The estimated seroprevalence of hepatitis C virus (HCV) in Spain is 1.7%, but is much higher in the at-risk population. The most efficient national screening strategy is unclear.
AimsTo estimate the prevalence of HCV among the at-risk population seen in primary care (PC), and to determine their epidemiological profile.
Materials and methodsCross-sectional descriptive prevalence study that included adult patients with risk factors for HCV infection seen in PC in the southwest Madrid region between 2010 and 2012.
ResultsA total of 158 patients (men=51.3%), mean age 46 years (SD=16.6), were included. The most common risk factors were hypertransaminasaemia (44.3%) and major surgery (13.3%). Immigration, unsafe sexual practices, and tattoos or body piercing were more prevalent in patients younger than 45 years of age. Fifteen patients (9.5%) were positive for anti-HCV; 9 of these (5.7%) were HCV-ARN positive. Of the positive patients, 4 (44.4%) had significant fibrosis at diagnosis (F3–F4).
Male patients had a higher rate of positive anti-HCV results (13.8 vs. 5.3%; p=0.072), as did patients older than 45 years of age (12.8 vs. 6.3%; p=0.167). Intravenous and intranasal drug use were associated with a higher rate of positive anti-HCV results (50 vs. 8.5%; p=0.005 and 66.7 vs. 8.4%; p=0.001, respectively).
ConclusionsPatients with risk factors for HCV infection have high seroprevalence. Screening programmes must therefore be implemented to detect HCV infection in this population in PC.
La seroprevalencia estimada del VHC en España es del 1,7%, cifra que es muy superior en la población con factores de riesgo. Se desconoce cuál sería la estrategia de cribado más eficiente en nuestro país.
ObjetivosEstimar la prevalencia del VHC en la población con factores de riesgo atendida en Atención Primaria (AP) y conocer su perfil epidemiológico.
Material y métodosEstudio descriptivo transversal de prevalencia que incluyó a pacientes adultos con factores de riesgo de infección por VHC asistidos en AP de la zona suroeste de la Comunidad de Madrid entre 2010 y 2012.
ResultadosSe incluyó a 158 pacientes (H: 51,3%) con una edad media de 46 años (DE=16,6). Los factores de riesgo más frecuentes fueron la hipertransaminasemia (44,3%) y cirugía mayor (13,3%). La inmigración, las prácticas sexuales de riesgo y los tatuajes o piercing fueron más prevalentes en los menores de 45 años. Del total de pacientes, 15 (9,5%) presentaron anti-VHC positivo, de ellos 9 tenían ARN-VHC positivo (5,7%). De los pacientes positivos, 4 (44,4%) presentaron fibrosis significativa al diagnóstico (F3-F4).
Los pacientes varones presentaron una mayor tasa de anti-VHC positivo (13,8 vs. 5,3%; p=0,072), y también los pacientes mayores de 45 años (12,8 vs. 6,3%; p=0,167). El uso de drogas parenterales se asoció a mayor tasa de anti-VHC positivo (50 vs. 8,5%; p=0,005), así como el uso de drogas vía nasal (66,7 vs. 8,4%; p=0,001).
ConclusionesLos pacientes con factores de riesgo de infección por VHC presentan una elevada seroprevalencia. Por tanto, es necesario implantar programas de detección de la infección VHC en esta población en AP.
Hepatitis C virus (HCV) affects more than 180 million people worldwide, according to World Health Organization figures, with an annual incidence of 3–4 million cases. Mean global prevalence of chronic hepatitis C is 2–3%, and the disease is responsible for 350000 deaths annually due to cirrhosis, hepatocarcinoma and acute liver failure.1
The estimated seroprevalence of HCV in the adult population in Spain is around 1.7% and 1.2% for viraemia.2–4 Prevalence varies according to isolated studies in different geographical areas, such as Catalonia,5,6 Murcia,7 Castilla and Leon,8 Asturias9 and northern Spain10; a seroprevalence of 1.8% was recently reported in the general population aged between 16 and 80 years in the Madrid region between 2008 and 2009.11 Prevalence is higher in the at-risk population, such as intravenous drug users (IVDU), recipients of blood products before 1992, patients on haemodialysis, individuals with sexual partners infected with HCV, persons with tattoos, body piercing or other procedures undertaken without adequate health controls, and exposed healthcare workers. There is broad consensus on the need to carry out HCV screening in certain risk groups,1,12 where prevalence may be as high as 10%.13 Furthermore, migratory movements from areas of high prevalence such as eastern European countries, north Africa and southeast Asia have led to an increase in the diagnosis of new cases of hepatitis C.3,13–15
In Spain, the target population for HCV screening is unknown. It has been proposed that all those born before 1975 should be screened,2 but there are as yet no data to support this suggestion. Despite these recommendations, there are no screening programmes in primary care (PC) for at-risk individuals, nor is the prevalence of the infection in this population known. For this reason, the aim of this study was to determine the seroprevalence of HCV among the at-risk population seen in PC, and to determine the epidemiological profile in a healthcare area in the southwest Madrid region of Spain.
Materials and methodsA cross-sectional descriptive prevalence study was conducted to determine the prevalence of HCV infection among the at-risk population seen in PC. To that end, the study included patients seen at 4 health centres in a city in the south west of the Madrid metropolitan area between January 2010 and December 2012, which serve a target population of approximately 100000 inhabitants.
The study was carried out in accordance with the principles of the Declaration of Helsinki, and was approved by the Hospital Universitario Fundación Alcorcón Clinical Research Ethics Committee and the regional healthcare technicians.
Patients were selected using a multi-stage sampling method: in the first stage, patients consulting a doctor were randomly selected from each PC centre. This was followed by a systematic randomised sampling stage (sampling constant k=5) in which at-risk patients were invited to participate in the study.
In order to calculate the sample size, it was estimated that the HCV prevalence in at-risk patients was 10%,13 and that at least 1000 people in the study area had risk factors. Based on these assumptions, we calculated that 122 patients with a valid anti-HCV test were required to reach the expected proportion with 5% precision and a 95% confidence interval (CI). Considering a 15% loss of evaluable individuals, 144 patients needed to be included.
Inclusion criteria were patients over 18 years who visited their PC physician and who presented risk factors for HCV infection, identified as sustained hypertransaminasaemia, history of transfusion of blood products and major surgery before 1992, body piercing or tattoos, intravenous or intranasal drug use, partners of patients with hepatitis C, unsafe sexual practices, haemodialysis, HIV-positive patients, exposed healthcare workers, and immigrants from areas with high HCV prevalence (central and eastern European countries, north Africa and southeast Asia). Hypertransaminasaemia was recorded as a risk factor in patients with no other known risk factors. Risk factors were identified by direct questioning based on the information included in the case report form. All patients received an information sheet and gave their written consent to participate in the study.
The primary endpoint was the presence of anti-HCV antibodies detected by third-generation ELISA, with a positive confirmatory test. Secondary endpoints were sociodemographic factors such as sex, age and occupation; anthropometric variables such as weight, height and body mass index; risk factors; and analytical variables such as alanine transaminase (ALT), aspartate aminotransferase (AST) and gamma-glutamyl transferase (GGT) values, and hepatitis B and HIV serology (hepatitis B surface antigen [HBsAg], anti-HIV). Anti-HCV positive patients were referred to the viral hepatitis clinic as per routine clinical practice for confirmation of the chronic hepatitis C diagnosis, where viral load, genotype and fibrosis stage (METAVIR scale or by Fibroscan) were recorded. Data on these patients were collected by the PC physicians in the participating health centres using case report forms created for this purpose. The forms were sent through the coordinator of each health centre to the principal investigator in the gastroenterology department of Hospital Universitario Fundación Alcorcón, where data related with the liver disease were added in the case of anti-HCV positive patients.
Patients with risk factors and known diagnosis of HCV infection were also included in the study.
Quantitative variables were expressed using measures of central tendency and dispersion (mean, standard deviation and 95% confidence intervals) and qualitative variables using frequency tables and percentages. Quantitative variables were compared using Student t-tests or Mann–Whitney tests, while qualitative variables were analysed using the Chi-squared test or Fisher's test. The odds ratios (OR) of the different risk factors associated with HCV infection were estimated, and logistic regression analysis was performed to determine the variables independently associated with the presence of anti-HCV. All analyses were performed using SPSS statistics program version 21.0.
ResultsThe study included a total of 158 patients with risk factors for HCV infection seen in PC between 2012 and 2014.
Of these, 15 were anti-HCV positive, which corresponds to a seroprevalence of 9.5%. Only 3 of the 15 patients (20%) had hitherto been diagnosed. HCV-RNA testing was obtained in 14 patients: 9 of these presented positive HCV-RNA (64.3%; 5.7% of the total); of these, 4 patients were genotype 1a and 5 patients genotype 1b. In addition, 4 of the patients with chronic hepatitis C (44.4%) presented significant fibrosis (F3-F4) at diagnosis.
Baseline demographic data and risk factors are shown in Table 1. As can be seen, 51.3% of the patients were men. Mean age was 46.2 years (SD 16.6), and 50.6% of the patients were aged over 45 years. BMI>25kg/m2 was noted in 41.8% of patients. The most common risk factors for hepatitis C development were hypertransaminasaemia in 70 patients (44.3%), history of major surgery in 21 patients (13.3%), immigration from an area of high prevalence in 15 patients (9.5%), unsafe sexual practices in 15 cases (9.5%) and having body piercing or tattoos in 14 cases (8.9%).
General characteristics of the sample.
| No. total | 158 |
| Sex M/F; n (%) | 81 (51.3)/76 (48.1) |
| Age (years), mean (SD) | 46.22 (16.6) |
| Age range, n (%) | |
| <45 y | 80 (50.6) |
| >45 y | 78 (49.4) |
| Weight (kg), mean (SD) | 73.03 (14.3) |
| BMI (kg/m2), mean (SD) | 26.27 (4.7) |
| Risk factors, n (%) | |
| Hypertransaminasaemia | 70 (44.3) |
| Exposed healthcare workers | 2 (1.3) |
| Immigrant from area of high prevalence | 15 (9.5) |
| Blood products | 8 (5.1) |
| Major surgery | 21 (13.3) |
| Body piercing or tattoos | 14 (8.9) |
| IVDU | 4 (2.5) |
| Intranasal drugs | 3 (1.9) |
| Partner of patient with HCV | 6 (3.8) |
| Unsafe sexual practices | 15 (9.5) |
| Liver biochemistry IU, mean (SD) | |
| ALT | 40.95 (34.7) |
| AST | 34.21 (37.3) |
| GGT | 59.92 (97.8) |
| Anti-HIV+ n (%) | 0 (0) |
| HbsAg+ n (%) | 3 (1.9) |
| Anti-HCV, n (%) | |
| Positive | 15 (9.5) |
| Negative | 143 (91.5) |
Distribution of risk factors differed according to patient age. Thus, among patients aged over 45 years, transfusion of blood products, hypertransaminasaemia and major surgery were the most common, while immigration, unsafe sexual practices and tattoos or body piercing were more prevalent among those aged under 45 years (Table 2).
Distribution of risk factors by age.
| Risk factors, n (%) | <45 years (n=80) | >45 years (n=78) | p |
|---|---|---|---|
| Hypertransaminasaemia | 25 (31.3) | 45 (57.7) | 0.001 |
| Blood products | 2 (2.5) | 6 (7.7) | 0.13 |
| Major surgery | 7 (8.8) | 14 (17.9) | 0.089 |
| Body piercing or tattoos | 14 (17.5) | 0 | <0.001 |
| IVDU | 2 (2.5) | 2 (2.6) | 0.98 |
| Intranasal drugs | 2 (2.5) | 1 (1.3) | 0.51 |
| Partner of patient with HCV | 0 | 6 (7.6) | 0.13 |
| Unsafe sexual practices | 13 (16.3) | 2 (2.6) | 0.003 |
| Exposed healthcare worker | 1 (1.3) | 1 (1.3) | 0.745 |
| Immigrant from area of high prevalence | 14 (17.5) | 1 (1.3) | 0.001 |
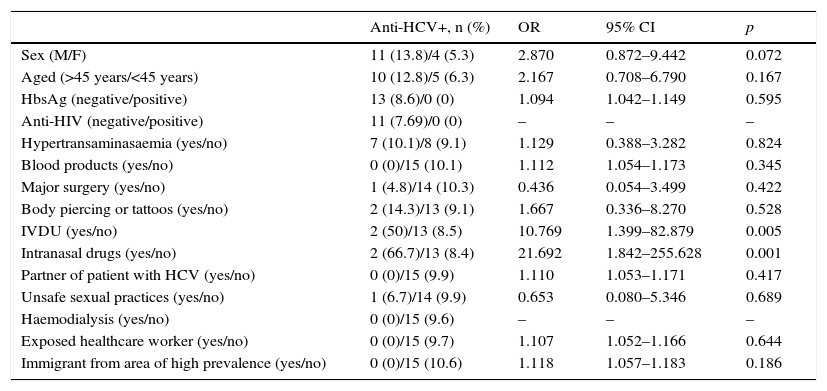
Male patients had a higher rate of positive anti-HCV tests than women (13.8% vs. 5.3%; OR=2.870; 95% CI: 0.872–9.442; p=0.072), as did patients over 45 years of age (12.8% vs. 6.3%; OR=2.167; 95% CI: 0.708–6.790; p=0.167), although these differences did not reach statistical significance. No significant association was found with HIV or HBV co-infection. Among the risk factors evaluated, intranasal and intravenous drug use were associated with a higher rate of positive anti-HCV tests (66.7% vs. 8.4%; OR=21.692; 95% CI: 1.842–255.628; p=0.001 and 50% vs. 8.5%; OR=10.769; 95% CI: 1.399–82.879; p=0.005, respectively). Patients with body piercing or tattoos also presented a high rate of seroprevalence (14.3%), followed by patients with hypertransaminasaemia (10.1%), unsafe sexual practices (6.7%) and those with a history of major surgery (4.8%) (Table 3).
Factors associated with seroprevalence.
| Anti-HCV+, n (%) | OR | 95% CI | p | |
|---|---|---|---|---|
| Sex (M/F) | 11 (13.8)/4 (5.3) | 2.870 | 0.872–9.442 | 0.072 |
| Aged (>45 years/<45 years) | 10 (12.8)/5 (6.3) | 2.167 | 0.708–6.790 | 0.167 |
| HbsAg (negative/positive) | 13 (8.6)/0 (0) | 1.094 | 1.042–1.149 | 0.595 |
| Anti-HIV (negative/positive) | 11 (7.69)/0 (0) | – | – | – |
| Hypertransaminasaemia (yes/no) | 7 (10.1)/8 (9.1) | 1.129 | 0.388–3.282 | 0.824 |
| Blood products (yes/no) | 0 (0)/15 (10.1) | 1.112 | 1.054–1.173 | 0.345 |
| Major surgery (yes/no) | 1 (4.8)/14 (10.3) | 0.436 | 0.054–3.499 | 0.422 |
| Body piercing or tattoos (yes/no) | 2 (14.3)/13 (9.1) | 1.667 | 0.336–8.270 | 0.528 |
| IVDU (yes/no) | 2 (50)/13 (8.5) | 10.769 | 1.399–82.879 | 0.005 |
| Intranasal drugs (yes/no) | 2 (66.7)/13 (8.4) | 21.692 | 1.842–255.628 | 0.001 |
| Partner of patient with HCV (yes/no) | 0 (0)/15 (9.9) | 1.110 | 1.053–1.171 | 0.417 |
| Unsafe sexual practices (yes/no) | 1 (6.7)/14 (9.9) | 0.653 | 0.080–5.346 | 0.689 |
| Haemodialysis (yes/no) | 0 (0)/15 (9.6) | – | – | – |
| Exposed healthcare worker (yes/no) | 0 (0)/15 (9.7) | 1.107 | 1.052–1.166 | 0.644 |
| Immigrant from area of high prevalence (yes/no) | 0 (0)/15 (10.6) | 1.118 | 1.057–1.183 | 0.186 |
Both intravenous and intranasal drug use were independently associated with HCV infection after adjusting for sex and age (Table 4).
DiscussionThe prevalence of anti-HCV positive patients in this at-risk population in the southwest Madrid region seen in PC is close to 10%, similar to that described in other published series13 and much higher than the estimated prevalence in the general population of 1.6–1.8%.6,11,16–19
These findings confirm that this population should be a priority target in screening programmes. Despite this, there are no specific PC programmes to detect infection in the recommended risk groups. Of the 15 anti-HCV positive patients identified in the study, 12 were unaware of the diagnosis (80%), around 65% had chronic infection and half of these had significant fibrosis at diagnosis, which shows the importance of screening for the infection in this target population.
The introduction of new direct antiviral agents in Spain, which have increased the efficacy and tolerability of therapeutic regimens, means that the vast majority of patients diagnosed can achieve cure. There is abundant evidence on the impact of these measures in terms of a reduction in morbidity and mortality and costs associated with the disease.20,21
The recently published Spanish national strategic plan22 includes an epidemiological approach to the disease, prioritising actions such as carrying out a survey on seroprevalence in the adult population and drawing up guidelines with recommendations for early diagnosis of the priority population in PC. A cost-effective strategy would consist in screening individuals born before 1975 who present a higher risk of infection due to the use of non-disposable syringes and absence of blood product screening.2
As in previous studies conducted in the general population, the male patients over 45 years old included in our study had a higher risk of presenting anti-HCV antibodies,11 possibly due to the decrease in the incidence of the infection in recent years. All patients aged over 45 years and only a small number of those under this age were born before 1975, suggesting that the prevalence of individuals born before this date is also higher in the at-risk population. It is not known if screening of the general population born before 1975 would be more worthwhile, so studies are needed to analyse this point.
The epidemiology of HCV infection in Europe is also changing due to several factors. First of all, the detection of anti-HCV antibodies in blood products since 1990 has meant that transfusion-related transmission has been virtually eradicated in Spain. Intravenous drug use has also fallen in Spain in recent years. A study conducted between 1994 and 1996 showed that 25% of anti-HCV positive patients had received transfusion of blood products and 10% had a history of IVDU.6 In recent years, cases of acute infection have been associated with nosocomial infection, with 73% of cases with a history of recent diagnostic and therapeutic procedures.23
This aspect was confirmed in our study. Immigration, intranasal drug use, unsafe sexual practices, tattoos and body piercing are the most common risk factors in the population aged under 45 years with respect to that over 45 years, in which hypertransaminasaemia, transfusion of blood products and major surgery predominated.
In our series, patients with a history of intravenous drug use and especially intranasal use presented a high anti-HCV prevalence rate. The rate of anti-HCV positivity in this population varies between 42% and 98% according to the series.24,25 Series published in Spain also report an OR>1 in patients with a history of IVDU.16–18 Recent studies have shown an increase in transmission in HIV patients with unsafe sexual practices (men who have sex with men), with a higher frequency of intranasal cocaine use during the sexual practices.26 In our study, the seroprevalence in the group with unsafe sexual practices was 6.8%, although the type of sexual practice was not recorded.
Likewise, recent migratory movements in Spain, with an increase in the influx of people from eastern Europe, southeast Asia and north Africa, has led to more diagnoses of chronic HCV infection due to the high prevalence of HCV in these countries, associated with poorer health and hygiene conditions. According to the Spanish Statistical Office (INE), the largest immigrant population in Spain is from Romania (773122 persons) followed by Morocco (756946 persons). The prevalence of HCV in Romania has been estimated to be 6%27 and 2% in Morocco.28 However, in our study, none of the immigrant patients from more prevalent areas were anti-HCV positive, although the country of origin was not recorded and the immigrant population sample was small. A recent study of the working population of Murcia and Madrid in Spain, which included more than 5000 patients, also failed to show an association between immigration and HCV seroprevalence.19
Chief among the limitations of our study is the small number of patients representing some risk categories such as drug addiction or exposed healthcare workers, a factor that prevented us from drawing definitive conclusions.
Another limitation is the possibility of selection bias. Despite the fact that patient selection was performed systematically and that health centres located in areas with different social-economic levels were included, the population that inhabits the city centre predominates over that of residential areas. The number of visits in each shift was not monitored. Taken together, these two factors plus the fact that not all patients with inclusion criteria agreed to participate in the study, could lead to over- or under-representation of certain risk factors.
Despite its limitations, this is the first study to show high HCV seroprevalence in the at-risk population in PC, and has led to de novo diagnosis in 8% of the population included. It can be considered, therefore, as a pilot experiment that could lead to the implementation of screening programmes.
In summary, patients with risk factors for HCV infection have high seroprevalence. For these reasons, pending an as yet undetermined strategic population screening plan, programmes for detecting HCV infection in the at-risk population in PC must be implemented.
Conflict of interestsThe authors declare that they have no conflict of interests.
Please cite this article as: Alonso López S, Agudo Fernández S, García del Val A, Martínez Abad M, López Hermosa Seseña P, Izquierdo MJ, et al. Seroprevalencia de hepatitis C en población con factores de riesgo del suroeste de la Comunidad de Madrid. Gastroenterol Hepatol. 2016;39:656–662.