We present the case of a 77-year-old patient with a personal history of arterial hypertension controlled with hygienic-dietary measures and a jejunal GIST (diffuse c-kit positive, focal CD34-positive, S100-negative), diagnosed from the study of a lower gastrointestinal haemorrhage in 2012. The patient underwent successful surgery that same year and started treatment with imatinib 400mg/day, but presented a recurrence in the form of a single liver metastasis, for which the dose was increased to 800mg/day in 2014. In August 2019, treatment was changed to sunitinib due to poor gastrointestinal tolerance, presenting at that time stable disease according to RECIST criteria.
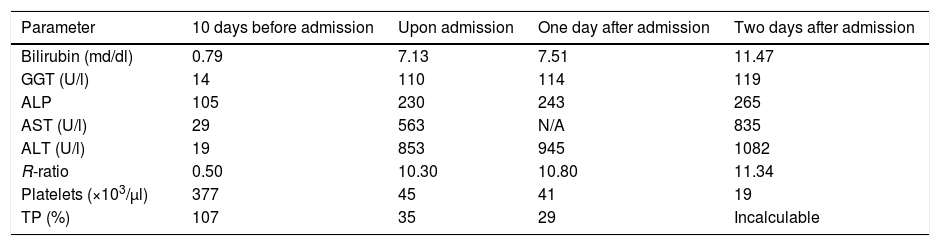
The patient went to A&E seven months after starting sunitinib, referred by his primary care physician, for presenting signs of asthenia, malaise and mucocutaneous jaundice, without any other associated symptoms (including encephalopathy), including fever, shivering, abdominal pain or alteration of gastrointestinal rhythm. The analytical values prior to the onset of the symptoms, upon arrival at A&E and during admission, can be seen in Table 1. In these analytical data we can see the predominance of cytolytic damage over cholestatic damage, with an R-ratio that it is clearly above 5, the threshold for defining a cytolytic pattern.
Analytical values before and during admission. A clear predominance of cytolysis (R-ratio >5) can be seen, consistent with the alterations described in treatment with sunitinib.
| Parameter | 10 days before admission | Upon admission | One day after admission | Two days after admission |
|---|---|---|---|---|
| Bilirubin (md/dl) | 0.79 | 7.13 | 7.51 | 11.47 |
| GGT (U/l) | 14 | 110 | 114 | 119 |
| ALP | 105 | 230 | 243 | 265 |
| AST (U/l) | 29 | 563 | N/A | 835 |
| ALT (U/l) | 19 | 853 | 945 | 1082 |
| R-ratio | 0.50 | 10.30 | 10.80 | 11.34 |
| Platelets (×103/μl) | 377 | 45 | 41 | 19 |
| TP (%) | 107 | 35 | 29 | Incalculable |
The patient denied at all times any consumption of alcohol or other drugs, including NSAIDs, paracetamol and antibiotics, as well as herbal products or any other substance. Hepatotropic virus serologies, including HCV, HBV, HAV, HEV, CMV, EBV, VES, and VZV were all negative. A vascular Doppler ultrasound was also performed, which ruled out portal or suprahepatic thrombotic pathology, as well as progression of the neoplastic disease. The autoimmunity study carried out during admission, the results of which were obtained later, was negative.
Treatment with corticosteroids and N-acetylcysteine infusion was started empirically due to the possibility of an aetiology other than pharmacological toxicity. Despite this, the patient's condition worsened progressively, both clinically and in tests, developing grade III–IV hepatic encephalopathy, AKI-III renal failure and respiratory failure, and he died three days after arriving at A&E. Given the patient's rapid worsening, advanced age and active oncological pathology, it was decided in session, in agreement with medical oncology, that the patient was not a candidate for transplantation or plasma exchange in the context of a clinical trial.
Fulminant liver failure is a rare complication, but especially dangerous in patients with oncological disease, since this is a contraindication for liver transplantation, the only therapeutic option in many cases. Sunitinib is a tyrosine kinase inhibitor, which is used in the treatment of renal cell carcinoma, pancreatic neuroendocrine tumours and gastrointestinal stromal tumours. A 2013 meta-analysis1 reported 40% of patients had elevated liver enzymes (predominantly with cytolysis pattern) during treatment with sunitinib, which were grade III/IV in 3% of patients.
However, up to five cases of liver failure associated with sunitinib treatment have already been described: three in patients with renal cell cancer, one in a patient with stromal tumours2 and another in a patient with ovarian cancer,3 the latter two being fatal.According to the Naranjo4 and CIOMS/RUCAM scales of probability of adverse effects and drug-related hepatotoxicity, respectively, the adverse reaction is classified as “probable” in both, taking into account that the speed and severity of the same did not allow for evaluating the response to the suspension of the drug, the use of placebo or the reintroduction of the drug.
Sunitinib liver failure is a rare and unlikely adverse effect that could be classified within the type B adverse reactions, that is, idiosyncratic, unpredictable, dose independent and not related to the pharmacology of the drug. A mechanism of ischaemic damage has been proposed as the cause of liver failure, rather than direct liver damage being responsible for liver failure.5
Please cite this article as: Casas Deza D, Gascón Ruiz M, Lamuela Calvo LJ, Sierra Gabarda O, Betoré Glaria E, Bernal Monterde V. Fallo hepático fulminante en paciente en tratamiento con sunitinib por tumor del estroma gastrointestinal metastásico. Gastroenterol Hepatol. 2021;44:424–425.