 months before starting treatment with DAA and the third had received a liver transplant 31 months before receiving antiviral treatment. The characteristics of HCC prior to treatment with DAA are summarised in Table 4.
Evolution of patients with HCC prior to treatment with DAA.
| Date of diagnosis of HCC | SOL largest size | Number of SOL | BCLC | Type of HCC treatment | Pre-DAA comorbidities | DAA date | Recurrence | Time between DAA and recurrence | Observations |
|---|---|---|---|---|---|---|---|---|---|
| 19/3/14 | 4 | B | Surgery + intraoperative RF (×3) | Former-drinker/DM | 14/1/15 | Yes | 10 months | ||
| 20/8/07 | 33 | 1 | A | RF ablation | No | 19/12/15 | Yes | 4 months | |
| 8/6/16 | 18 | 1 | 0 | RF ablation | ExHPDA | 15/6/16 | Yes | 19 months | DAA before treatment for HCC |
| 5/5/11 | 26 | 1 | A | OLT | DM | 27/1/15 | No | ||
| 14/11/13 | 14 | 1 | 0 | Surgery | DM | 30/1/15 | No | ||
| 2005 | Surgery | No data available | 23/6/16 | No | No data available for baseline HCC |
DAA: direct-acting antivirals; DM: diabetes mellitus; ExHPDA: history of parenteral drug addiction; former-drinker: history of risky alcohol consumption; HCC: hepatocellular carcinoma; LT: liver transplant; OLT: orthotopic liver transplantation; RF: radiofrequency ablation; SOL: space-occupying lesion; SR: surgical resection.
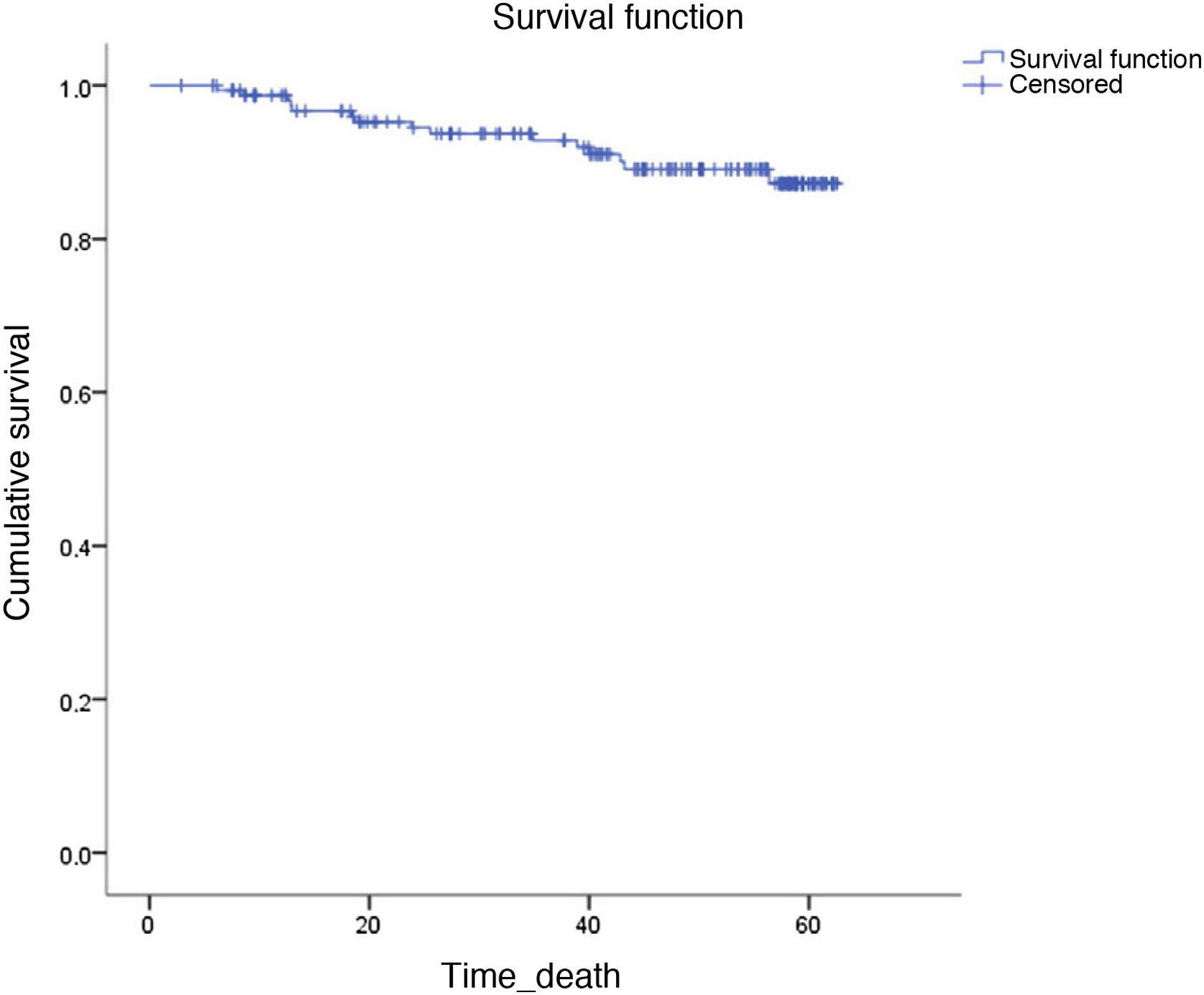
Fifteen patients died during follow-up (9.2%). Four died from HCC progression, 3 from infections, 2 from other tumours (cholangiocarcinoma and adenocarcinoma of the lung), 2 from progression of their liver disease, and the cause of death in the other 4 is unknown.
The probability of survival at 60 months was >85% (Fig. 2).
DiscussionThe introduction of DAA has heralded a revolution in the treatment of patients with hepatitis C, making it possible not only to treat it but also to cure a large number of patients who previously did not have this option. The aetiological treatment of all liver diseases has had a beneficial impact on disease evolution, with an improvement in fibrosis and in patient prognosis. In the case of hepatitis C, curing through treatment with IFN had already demonstrated an improvement in fibrosis10 and increased survival.3 Recent treatments with DAA will be likely to have the same long-term beneficial effect, which needs to be proven. This was the objective of our study.
Our data illustrate an overall improvement in all the laboratory parameters related to inflammation, fibrosis and liver function once SVR has been achieved. What is striking is that this improvement occurs very soon after SVR, at 12 w, is subsequently maintained and even continues to improve, albeit more discreetly, over time. This suggests that the elimination of the virus is a major milestone in the natural history of the disease and that once it is achieved, the disease improves rapidly and this beneficial effect lasts over time.
This benefit not only affects transaminases and other laboratory data that are surrogate markers of liver function (albumin, bilirubin) or portal hypertension (platelets), but as we have observed, it translates into an improvement in liver fibrosis measured by non-invasive methods after treatment. It should be noted that the improvement of fibrosis is independent of the status of baseline fibrosis (F3-F4), as well as transaminases, which are indicators of liver inflammation. In contrast, bilirubin, albumin, and platelets, which are associated with more advanced disease, only improve significantly in F4 patients, probably because these patients have more altered baseline values and the improvement is more noticeable.
Parallel to the improvement of transaminases, our study also found a significant decrease in fibrosis at 12 w as measured by serological tests (APRI and FIB 4). This reduction in fibrosis estimated by non-invasive serological tests such as APRI or FIB-4 is also described in the literature.11 These tests seem to be quite accurate in assessing liver stiffness after SVR, compared with biopsy, in patients with advanced fibrosis prior to treatment. However, the cut-off values used before treatment are known to be invalid once SVR has been achieved, meaning that these cut-off points after treatment with DAA have yet to be determined.11 Regarding fibrosis measured by TE, TE values also diminish after treatment, and this improvement is observed mainly between baseline and 12 w visits, with a median decrease of 25.7% (IQR 11.85–40.56). These results are similar to others published in the literature.12,13 In a meta-analysis that included 24 studies comparing fibrosis measured by TE before and after treatment,13 a mean decrease in fibrosis of 28% (IQR 21.8–34.8) was described between baseline and 6–12 months following the end of treatment in patients with SVR, although most of the studies included were conducted with treatments with IFN. Despite this, the gold standard for the determination of fibrosis is the liver biopsy, and due to the lack of comparative studies with pre- and post-treatment paired liver biopsies it cannot be determined whether the improvement in the hepatic stiffness parameters measured by TE is due to resolution of liver inflammation or the regression of fibrosis. This is why the recently-published EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis11 acknowledge that at present and with the available evidence, TE is not valid for detecting fibrosis regression after SVR in patients with hepatitis C and advanced compensated liver disease prior to the initiation of antiviral therapy.
In an attempt to assess whether this biochemical improvement and improvement in non-invasive fibrosis markers have clinical implications, some studies have shown a significant reduction in decompensation after SVR is achieved with IFN-based treatments in patients with compensated cirrhosis.14 Most of these patients had Child–Pugh A due to the limitations of the treatment with IFN, which conditions a low rate of decompensation regardless of the response to antiviral therapy.15 With the use of DAA, these limitations have diminished significantly, hence patients with more advanced cirrhosis have been treated. The results in patients treated with DAA are more heterogeneous, although16 a lower incidence of decompensations in patients treated and cured with DAA has been described in the literature.
Some data seem to suggest that this improvement in the rate of decompensations occurs mostly in cured patients with Child–Pugh A, which may lead us to suspect that antiviral treatment is less beneficial in patients with more advanced liver disease.17
The cumulative incidence of de novo decompensations in our cohort is higher than that described in other studies,16 although it remains significantly lower than the incidence described in historical cohorts of patients treated with IFN or untreated patients.18 The high percentage of patients from our cohort with genotype 3 among patients with de novo decompensations is noteworthy. The increased risk of liver disease progression in these patients is known,19 and it will probably be particularly important to treat patients with genotype 3 before they develop advanced fibrosis.
The data from our study also indicate that in Child–Pugh A patients, the rate of progression to more advanced stages after SVR is lower than in Child–Pugh B patients, which seems to endorse the findings of previous studies that suggest a more beneficial effect in patients with earlier stages.17 However, although only two Child–Pugh C patients were treated in our study, which makes it difficult to draw conclusions, we observed a significant clinical and analytical improvement in one of these patients, with the disease regressing to Child–Pugh class A, which could suggest that the clinical benefit for the patient may appear at any stage of the disease.
With regard to the incidence of HCC, several studies have confirmed that achieving SVR after treatment with IFN reduces the risk of HCC by 0.5%–1% a year.20,21 However, we know that SVR rates were markedly lower with this treatment and that tolerance much worse than with the new DAA due to the appearance of multiple adverse effects.22 As a result, patients who were more severe and had a greater risk of developing HCC could not be treated. With the advent of DAA, two studies were initially published23,24 in which a high risk of incidence and recurrence after treatment with the new DAA was described. Numerous studies were subsequently conducted to attempt to clarify the true relationship between treatment with DAA and the appearance of HCC. There is a recently-published review22 that discusses the different studies conducted on the incidence and recurrence of HCC with DAA. In relation to the incidence of HCC, the data provided are very variable, ranging from an incidence of 0.93% in the Spanish study by Calleja et al.25 to one of 9.1% in the study by Ravi et al.26 The different studies presented are methodologically very different, although current evidence does seem to suggest that a lower incidence rate of HCC after SVR is achieved by treatment with DAA.22
In our cohort, the incidence rate is within the literature reports, although the high percentage of patients with HCC in a non-cirrhotic liver (F3 in TE) is striking, which would seem to support the need to continue with six-monthly screening in all F3 patients (apart from patients with cirrhosis), as currently recommended by guidelines.27
It is striking that the majority of patients who present de novoHCC are overweight, particularly the two patients with HCC with baseline F3. The relationship between HCC and metabolic syndrome has been described,27 and although our cohort is small, it appears to be a clearly related comorbidity. It should therefore be considered as an important factor to treat and to emphasise once the hepatitis C has been cured.
In 2016, there was alarm about the high rate of recurrence of HCC after treatment with DAA following the publication of the two studies mentioned above,23,24 which reported recurrence rates of 27.6% and 28.81%, respectively. Several studies have subsequently been published28–30 comparing the recurrence rate in patients treated with DAA and those not receiving antiviral therapy, concluding that treatment with DAA is not associated with a higher recurrence rate of HCC. On the contrary, it results in increased survival in patients treated with DAA,28 and a reduction of >60% in the recurrence of HCC is defined in treated versus untreated patients.30 Thus, current data would seem to support the idea that – as occurs with incident HCC –, there is a lower recurrence rate of HCC after SVR achieved by treatment with DAA.22
In our study, the HCC recurrence rate in patients with an SVR is similar to that described in the aforementioned studies. In addition, we can see that the 3 patients with no recurrence during follow-up had received treatment with curative intent (surgical resection or liver transplantation), had a single small SOL (BCLC 0 or A), antiviral treatment began years after the targeted treatment for HCC, and a complete response was confirmed for years, so these factors could be associated with a reduction in HCC recurrence.
The main limitations of this study are, first of all, its unicentric design and small sample size, and conclusions should therefore be approached with caution. Secondly, although some patients have a long follow-up time, most patients have not completed four years of follow-up. Thirdly, only data from SVR patients have been analysed, so we cannot make comparisons with patients who were not cured. Finally, we used TE to assess the evolution of fibrosis after SVR, although to date we do not have sufficient long-term data comparing paired biopsies that support the use of FibroScan for this purpose. The strong points of our study are, firstly, that all F3 and F4 patients cured during the study period are included, so it is a real-life cohort that yields data that can probably be extrapolated to other patient cohorts in our setting. Secondly, the improvement in laboratory parameters and fibrosis markers occurred very early, so increasing the follow-up period would be unlikely to change these results.
ConclusionsOn the strength of our study data, we may conclude that there is a general improvement in all laboratory parameters and liver fibrosis measured by non-invasive methods in patients with advanced fibrosis or cirrhosis presenting SVR after treatment with DAA. Despite antiviral treatment, the risk of decompensation and the onset of HCC (both de novo and recurrent) is not negligible, so these patients should be followed up.
Ethical considerationsThe study was approved by the Ethics Committee of our centre and was carried out in accordance with the Declaration of Helsinki. The participants signed an informed consent form in order to take part in the study.
FundingThis study did not require funding.
Conflicts of interestThe authors declare that they have no conflicts of interest.