Idiopathic non-cirrhotic portal hypertension (INCPH) is a rare condition that involves intrahepatic portal hypertension without cirrhosis or other causes of liver disease.1,2 It requires the presence of portal hypertension (PHT) with permeability of the splenoportal axis and suprahepatic veins. The aetiopathogenesis of this entity is unknown3 but is associated with infections, toxins, immunological disorders, hypercoagulation and human immunodeficiency virus (HIV).
We present the case of a 39-year-old male with a pre-existing diagnosis of stage C3 HIV infection who attended A&E having presented melaena for the previous three days. He had not taken NSAIDs or other gastro-damaging agents. He presented asthenia of recent onset with no abdominal pain. He was on antiretroviral therapy, having received various drugs since 1999 (including didanosine for 42 months). At the physical examination, the patient presented pale skin and mucosa, tachycardia at 110bpm and blood pressure of 130/90mmHg. The abdominal examination revealed non-painful hepatosplenomegaly.
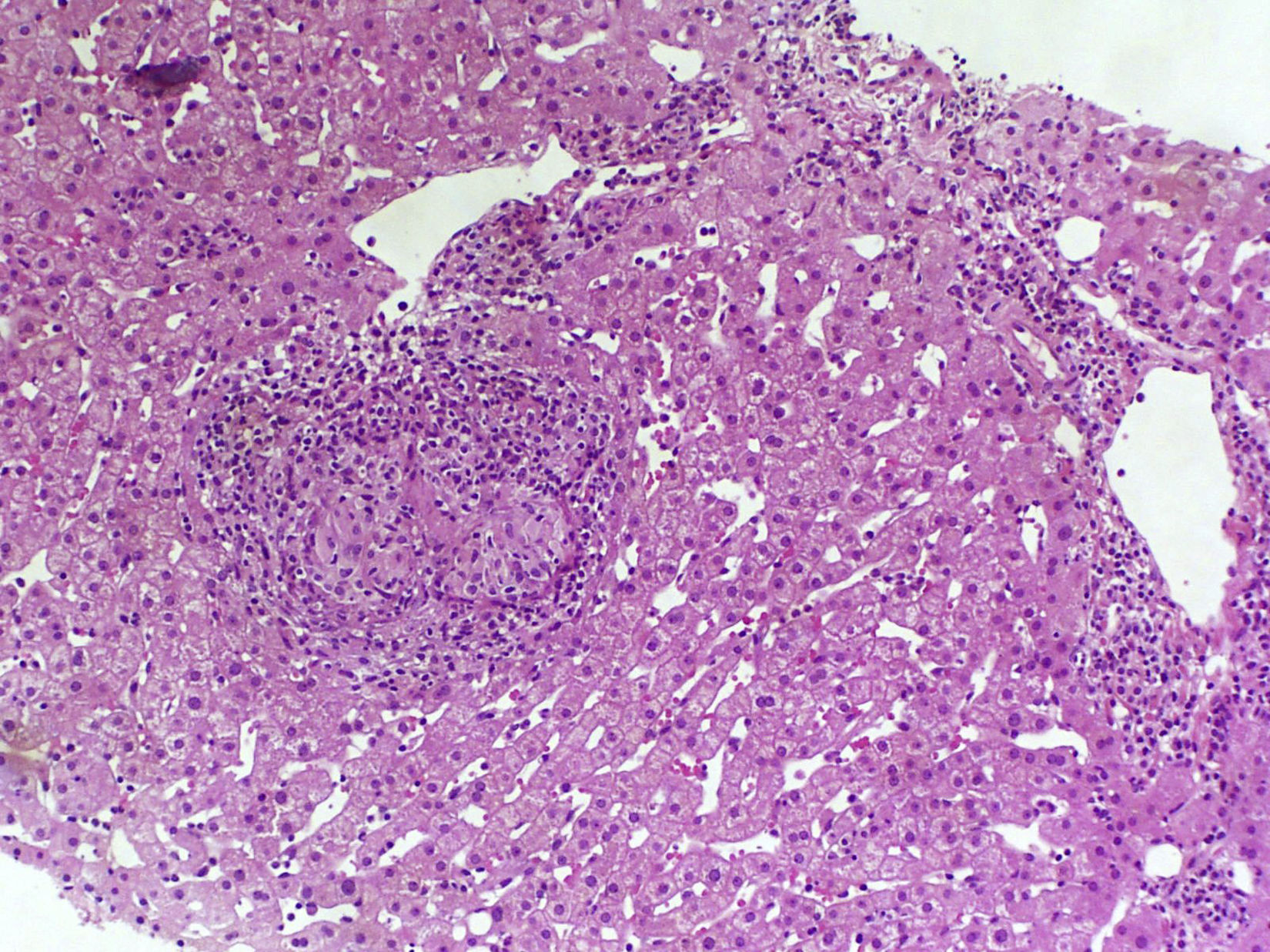
The complete blood count found haemoglobin levels of 6.4g/dl (normal range: 13–18g/dl) and a normal platelet count (168,000U/ml). The clinical chemistry found bilirubin levels of 0.8mg/dl, albumin 4g/dl, aspartate aminotransferase 38U/l, alanine aminotransferase 25U/l and alkaline phosphatase 98U/l. The coagulation study found an INR of 1. The HIV viral load was undetectable with a CD4 count of 188cell/μl. Complementary examinations conducted included an early upper gastrointestinal endoscopy, which revealed large oesophageal varices with no signs of bleeding or recent haemostasis. Three packed red blood cell units were transfused and the patient was admitted for examination. Bloods were conducted that included: viral serologies, autoimmunity study, ferrokinetic studies, copper, ceruloplasmin, α-1 antitrypsin and porphyria, and all results were normal. The abdominal Doppler ultrasound and chest/abdominal CT scan revealed signs of PHT (hepatomegaly, splenomegaly, portal vein dilatation and significant collateral circulation). The permeability of the splenic-mesenteric-portal venous system was confirmed. A liver elastography was performed, yielding a result of 13.8kPa. The haemodynamic study revealed a hepatic venous pressure gradient (HVPG) of 10mmHg. A transjugular liver biopsy was performed during the procedure, with insufficient material to conduct an anatomopathological study. As a result, a percutaneous liver biopsy was subsequently performed. The anatomopathological study with haematoxylin and eosin staining, Masson's trichrome staining and reticulin staining did not reveal significant histological abnormalities. Only minimal sinusoidal dilatation was found (Fig. 1). Although no active bleeding was found during the endoscopy, the patient was deemed to have presented variceal upper gastrointestinal bleeding. As such, secondary prophylaxis with beta blockers and endoscopic ligation of the oesophageal varices was initiated.
At two-months follow-up (which found the patient to be asymptomatic), an abdominal Doppler ultrasound was performed, showing partial thrombosis of the proximal portion of the right portal vein and superior mesenteric vein. A thrombophilia study was ordered, which revealed factor V Leiden heterozygous mutation. As a result, anticoagulation therapy was started, initially with LMWH and then with acenocoumarol. Following initiation of anticoagulation therapy, subsequent CT scans revealed correct opacification of the splenic-mesenteric-portal venous system with a persistent minor peripheral repletion defect in the right portal branch smaller than found in previous studies. The patient is currently asymptomatic at 48-months follow-up. Endoscopic band ligation was performed six times and the patient continues to be treated with propranolol and acenocoumarol.
INCPH is a rare condition of uncertain aetiology characterised by PHT without liver disease. Although it can be found all over the world, it is most prevalent in developing countries and is associated with a lower socioeconomic status.1,4 The aetiology of INCPH is unknown. A potential cause that has been hypothesised is the presence of persistent digestive tract infections or exposure to certain toxins (arsenic, vinyl, copper sulphate or azathioprine). HIV infection has been associated with the pathogenesis of INCPH, particularly in patients who have received antiviral therapy (notably following prolonged exposure to didanosine and/or stavudine), or as a direct result of HIV itself.2,5,6 It has recently been postulated that these patients are more susceptible to prothrombotic abnormalities.7,8 Portal vein thrombosis is more prevalent than in patients with liver cirrhosis, especially in patients with HIV-related INCPH.1,2
A subgroup of patients with HIV and PHT without liver disease has recently been identified. These patients are characterised by preserved liver function and a lack of histological cirrhosis.9 Significant splenomegaly is also typical. It is noteworthy that these patients present normal or slightly elevated HVPG values in the haemodynamic study, which underestimates the actual portal venous pressure. This is due to increased presinusoidal hepatic resistance and the presence of intrahepatic venous collaterals.1 Despite the fact that our patient manifested unequivocal signs of PHT (oesophageal varices, splenomegaly, collaterals), an HVPG of 10mmHg was found. Liver biopsy is vital to rule out cirrhosis and other causes of PHT. Histological abnormalities are varied and are not pathognomonic. Those that typically manifest include portal vein sclerosis, sinusoidal dilatation and nodular regenerative hyperplasia.1,10
There is no specific treatment for the condition, which is limited to managing the complications of portal hypertension.7 Although the natural history of this condition is unknown, mortality in these patients is usually caused by variceal bleeding. Mortality rates are lower than for cirrhosis, probably due to preserved liver function.
Please cite this article as: Gómez Alonso M, Goñi Esarte S, Bolado Concejo F, Urman Fernández JM, Basterra Ederra M, Kutz Leoz M, et al. Hipertensión portal idiopática no cirrótica y trombosis portal en paciente con infección por VIH. Gastroenterol Hepatol. 2017;40:353–354.