Portal hypertension is a hemodynamic abnormality that complicates the course of cirrhosis, as well as other diseases that affect the portal venous circulation. The development of portal hypertension compromises prognosis, especially when it rises above a certain threshold known as clinically significant portal hypertension (CSPH). In the consensus conference on Portal Hypertension promoted by the Spanish Association for the Study of the Liver and the Hepatic and Digestive diseases area of the Biomedical Research Networking Center (CIBERehd), different aspects of the diagnosis and treatment of portal hypertension caused by cirrhosis or other diseases were discussed. The outcome of this discussion was a set of recommendations that achieved varying degrees of consensus among panelists and are reflected in this consensus document. The six areas under discussion were: the relevance of clinically significant portal hypertension and the non-invasive methods used for its diagnosis and that of cirrhosis, the prevention of the first episode of decompensation and its recurrence, the treatment of acute variceal bleeding and other complications of portal hypertension, the indications for the use of TIPS, and finally, the diagnosis and treatment of liver vascular diseases.
La hipertensión portal es una anomalía hemodinámica que complica el curso de la cirrosis, así como de otras enfermedades que afectan a la circulación venosa portal. El desarrollo de hipertensión portal grava negativamente el pronóstico, especialmente cuando asciende por encima de una determinada cuantía conocida como hipertensión portal clínicamente significativa (HPCS). En la conferencia de consenso en Hipertensión Portal promovida por la Asociación Española para el Estudio del Hígado y el área de enfermedades hepáticas y digestivas del Centro de Investigación Biomédica en Red (CIBERehd) se han discutido diferentes aspectos del diagnóstico y tratamiento de la hipertensión portal causada por la cirrosis o por enfermedades diferentes a ésta. El resultado de esta discusión fue la redacción de un conjunto de recomendaciones que alcanzaron diferentes grados de consenso entre los panelistas y que se han plasmado en el presente documento de consenso. Las seis áreas objeto de la discusión han sido: la relevancia de la hipertensión portal clínicamente significativa y los métodos no invasivos utilizados para su diagnóstico y el de la cirrosis, la prevención del primer episodio de descompensación y de su recurrencia, el tratamiento de la hemorragia aguda por varices y de otras complicaciones de la hipertensión portal, las indicaciones del uso del TIPS y, por último, el diagnóstico y tratamiento de las enfermedades vasculares del hígado.
Portal hypertension is the most common and severe complication in patients with cirrhosis, occurring when the portal pressure gradient (PPG, pressure difference between the portal vein and the inferior vena cava) is greater than 5 mmHg. The hepatic venous pressure gradient (HVPG) is an excellent surrogate marker of PPG which can be estimated in clinical practice by catheterisation of the suprahepatic veins. HVPG is calculated as the difference between wedged hepatic venous pressure (WHVP) and free hepatic venous pressure (FHVP). In cirrhosis, especially in cirrhosis of alcoholic or viral origin, WHVP faithfully reflects portal pressure.1 HVPG measurement is a simple, safe and minimally invasive procedure well tolerated by the patient, as it is not necessary to puncture the portal vein. In addition, measurement of HVPG may be useful in portal hypertension other than sinusoidal hypertension, as it can differentiate pre-hepatic/pre-sinusoidal portal hypertension from hepatic and post-hepatic portal hypertension, and can be complemented by transjugular liver biopsy or right heart catheterisation.2
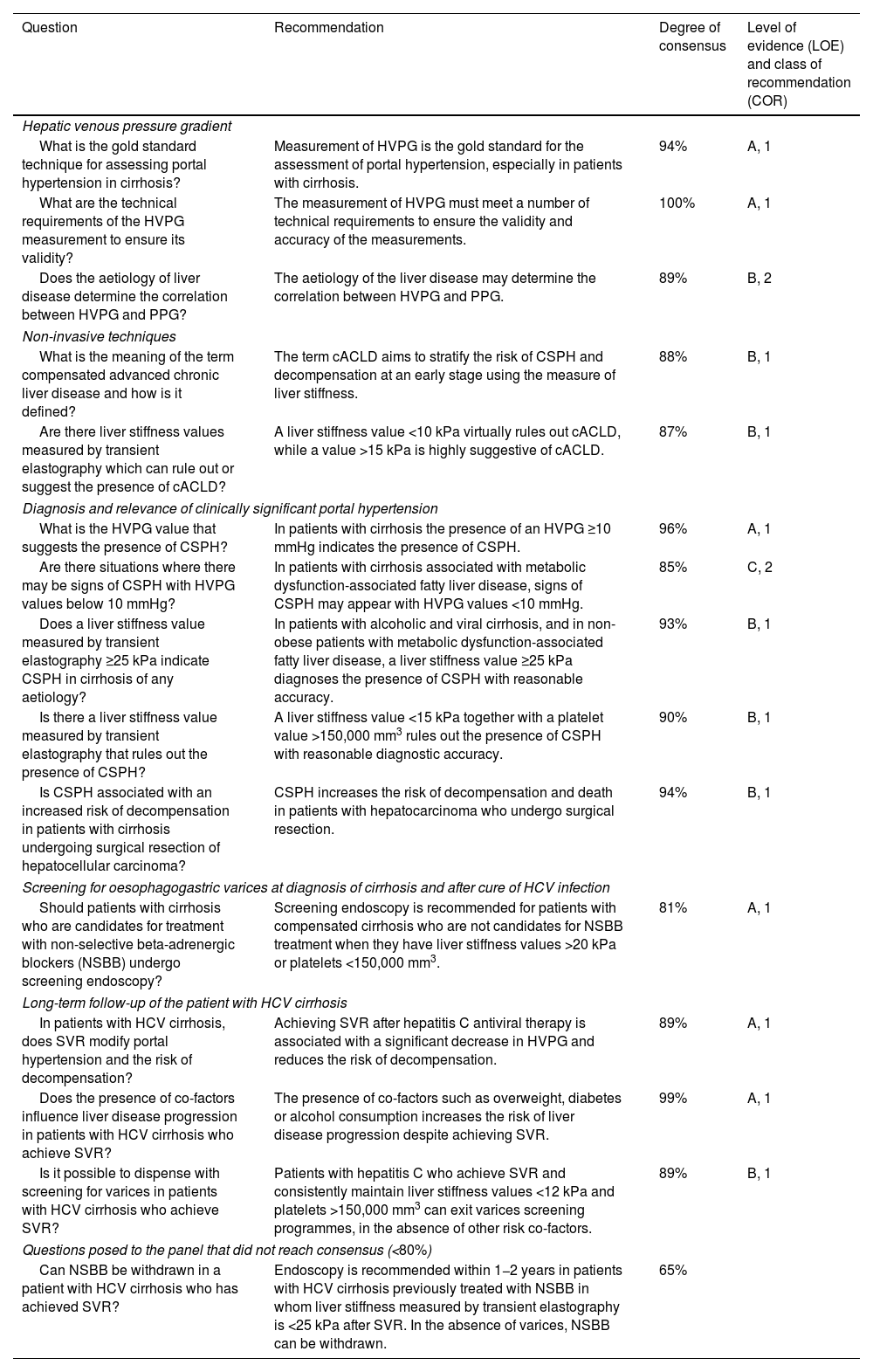
Recommendations on the diagnosis of cirrhosis and portal hypertension (session 1).
| Question | Recommendation | Degree of consensus | Level of evidence (LOE) and class of recommendation (COR) |
|---|---|---|---|
| Hepatic venous pressure gradient | |||
| What is the gold standard technique for assessing portal hypertension in cirrhosis? | Measurement of HVPG is the gold standard for the assessment of portal hypertension, especially in patients with cirrhosis. | 94% | A, 1 |
| What are the technical requirements of the HVPG measurement to ensure its validity? | The measurement of HVPG must meet a number of technical requirements to ensure the validity and accuracy of the measurements. | 100% | A, 1 |
| Does the aetiology of liver disease determine the correlation between HVPG and PPG? | The aetiology of the liver disease may determine the correlation between HVPG and PPG. | 89% | B, 2 |
| Non-invasive techniques | |||
| What is the meaning of the term compensated advanced chronic liver disease and how is it defined? | The term cACLD aims to stratify the risk of CSPH and decompensation at an early stage using the measure of liver stiffness. | 88% | B, 1 |
| Are there liver stiffness values measured by transient elastography which can rule out or suggest the presence of cACLD? | A liver stiffness value <10 kPa virtually rules out cACLD, while a value >15 kPa is highly suggestive of cACLD. | 87% | B, 1 |
| Diagnosis and relevance of clinically significant portal hypertension | |||
| What is the HVPG value that suggests the presence of CSPH? | In patients with cirrhosis the presence of an HVPG ≥10 mmHg indicates the presence of CSPH. | 96% | A, 1 |
| Are there situations where there may be signs of CSPH with HVPG values below 10 mmHg? | In patients with cirrhosis associated with metabolic dysfunction-associated fatty liver disease, signs of CSPH may appear with HVPG values <10 mmHg. | 85% | C, 2 |
| Does a liver stiffness value measured by transient elastography ≥25 kPa indicate CSPH in cirrhosis of any aetiology? | In patients with alcoholic and viral cirrhosis, and in non-obese patients with metabolic dysfunction-associated fatty liver disease, a liver stiffness value ≥25 kPa diagnoses the presence of CSPH with reasonable accuracy. | 93% | B, 1 |
| Is there a liver stiffness value measured by transient elastography that rules out the presence of CSPH? | A liver stiffness value <15 kPa together with a platelet value >150,000 mm3 rules out the presence of CSPH with reasonable diagnostic accuracy. | 90% | B, 1 |
| Is CSPH associated with an increased risk of decompensation in patients with cirrhosis undergoing surgical resection of hepatocellular carcinoma? | CSPH increases the risk of decompensation and death in patients with hepatocarcinoma who undergo surgical resection. | 94% | B, 1 |
| Screening for oesophagogastric varices at diagnosis of cirrhosis and after cure of HCV infection | |||
| Should patients with cirrhosis who are candidates for treatment with non-selective beta-adrenergic blockers (NSBB) undergo screening endoscopy? | Screening endoscopy is recommended for patients with compensated cirrhosis who are not candidates for NSBB treatment when they have liver stiffness values >20 kPa or platelets <150,000 mm3. | 81% | A, 1 |
| Long-term follow-up of the patient with HCV cirrhosis | |||
| In patients with HCV cirrhosis, does SVR modify portal hypertension and the risk of decompensation? | Achieving SVR after hepatitis C antiviral therapy is associated with a significant decrease in HVPG and reduces the risk of decompensation. | 89% | A, 1 |
| Does the presence of co-factors influence liver disease progression in patients with HCV cirrhosis who achieve SVR? | The presence of co-factors such as overweight, diabetes or alcohol consumption increases the risk of liver disease progression despite achieving SVR. | 99% | A, 1 |
| Is it possible to dispense with screening for varices in patients with HCV cirrhosis who achieve SVR? | Patients with hepatitis C who achieve SVR and consistently maintain liver stiffness values <12 kPa and platelets >150,000 mm3 can exit varices screening programmes, in the absence of other risk co-factors. | 89% | B, 1 |
| Questions posed to the panel that did not reach consensus (<80%) | |||
| Can NSBB be withdrawn in a patient with HCV cirrhosis who has achieved SVR? | Endoscopy is recommended within 1−2 years in patients with HCV cirrhosis previously treated with NSBB in whom liver stiffness measured by transient elastography is <25 kPa after SVR. In the absence of varices, NSBB can be withdrawn. | 65% | |
cACLD: compensated advanced chronic liver disease; CSHP: clinically significant portal hypertension; HCV: hepatitis C virus; HVPG: hepatic venous pressure gradient; kPa: kilopascals; NSBB: nonselective beta-adrenergic blockers; PPG: portal pressure gradient; SVR: sustained viral response.
To ensure that the HVPG measurement is correct, it is important to standardise the technique so that the results are valid, accurate and reproducible.3 For this purpose, digital equipment that allows continuous tracings at low speed (maximum 7.5 mm/s) should be used to calculate the average pressure of a representative segment. For WHVP measurement, a catheter/balloon should be used to occlude a representative sinusoidal territory and a small amount of radiological contrast injected to confirm correct occlusion of the vein and to check for the presence of hepatic veno-venous communications as, if present, if they prevent correct occlusion of the suprahepatic vein, the HVPG value may be underestimated.4 To measure WHVP correctly, a stabilisation period of the trace is necessary, so it is recommended to record at least 1 min with a minimum of 20−30 s of stable trace. In addition, to ensure that the WHVP measurement is correct and reproducible, it should be determined in triplicate. FHVP should be measured in the suprahepatic vein 2−3 cm from the confluence with the inferior vena cava. Calculation of HVPG using the right atrial or inferior vena cava pressure value instead of FHVP is less accurate and should be avoided.5,6 The HVPG value may be underestimated if the measurement is performed under deep sedation, both because of the possible hypotensive effect of the drugs and their effect on respiratory dynamics modifying intra-abdominal pressure, and should therefore be avoided.7 Other factors such as haemodynamic instability, treatment with vasoactive drugs, mechanical ventilation, polytransfusion or evacuative paracentesis with albumin replacement in the hours prior to the procedure may also alter the measurement of HVPG. Importantly, if the catheterisation technique and recording are performed correctly, the degree of both inter-observer and test-retest agreement is excellent.8,9
The aetiology of the liver disease may affect the correlation between HVPG and PPGWHVP accurately reflects portal pressure in cirrhosis of viral and alcoholic aetiology and the concordance between the PPG and HVPG is excellent in these aetiologies.10,11 In patients with cirrhosis due to fatty liver disease of metabolic origin, the concordance between the two gradients is likely to be lower. A recent study analysing the concordance of WHVP and directly measured portal pressure in patients with decompensated cirrhosis due to metabolic dysfunction-associated fatty liver disease (MAFLD) showed that WHVP underestimates portal pressure in up to one third of the patients analysed.12 It is not known whether this discordance exists in the compensated phase of the disease. In patients with pre-sinusoidal or pre-hepatic hypertension, the WHVP measurement is not a true reflection of portal pressure as it measures pressure in the sinusoidal territory, but is not able to capture changes beyond the sinusoid. Finally, in patients with cirrhosis secondary to primary biliary cholangitis, there is a component of presinusoidal portal hypertension without necessarily cirrhosis and which is not reliably captured by HVPG.13,14
2. Non-invasive techniquesThe term compensated advanced chronic liver disease (cACLD) aims to stratify the risk of clinically significant portal hypertension (CSPH) and decompensation early using the measure of liver stiffnessThe widespread use in clinical practice of transient elastography to measure liver stiffness and estimate the severity of liver fibrosis allows staging of chronic liver disease without the need for liver biopsy, but this makes it difficult to determine whether the patient has advanced fibrosis or cirrhosis. For this reason, the concept of cACLD was introduced at the Baveno VI Consensus Workshop using two cut-off points that selected two groups of patients with a different risk of developing CSPH and therefore liver decompensation. Two meta-analyses15,16 have shown that the risk of liver disease-related complications is substantially increased in patients with liver stiffness >10 kPa.
A liver stiffness value below 10 kPa virtually rules out cACLD, while a value above 15 kPa is highly suggestive of itThe prevalence of advanced fibrosis/cirrhosis in patients with liver stiffness below 10 kPa is very low, close to 10% in most studies comparing elastography with biopsy, although depending on the aetiology, the prevalence can vary between 4% and 20%.17–21 In terms of the incidence of events, the risk of liver disease-related complications in patients with liver stiffness <10 kPa (or cut-off points close to this value) is less than or equal to 1% at three years in most series.19,22–30 So, a liver stiffness value of less than 10 kPa virtually rules out cACLD.
A cut-off point above 15 kPa, however, selects a patient population with a prevalence of advanced fibrosis/cirrhosis above 80% for most aetiologies.17,18,20,21,31,32 A recent series33 showed that the prevalence of portal hypertension (HVPG > 5 mmHg) in patients with a degree of liver stiffness >15 kPa was over 90% in most aetiologies, except in patients with MAFLD who were obese, where the prevalence was 64%. Not many studies have studied the incidence of events in patients with liver stiffness >15 kPa, although several have shown that as stiffness increases so does the risk of events. In a prospective cohort of patients with alcohol-related liver disease.19 the risk of developing liver disease-related complications (including alcoholic hepatitis) at four years in patients with liver stiffness >15 kPa was 54% compared to 3% in patients with liver stiffness values <10 kPa.
3. Diagnosis and relevance of clinically significant portal hypertensionIn patients with cirrhosis, the presence of HVPG equal to or greater than 10 mmHg indicates CSHPThe natural history of cirrhosis can be divided into two phases: a long compensated phase and a shorter decompensated phase.34 Within the compensated phase, one of the most important milestones in terms of prognosis is the development of CSPH, defined as an HVPG ≥ 10 mmHg, as its presence determines the risk of clinical decompensation. The definition of CSPH comes from longitudinal studies involving patients with cirrhosis of viral and alcohol-related aetiology in which it was found that patients with an HVPG ≤ 10 mmHg did not develop oesophageal varices or complications of portal hypertension (ascites, variceal bleeding or hepatic encephalopathy), while the five-year risk of decompensation in patients with CSPH was approximately 40%.35 It has recently been shown that in patients with MAFLD-associated cirrhosis, the CSPH concept retains its prognostic capacity.36
In patients with MAFLD-associated cirrhosis, signs of CSPH may appear with HVPG values below 10 mmHgIn patients with cirrhosis of viral and alcoholic aetiology, the presence of oesophagogastric varices, portosystemic collaterals or clinical decompensation is anecdotal if HVPG is <10 mmHg. However, in patients with MAFLD cirrhosis it is possible to observe signs of CSPH or clinical decompensation with HVPG values <10 mmHg. A multicentre cross-sectional study showed that the prevalence of liver decompensation with an HVPG value <10 mmHg was 9% in patients with MAFLD cirrhosis compared to no decompensation among patients with hepatitis C virus cirrhosis, with ascites being the most frequently described complication.37 Additionally, a post hoc analysis of the 475 MAFLD patients with advanced disease (F3 and F4 fibrosis) included in simtuzumab trials identified seven cases (14%) of decompensation in patients with a baseline HVPG < 10 mmHg.36
In patients with alcoholic and viral cirrhosis and in non-obese patients with MAFLD, a liver stiffness value of 25 kPa or above diagnoses the presence of CSPH with reasonable accuracyA liver stiffness value below 15 kPa together with a platelet value above 150,000/mm3 rules out the presence of CSPH with reasonable diagnostic accuracy.
Following the Baveno VI Consensus Workshop, two publications contributed to refining and improving the assessment and stratification of patients with cACLD according to their risk of CSPH. The ANTICIPATE study38 provided risk prediction models for CSPH using the degree of liver stiffness measured by transient elastography plus platelet count in a population of patients with cACLD composed mainly of patients with viral and alcohol-related aetiology. These models were subsequently validated in a different cohort with a similar composition.33 In this multicentre study of more than 800 patients with cACLD, the prevalence of CSPH was 83.5%, 91% and 93.7% for liver stiffness values of 20, 25 and 30 kPa respectively. A liver stiffness value ≥25 kPa was chosen as the optimal cut-off point for diagnosing CSPH with a positive predictive value and specificity of over 90%. This liver stiffness cut-off was adequate for the diagnosis of CSPH in viral and alcohol-related cACLD, but not for patients with MAFLD cACLD and patients with obesity.
The exclusion of CSPH in patients with cACLD has been a difficult task. The use of the combination of liver stiffness and platelet count seems to perform better in ruling it out, and data from the above-mentioned multicentre study33 pointed in the same direction. Adding a platelet count ≥150,000/mm3 to a liver stiffness cut-off point ≤15 kPa could exclude CSPH with a negative predictive value and a sensitivity above 90% for most aetiologies (viral, alcohol-related and MAFLD).
These criteria for diagnosing and ruling out CSPH have subsequently been validated in numerous publications,39–41 including more than 500 patients, with positive and negative predictive values of 91.5% and 100% respectively. The recommendations for different cut-off points for liver stiffness by elastography have been established for patients with cACLD of viral aetiology and to a lesser extent alcohol or MAFLD, so values may vary for cACLD of other causes.
CSPH increases the risk of decompensation and death in patients with hepatocellular carcinoma (HCC) who undergo surgical resectionThe initial findings by Bruix et al.42 in a small series of cirrhotic patients with surgically resected HCC who had portal hypertension showed that they were at increased risk of postoperative decompensation. In a later study with a larger number of patients, CSPH and elevated bilirubin were shown to be the best predictors of mortality in the postoperative period after resection.43 Finally, in a systematic review of 11 studies, Berzigotti, et al.44 demonstrated that, in patients with compensated cirrhosis and HCC treated with surgery, the presence of CSPH almost doubled the three- and five-year mortality risk and tripled the risk of postoperative clinical decompensation.44
4. Screening for oesophagogastric varices at diagnosis of cirrhosis and after cure of hepatitis C virus infectionScreening endoscopy is recommended for patients with compensated cirrhosis who are not candidates for treatment with non-cardioselective beta-adrenergic blockers (NSBB) when they have liver stiffness values above 20 kPa or platelets below 150,000/mm3A controversial issue at present is when to perform endoscopic screening for oesophageal varices in patients with CSPH who cannot take NSBB. On the one hand, the PREDESCI45 study demonstrates the benefit, in terms of survival and decompensation, of treatment with NSBB in compensated patients with CSPH, which would already include adequate primary prophylaxis in those patients with at-risk varices. On the other, the ANTICIPATE study38 estimated that the likelihood of having varices at risk of bleeding requiring treatment is clearly lower in patients with a liver stiffness value <20 kPa and a platelet count >150,000/mm3. Lastly, patients with contraindications or intolerance to NSBB may benefit from treatment with endoscopic band ligation (EBL) as primary prophylaxis. Therefore, any patient with cACLD at risk for varices (especially those with liver stiffness >20 kPa or platelets <150,000/mm3) and contraindication or intolerance to NSBB should undergo screening endoscopy and EBL if appropriate. This recommendation has been taken up in the Baveno VII Consensus Workshop46 and in the European Society of Gastrointestinal Endoscopy (ESGE) consensus on the diagnosis and management of oesophago-gastric variceal bleeding.47
5. Long-term follow-up of the patient with hepatitis C virus cirrhosisAchieving sustained virologic response (SVR) after hepatitis C antiviral treatment is associated with a significant decrease in HVPG and reduces the risk of decompensationThe introduction of direct-acting antivirals (DAA), with greater efficacy and a better safety profile than previous generations of antivirals, has made it possible to treat and cure most patients with advanced hepatitis C virus (HCV) liver disease. The first study to show the impact of SVR achieved by DAA on portal pressure included 60 patients with HVPG measurement before and after antiviral treatment who were stratified according to baseline (6–9; 10–15; ≥16 mmHg).48 SVR improved portal hypertension in all HVPG strata, although HVPG reduction was less likely in patients with HVPG ≥ 16 mmHg and therefore more advanced liver disease. A prospective Spanish multicentre study included 226 patients with CSPH in whom HVPG was measured at baseline and six months after completion of treatment and achievement of SVR.49 This study showed a significant mean decrease of 2.1 ± 3.2 mmHg in this short period of time, despite which 78% of patients remained with CSPH and therefore at risk of decompensation. At two years, these patients underwent a new haemodynamic study and 53% continued to have CSPH. Long-term follow-up of the Austrian cohort50 and the Spanish cohort51 found that the incidence of de novo decompensation or re-decompensation after SVR was low. Having an elevated baseline and/or post-treatment HVPG value and a history of previous decompensation were associated with an increased risk of decompensation after SVR. It is important to note that, in these studies, no patient whose HVPG dropped below 10 mmHg after SVR developed decompensation after three to four years of follow-up.
The presence of cofactors such as overweight, diabetes or alcohol consumption increases the risk of liver disease progression despite obtaining SVRAfter SVR, 10% of patients may have fibrosis progression.52 The presence of overweight or obesity, diabetes and alcohol consumption are important contributors to liver disease progression even after elimination/suppression of the primary aetiological factor and should be carefully assessed.53,54
In the absence of other risk co-factors, patients with hepatitis C who achieve SVR and consistently maintain liver stiffness values <12 kPa and platelets >150,000/mm3 can exit varices screening programmes.
The utility of non-invasive techniques to detect the presence of CSPH has been studied predominantly in patients with active HCV infection. A multicentre study evaluated 324 patients with HCV-associated portal hypertension who achieved SVR and had a post-treatment HVPG measurement.55 The prevalence of CSPH before and after treatment was 80% and 54% respectively. The combination of liver stiffness value and platelet count after SVR had a high diagnostic accuracy for estimating CSPH. Following SVR, a liver stiffness value <12 kPa associated with a platelet count >150,000/mm3 ruled out the presence of CSPH with a sensitivity of 99.2%. A liver stiffness value ≥25 kPa was highly suggestive of CSPH despite having obtained SVR (93.6%). No patients with liver stiffness <12 kPa and a platelet count >150,000/mm3 developed decompensation during a median follow-up of 36 months. These criteria, however, do not preclude further HCC screening in patients with advanced fibrosis as the risk persists despite achieving SVR.
6. Questions posed to the panel that did not reach consensusEndoscopy is recommended within 1−2 years in patients with HCV cirrhosis previously treated with NSBB in whom liver stiffness measured by transient elastography is less than 25 kPa after SVR. In the absence of varices, NSBB can be withdrawnThere are robust data indicating that cure of HCV infection in patients with CSPH is accompanied by a significant decrease in portal pressure.51 However, despite this reduction, up to 77% of patients continue to have an HVPG above 10 mmHg, and are therefore at risk of decompensation. In one recent study, Pons et al.33 demonstrated that in a series of 572 patients with cACLD and SVR after antiviral treatment, followed up for an average of 2.8 years, the incidence of decompensation was less than 1%. All decompensated patients had liver stiffness at baseline >20 kPa, and in 80% it did not change after cure of the infection.
In that same vein, in a retrospective analysis of 418 patients with paired transient elastography and HVPG measurements, Semmler et al.55 found that the three-year risk of decompensation in patients with liver stiffness <12 kPa and platelets >150,000 was 0%. These authors were able to confirm the utility of transient elastography in the assessment of CSPH in patients with SVR after antiviral therapy, with an even higher efficacy than that observed in patients with active infection.
It would seem reasonable to check for the absence of varices in all patients virologically cured of hepatitis C and whose liver stiffness is <25 kPa, especially in those with values <12-15 kPa, before considering withdrawal of NSBB. In making this decision, and in the absence of definitive prospective information, the tolerance to NSBB, the absence of co-factors of liver damage (for example, obesity and alcohol consumption) and the patient's opinion should be taken into account.
SESSION 2. Prevention of the first decompensation and recurrence (Table 2)1. Definition of decompensationThe presence of minimal ascites, identified only by ultrasound, is not considered as a decompensation of cirrhosisThe prognostic impact of minimal ascites, detected exclusively by imaging tests, has been evaluated in a limited number of studies,56–61 which differ in terms of design, 50% being retrospective, and in inclusion criteria. In addition, two of the studies did not include a control group without ascites.57,59 There are other significant limitations, such as the small number of patients with ascites detected by ultrasound. Furthermore, in at least half of the studies, the group of patients with ultrasound-detected ascites included individuals who had experienced other episodes of cirrhosis decompensation. Lastly, not all studies could conclusively confirm the prognostic impact of minimal ascites,58,61 so the prognostic value of ascites detected by ultrasound remains unknown. Therefore, with the information currently available, it is not possible to consider minimal ascites as decompensation of cirrhosis..
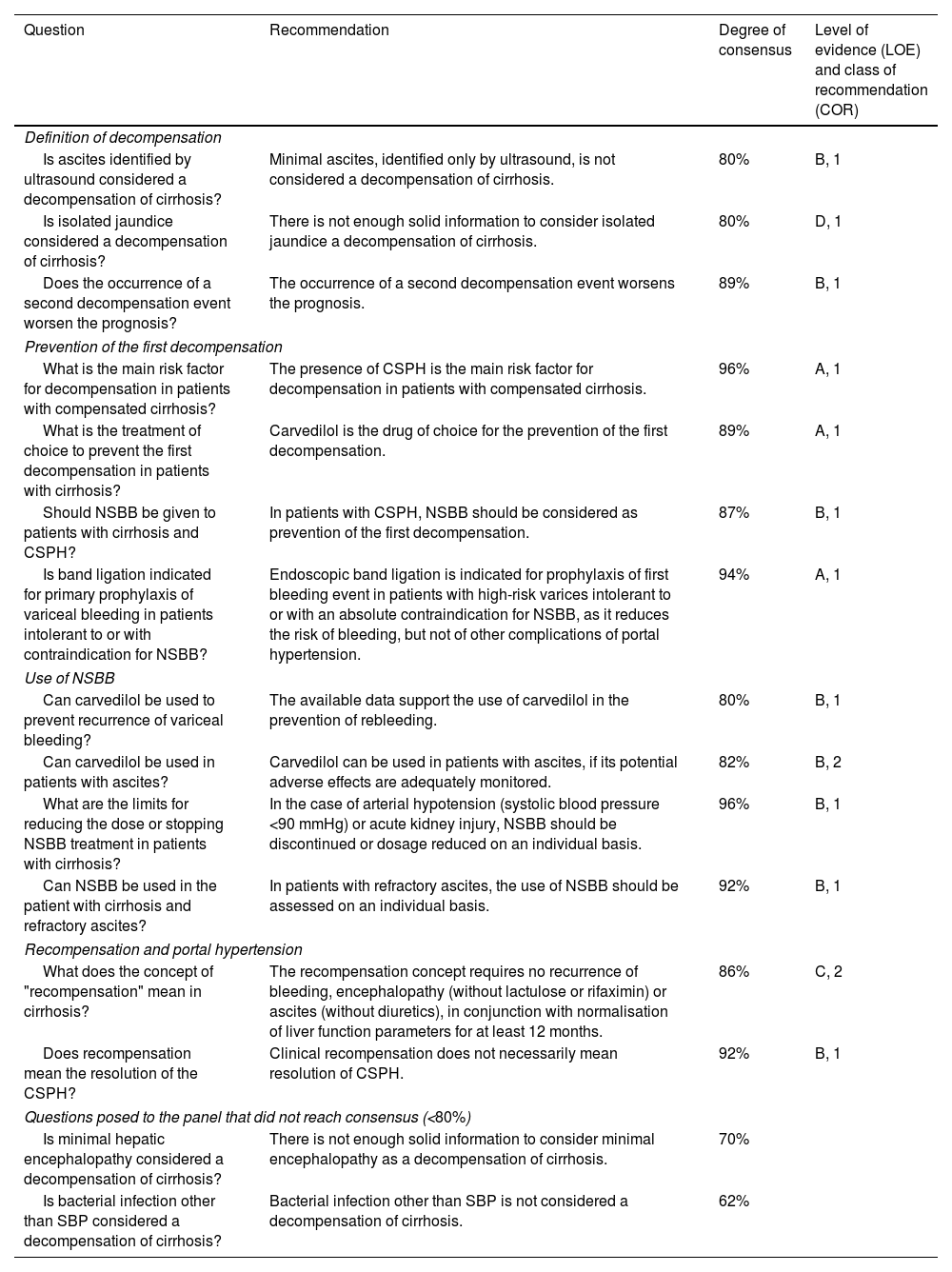
Recommendations on prevention of first decompensation and recurrence (session 2).
| Question | Recommendation | Degree of consensus | Level of evidence (LOE) and class of recommendation (COR) |
|---|---|---|---|
| Definition of decompensation | |||
| Is ascites identified by ultrasound considered a decompensation of cirrhosis? | Minimal ascites, identified only by ultrasound, is not considered a decompensation of cirrhosis. | 80% | B, 1 |
| Is isolated jaundice considered a decompensation of cirrhosis? | There is not enough solid information to consider isolated jaundice a decompensation of cirrhosis. | 80% | D, 1 |
| Does the occurrence of a second decompensation event worsen the prognosis? | The occurrence of a second decompensation event worsens the prognosis. | 89% | B, 1 |
| Prevention of the first decompensation | |||
| What is the main risk factor for decompensation in patients with compensated cirrhosis? | The presence of CSPH is the main risk factor for decompensation in patients with compensated cirrhosis. | 96% | A, 1 |
| What is the treatment of choice to prevent the first decompensation in patients with cirrhosis? | Carvedilol is the drug of choice for the prevention of the first decompensation. | 89% | A, 1 |
| Should NSBB be given to patients with cirrhosis and CSPH? | In patients with CSPH, NSBB should be considered as prevention of the first decompensation. | 87% | B, 1 |
| Is band ligation indicated for primary prophylaxis of variceal bleeding in patients intolerant to or with contraindication for NSBB? | Endoscopic band ligation is indicated for prophylaxis of first bleeding event in patients with high-risk varices intolerant to or with an absolute contraindication for NSBB, as it reduces the risk of bleeding, but not of other complications of portal hypertension. | 94% | A, 1 |
| Use of NSBB | |||
| Can carvedilol be used to prevent recurrence of variceal bleeding? | The available data support the use of carvedilol in the prevention of rebleeding. | 80% | B, 1 |
| Can carvedilol be used in patients with ascites? | Carvedilol can be used in patients with ascites, if its potential adverse effects are adequately monitored. | 82% | B, 2 |
| What are the limits for reducing the dose or stopping NSBB treatment in patients with cirrhosis? | In the case of arterial hypotension (systolic blood pressure <90 mmHg) or acute kidney injury, NSBB should be discontinued or dosage reduced on an individual basis. | 96% | B, 1 |
| Can NSBB be used in the patient with cirrhosis and refractory ascites? | In patients with refractory ascites, the use of NSBB should be assessed on an individual basis. | 92% | B, 1 |
| Recompensation and portal hypertension | |||
| What does the concept of "recompensation" mean in cirrhosis? | The recompensation concept requires no recurrence of bleeding, encephalopathy (without lactulose or rifaximin) or ascites (without diuretics), in conjunction with normalisation of liver function parameters for at least 12 months. | 86% | C, 2 |
| Does recompensation mean the resolution of the CSPH? | Clinical recompensation does not necessarily mean resolution of CSPH. | 92% | B, 1 |
| Questions posed to the panel that did not reach consensus (<80%) | |||
| Is minimal hepatic encephalopathy considered a decompensation of cirrhosis? | There is not enough solid information to consider minimal encephalopathy as a decompensation of cirrhosis. | 70% | |
| Is bacterial infection other than SBP considered a decompensation of cirrhosis? | Bacterial infection other than SBP is not considered a decompensation of cirrhosis. | 62% | |
CSPH: clinically significant portal hypertension; NSBB: non-selective adrenergic beta-blockers; SBP: spontaneous bacterial peritonitis.
The development of jaundice in patients with compensated cirrhosis has classically been regarded as a decompensation event, which rarely occurs in isolation as the first event, with the exception of chronic cholestatic disease.62 In the systematic review of the last Baveno VII Consensus Workshop, of the 116 studies evaluated in this regard, 32 included jaundice as a decompensation event.46 The incidence was reported in 18 of them,62–79 with 11 of them providing a heterogeneous definition (from >2 to ≥5 mg/dl). In the few studies (N = 9) where they differentiated whether or not jaundice was an isolated first event of decompensation, the rarity of this form of presentation was confirmed (0.7–3%), while in those where no such differentiation was made, the rate of jaundice development was much higher (2.9–73.9%). None of the studies specified the duration of the jaundice or whether a cause of superimposed liver damage (for example, bacterial/viral infections, hepatotoxicity or acute alcoholic hepatitis) was ruled out. Lastly, it cannot be ruled out that the development of jaundice reflected acute-on-chronic liver failure (ACLF), as the current concept and definition of this syndrome post-dated the publication of most of the earlier studies. Therefore, further prospective studies are needed to determine whether jaundice represents a first decompensation or instead reflects additional liver damage or an episode of acute liver failure on a chronic background.
The occurrence of a second decompensation greatly worsens the prognosisThe development of a second decompensation event, either by recurrence of the initial decompensation (for example, second episode of encephalopathy or variceal bleeding) or by ascites-related complications (for example, refractory ascites, spontaneous bacterial peritonitis and/or hepatorenal syndrome), is associated with a significant worsening of survival.62,80–85
2. Prevention of the first decompensationCSPH is the main risk factor for decompensation in patients with compensated cirrhosisAn HVPG ≥10 mmHg is defining of CSPH.46 This is because observational studies have established that HVPG, better than any other parameter, identifies patients with compensated cirrhosis at high risk of developing decompensation.35 The HVPG also identifies patients at risk of developing oesophagogastric varices and HCC.
Carvedilol is the drug of choice for the prevention of the first decompensationTreatment with NSBB is indicated in compensated patients with CSPH to prevent decompensation of cirrhosis. Randomised clinical studies have shown that in these patients NSBB significantly decreases the risk of developing a first decompensation, mainly by decreasing the risk of developing ascites, which is the most frequent complication in compensated patients.45 In patients with compensated cirrhosis, carvedilol is the NSBB of choice. Carvedilol has a vasodilatory effect due to its anti-〈-adrenergic action and so may attenuate the increased intrahepatic resistance that is a predominant mechanism for the development of portal hypertension in compensated cirrhosis.86 Carvedilol causes a greater decrease in HVPG than traditional NSBB and tends to be better tolerated.87 Clinical studies have shown a trend towards greater effectiveness in preventing decompensation than traditional NSBB. In addition, a meta-analysis of individual patient data from randomised studies versus placebo or EBL of varices in patients with at-risk varices found a significant improvement in survival in patients with compensated cirrhosis favouring the use of carvedilol,88 especially in patients with oesophageal varices.
In patients with CSPH, NSBB should be considered as prevention of the first decompensationPrevention of decompensation in patients with compensated cirrhosis is indicated in all patients with CSPH, whether or not they have varices.45 However, in patients with CSPH, the risk of decompensation is particularly high in those with varices, so the benefit obtained is also greater in these patients.45
EBL is indicated for prophylaxis of first bleeding event in patients with high-risk varices intolerant to or with an absolute contraindication for NSBB, as it reduces the risk of bleeding, but not of other complications of portal hypertension.
Both NSBB and EBL have been shown in randomised studies to decrease the risk of a first bleed in patients with at-risk oesophageal varices (large varices or small varices with red signs or in patients with advanced liver failure). A meta-analysis of studies comparing NSBB and EBL showed similar survival with both treatments in patients with at-risk varices.89 The risk of first bleeding event in these patients is also similar.90 A recent meta-analysis of comparative studies between NSBB and EBL, stratifying the results according to the presence or absence of decompensation, shows that NSBB achieves a significant improvement in survival over EBL in patients with compensated cirrhosis, mainly by reducing the risk of developing ascites. This suggests that in compensated patients with at-risk varices it is preferable to use NSBB. However, when these drugs are contraindicated, or when complications occur with treatment that require their withdrawal, EBL is the indicated treatment in both compensated and decompensated patients, as it significantly reduces the risk of bleeding.
3. Use of non-cardioselective beta-blockersAvailable data support the use of carvedilol in the prevention of rebleedingEBL in combination with NSBB therapy such as propranolol and carvedilol represents the treatment of choice for secondary prophylaxis of variceal bleeding. In relation to the prevention of rebleeding and mortality risk, the use of NSBB is the key element of secondary prophylaxis.91,92 Randomised studies on prophylaxis for rebleeding have shown that carvedilol monotherapy has similar efficacy to EBL93 and to combination therapy with nadolol and isosorbide mononitrate.94 However, the likelihood of rebleeding is higher in patients treated with single modality therapy versus combination therapy. Both the combinations of carvedilolol and EBL and propranolol/nadolol and EBL have shown greater efficacy in the prevention of rebleeding and in the prevention of other non-haemorrhagic decompensations,95 as well as a greater decrease in HVPG in the short term (first month post-bleed),96 with a better haemodynamic response rate (53% vs. 29%) and longer-term survival.97
Carvedilol can be used in patients with ascites if its potential adverse effects are adequately monitoredAscites is the most common complication in the natural history of cirrhosis and its occurrence is a progression from the compensated to the decompensated stage. A meta-analysis of 15 clinical trials, including 452 patients with ascites treated with NSBB, showed that patients with haemodynamic response (HVPG reduction <12 mmHg or >20% from baseline) were less likely to develop decompensated cirrhosis than non-responders.98 It should also be noted that treatment with NSBB can have a significant impact on cardiocirculatory status, which may affect patient survival. Related to this, a single-centre study in patients with ascites on primary prophylaxis with NSBB demonstrated an increased risk of refractory ascites and poorer short-term survival in those with cardiac output or cardiac index below 5 and 3 l/min.99 However, the available information on the efficacy and safety of carvedilol treatment in patients with ascites is limited, as it is based on only two retrospective studies. A first single-centre study of 264 patients with ascites treated with carvedilol at a dose of 12.5 mg showed better long-term survival in those treated with carvedilol.100 In the same vein, retrospective analysis of a multicentre clinical trial comparing the long-term effect of treatment with carvedilol (dose: 6.25–12.5 mg; 49% with ascites) versus EBL (53% with ascites) showed longer survival in patients treated with carvedilol (7.8 vs. 4.2 years).101
In the case of hypotension (systolic blood pressure <90 mmHg) or acute kidney injury, NSBB should be discontinued or the dose reducedAs cirrhosis progresses, compensatory cardiac mechanisms help maintain renal perfusion in patients with diuretic-sensitive ascites. However, when ascites is refractory, these cardiac mechanisms can no longer compensate for the worsening arterial vasodilation, leading to a reduction in organ perfusion.102 Due to their negative inotropic effect, NSBB may upset the fragile cardiodynamic balance and impair renal perfusion, so careful review of dosage is advised in these patients. Based on the results of a study in patients with diuretic-refractory and diuretic-responsive ascites treated with NSBB,103 the Baveno VII Consensus Workshop recommended that in patients with persistent hypotension (mean arterial blood pressure <65 mmHg or systolic blood pressure <90 mmHg) or hepatorenal syndrome, NSBB should be discontinued and reintroduced at lower doses with careful monitoring. In severe infection, such as spontaneous bacterial peritonitis (SBP), it has also been suggested that maintaining NSBB treatment may increase the risk of hepatorenal syndrome and death.104 Furthermore, in decompensated patients on the transplant list, treatment with NSBB has been associated with an increased risk of acute kidney injury,105 mainly in patients with ascites and previous kidney failure106 or worse liver function (Child-Pugh C).107 However, in patients on the transplant list, the use of NSBB has been associated with improved short-term survival. Therefore, it is suggested that the use of NSBB in this population (with infection, hypotension or acute kidney injury) should be individually tailored to the changing circumstances observed in these patients and should be reserved for those with better cardiac and haemodynamic reserve.108
In patients with refractory ascites, the use of NSBB should be assessed on an individual basisRefractory ascites is a common decompensation in the later stages of decompensated cirrhosis.34 In patients with refractory ascites, NSBB block compensatory cardiac mechanisms and promote worsening peripheral vasodilation, reducing renal perfusion pressure, which promotes the development of renal failure and reduces survival.103 However, the harmful effect occurs when NSBB are used at high doses. A single-centre, prospective, observational study, and another with a crossover design, carried out in patients with refractory ascites both found that the use of high-dose propranolol (160 mg/day) increased the mortality risk 2.6-fold compared to patients not treated with NSBB, mainly due to increased circulatory dysfunction after large-volume ascites.109,110 In the same vein, four retrospective studies showed the possible harmful effect of NSBB use in different clinical scenarios such as Child-Pugh C patients111 and those with refractory ascites,112 mainly due to the risk of acute kidney injury.105 Therefore, in these groups, it is recommended to discontinue NSBB or to reduce the dose, adapting it to the individual conditions of each patient.
In contrast, treatment with low-dose NSBB has been shown to reduce mortality rates113 in patients on the liver transplant list. In patients with infections such as SBP, NSBB reduce the mortality rate114 or do not increase it, especially if a mean blood pressure above 65 mmHg is maintained. There may therefore be a haemodynamic window for maintaining NSBB in these contexts.115 Lastly, the prospective CANONIC study showed that maintaining NSBB treatment in patients with ACLF modifies the inflammatory response and is associated with lower mortality rates.116 Therefore, in patients with refractory ascites, with a resulting fragile cardiodynamic balance, personalised treatment is recommended.
4. Recompensation and portal hypertensionThe recompensation concept requires no recurrence of bleeding, encephalopathy (without lactulose/rifaximin) or ascites (without diuretics), in conjunction with normalisation of liver function parameters for at least 12 monthsThere is growing evidence to support the fact that adequate control of the cause of liver disease has a significant impact on the natural history of cirrhosis. Aetiological treatment has the potential to halt disease progression and dramatically reduce the likelihood of experiencing future episodes of decompensation.
In this regard, hepatic recompensation has been defined as the absence of episodes of hepatic decompensation such as variceal bleeding, hepatic encephalopathy (without lactulose/rifaximin) or ascites (without diuretics) combined with normalisation of liver function for at least 12 months. The possibility of recompensation has been described in patients with prolonged abstinence from alcohol use or in those with viral hepatitis where control or elimination of the aetiological agent has been achieved (suppression of hepatitis B virus replication in the absence of delta virus co-infection; HCV clearance with SVR). This definition is based on the available data from numerous studies, which have shown that some of the patients with alcohol-related cirrhosis on the waiting list for liver transplantation can be removed from the waiting list due to improved liver function.117,118 Similarly, after elimination of C virus or suppression of B virus replication, there is a significant improvement in liver function with a marked reduction in the likelihood of decompensation.119–123 Although the concept of recompensation has been defined in the context of alcoholic liver disease and viral hepatitis, recent evidence suggests that it may also extend to diseases such as metabolic dysfunction-associated fatty liver disease.124 The improvement in liver function, as well as the decrease in the likelihood of decompensation is not universal after elimination of the aetiological factor, so a minimum time of 12 months has been established before a diagnosis of recompensation can be made.
Clinical recompensation does not necessarily mean resolution of the CSPHHaemodynamic studies in patients with HCV cirrhosis50,51 have shown persistence of CSPH after SVR in 50% of patients with an elevated HVPG (≥16 mmHg) at baseline. It should not therefore be assumed that the risk of decompensation will disappear universally after elimination of the aetiological agent, despite an improvement in liver function. However, in patients in whom an SVR results in an HVPG <10 mmHg, the likelihood of decompensation during follow-up is virtually zero, provided that other factors that might influence the natural history of cirrhosis (for example, drug use and metabolic syndrome) are adequately controlled.
5. Questions posed to the panel that did not reach consensusThere is not enough solid information to consider minimal encephalopathy as a decompensation of cirrhosisStudies which have assessed the prognostic impact of minimal hepatic encephalopathy have a number of limitations.78,125–136 First, the population included in the studies was heterogeneous with respect to age, aetiology, comorbidities, or stage of cirrhosis. Three studies included patients with occult hepatic encephalopathy (minimal and grade 1 of the West-Haven classification)128,132,133 and most included both compensated and decompensated patients (including previous overt hepatic encephalopathy). In the only study that looked at them separately, the number of compensated patients was relatively small and in one third of patients with compensated cirrhosis and minimal hepatic encephalopathy who progressed to more advanced stages, it was due to the development of oesophageal varices rather than decompensation events.78 Second, the diagnostic tests varied between studies and the statistical methodology for assessing prognostic impact was heterogeneous and mostly suboptimal, with competing risk analyses performed in only two studies.78,136 Finally, follow-up was generally short (<14 months in 67% of studies) for assessing decompensation and/or mortality in patients with compensated cirrhosis and not all studies were able to confirm its prognostic impact.126,127,132 Further prospective studies are therefore needed to clarify the prognostic impact of minimal encephalopathy and to determine whether it can be considered a decompensation of cirrhosis.
Bacterial infection other than SBP is not considered a decompensation of cirrhosisIn a cohort of 1,672 patients with compensated cirrhosis of viral origin followed up prospectively, bacterial infections were found to be a common event and were associated with an increased risk of decompensation and death. However, it was not discerned whether the increased mortality risk occurred once the patient had a decompensation event.137 Two recent studies have provided information on this issue. A nested analysis of the PREDESCI study45 confirmed the high prevalence of bacterial infections in compensated cirrhosis and their prognostic impact, although mortality occurred once the decompensated phase developed.138 Similar results were found in a secondary analysis of a prospective two-centre study in which the development of isolated infection (i.e. without associated decompensation) was not associated with an increase in the mortality rate.79 These studies support bacterial infections being a trigger for decompensation, but in the absence of decompensation there is no precise information about their impact on mortality rates and, consequently, they cannot be considered a form of decompensation. There are even fewer data on the prevalence and impact of bacterial infections in patients with compensated cirrhosis without CSPH.
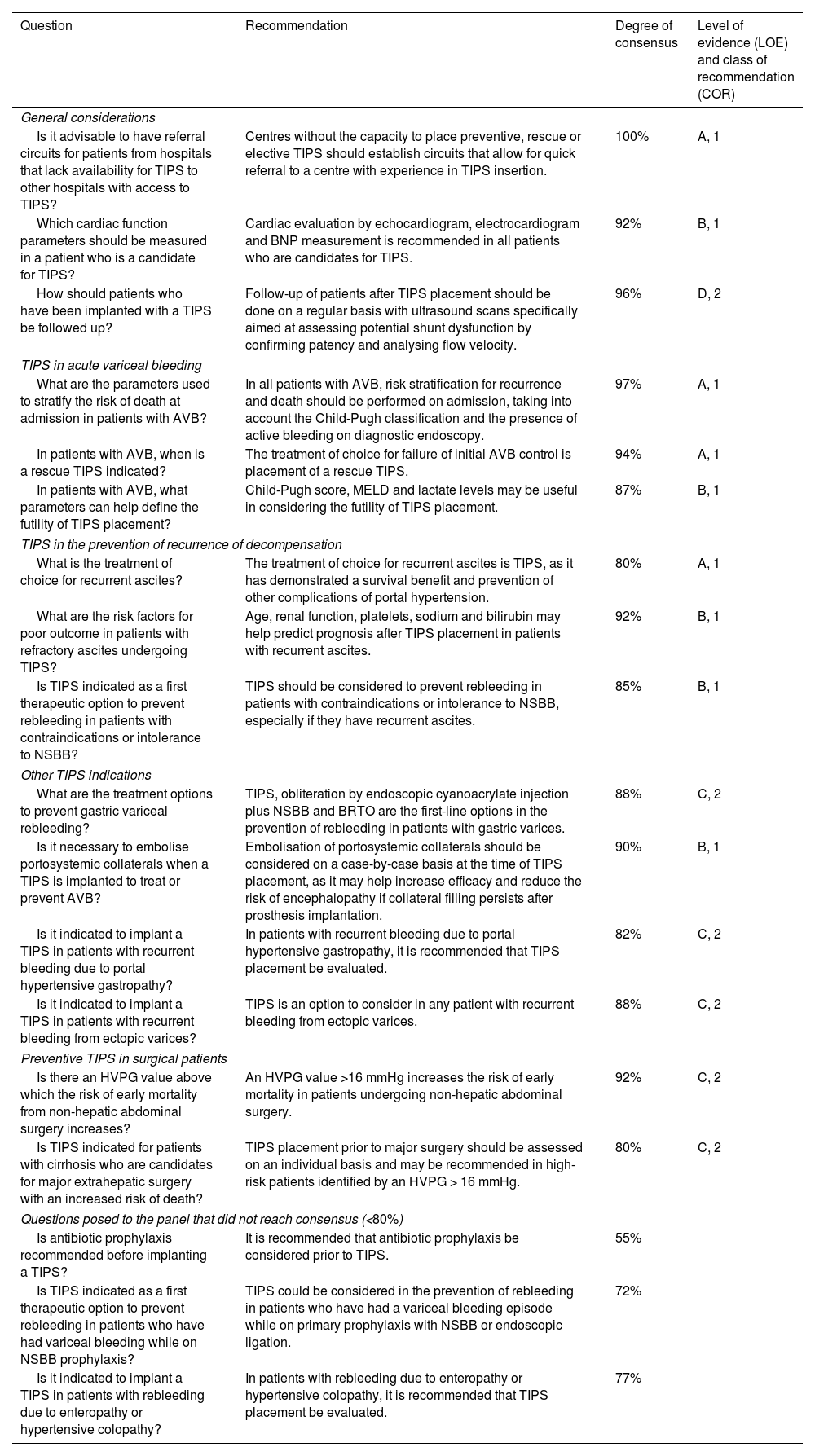
SESSION 3. Acute oesophagogastric variceal bleeding (Table 3)1. General managementIt is recommended that patients with acute variceal bleeding (AVB) be treated in intensive care units or specific intermediate unitsConsidering the still high mortality rate for AVB,139 different expert opinions indicate that the treatment of these patients should be carried out in units with specialised staff and capacity for the treatment of critically ill patients.140,141
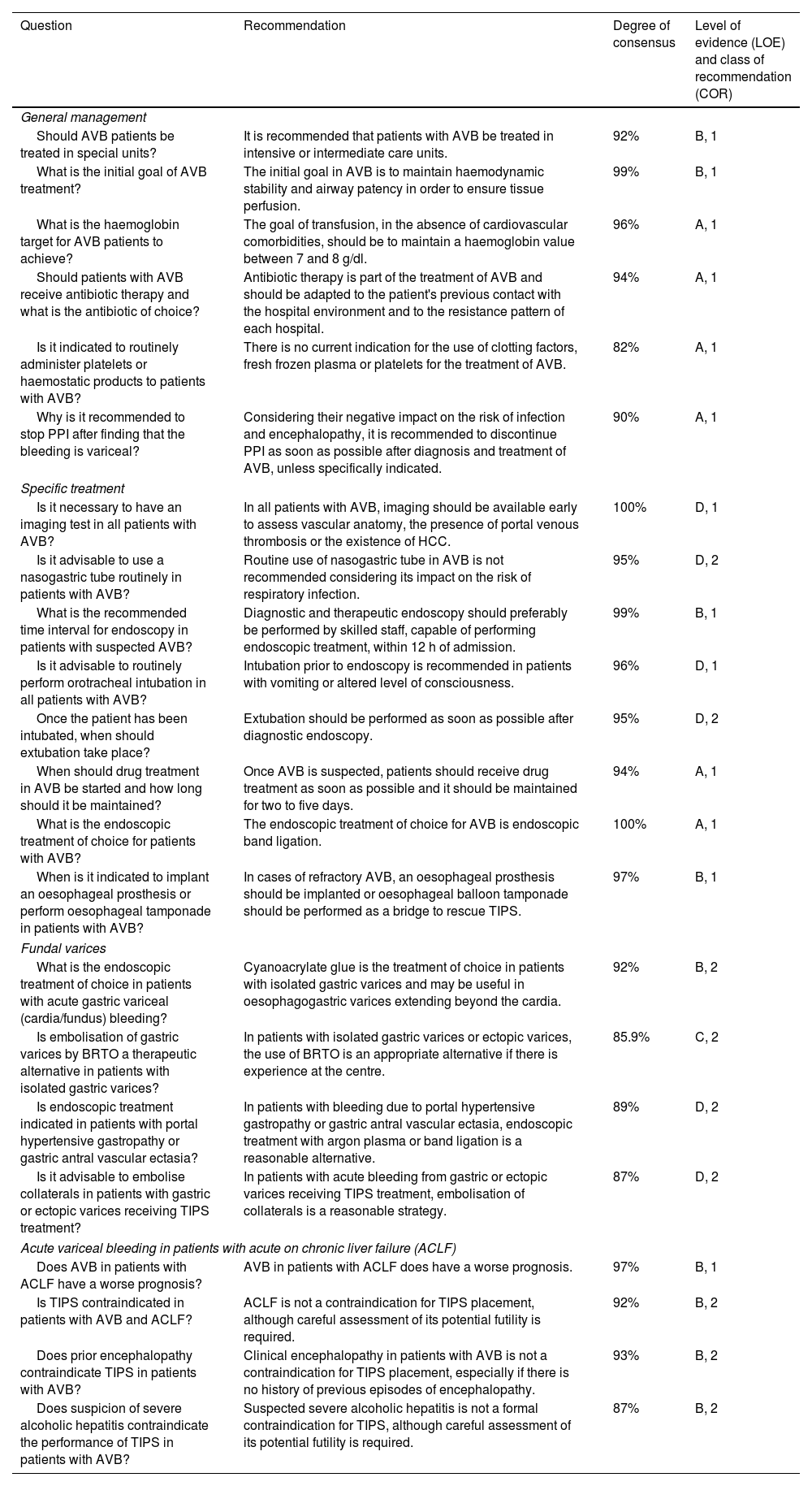
Recommendations on acute oesophagogastric variceal bleeding (session 3).
| Question | Recommendation | Degree of consensus | Level of evidence (LOE) and class of recommendation (COR) |
|---|---|---|---|
| General management | |||
| Should AVB patients be treated in special units? | It is recommended that patients with AVB be treated in intensive or intermediate care units. | 92% | B, 1 |
| What is the initial goal of AVB treatment? | The initial goal in AVB is to maintain haemodynamic stability and airway patency in order to ensure tissue perfusion. | 99% | B, 1 |
| What is the haemoglobin target for AVB patients to achieve? | The goal of transfusion, in the absence of cardiovascular comorbidities, should be to maintain a haemoglobin value between 7 and 8 g/dl. | 96% | A, 1 |
| Should patients with AVB receive antibiotic therapy and what is the antibiotic of choice? | Antibiotic therapy is part of the treatment of AVB and should be adapted to the patient's previous contact with the hospital environment and to the resistance pattern of each hospital. | 94% | A, 1 |
| Is it indicated to routinely administer platelets or haemostatic products to patients with AVB? | There is no current indication for the use of clotting factors, fresh frozen plasma or platelets for the treatment of AVB. | 82% | A, 1 |
| Why is it recommended to stop PPI after finding that the bleeding is variceal? | Considering their negative impact on the risk of infection and encephalopathy, it is recommended to discontinue PPI as soon as possible after diagnosis and treatment of AVB, unless specifically indicated. | 90% | A, 1 |
| Specific treatment | |||
| Is it necessary to have an imaging test in all patients with AVB? | In all patients with AVB, imaging should be available early to assess vascular anatomy, the presence of portal venous thrombosis or the existence of HCC. | 100% | D, 1 |
| Is it advisable to use a nasogastric tube routinely in patients with AVB? | Routine use of nasogastric tube in AVB is not recommended considering its impact on the risk of respiratory infection. | 95% | D, 2 |
| What is the recommended time interval for endoscopy in patients with suspected AVB? | Diagnostic and therapeutic endoscopy should preferably be performed by skilled staff, capable of performing endoscopic treatment, within 12 h of admission. | 99% | B, 1 |
| Is it advisable to routinely perform orotracheal intubation in all patients with AVB? | Intubation prior to endoscopy is recommended in patients with vomiting or altered level of consciousness. | 96% | D, 1 |
| Once the patient has been intubated, when should extubation take place? | Extubation should be performed as soon as possible after diagnostic endoscopy. | 95% | D, 2 |
| When should drug treatment in AVB be started and how long should it be maintained? | Once AVB is suspected, patients should receive drug treatment as soon as possible and it should be maintained for two to five days. | 94% | A, 1 |
| What is the endoscopic treatment of choice for patients with AVB? | The endoscopic treatment of choice for AVB is endoscopic band ligation. | 100% | A, 1 |
| When is it indicated to implant an oesophageal prosthesis or perform oesophageal tamponade in patients with AVB? | In cases of refractory AVB, an oesophageal prosthesis should be implanted or oesophageal balloon tamponade should be performed as a bridge to rescue TIPS. | 97% | B, 1 |
| Fundal varices | |||
| What is the endoscopic treatment of choice in patients with acute gastric variceal (cardia/fundus) bleeding? | Cyanoacrylate glue is the treatment of choice in patients with isolated gastric varices and may be useful in oesophagogastric varices extending beyond the cardia. | 92% | B, 2 |
| Is embolisation of gastric varices by BRTO a therapeutic alternative in patients with isolated gastric varices? | In patients with isolated gastric varices or ectopic varices, the use of BRTO is an appropriate alternative if there is experience at the centre. | 85.9% | C, 2 |
| Is endoscopic treatment indicated in patients with portal hypertensive gastropathy or gastric antral vascular ectasia? | In patients with bleeding due to portal hypertensive gastropathy or gastric antral vascular ectasia, endoscopic treatment with argon plasma or band ligation is a reasonable alternative. | 89% | D, 2 |
| Is it advisable to embolise collaterals in patients with gastric or ectopic varices receiving TIPS treatment? | In patients with acute bleeding from gastric or ectopic varices receiving TIPS treatment, embolisation of collaterals is a reasonable strategy. | 87% | D, 2 |
| Acute variceal bleeding in patients with acute on chronic liver failure (ACLF) | |||
| Does AVB in patients with ACLF have a worse prognosis? | AVB in patients with ACLF does have a worse prognosis. | 97% | B, 1 |
| Is TIPS contraindicated in patients with AVB and ACLF? | ACLF is not a contraindication for TIPS placement, although careful assessment of its potential futility is required. | 92% | B, 2 |
| Does prior encephalopathy contraindicate TIPS in patients with AVB? | Clinical encephalopathy in patients with AVB is not a contraindication for TIPS placement, especially if there is no history of previous episodes of encephalopathy. | 93% | B, 2 |
| Does suspicion of severe alcoholic hepatitis contraindicate the performance of TIPS in patients with AVB? | Suspected severe alcoholic hepatitis is not a formal contraindication for TIPS, although careful assessment of its potential futility is required. | 87% | B, 2 |
ACLF: acute-on-chronic liver failure; AVB: acute variceal bleeding; BRTO: balloon-occluded retrograde transvenous obliteration; HCC: hepatocellular carcinoma; PPI: proton pump inhibitors; TIPS: transjugular intrahepatic portosystemic shunt.
Initial management of AVB should be aimed at haemodynamic and general stabilisation of the patient, with attention to careful monitoring of blood pressure, urine output and oxygen saturation.140,141 Volume replacement should be initiated early with the goal of maintaining a mean arterial pressure >65 mmHg. Crystalloids are the solutions of choice as they have fewer haemostasis disturbances and less anaphylactic reactions. It is essential to protect the airway, especially in patients with altered levels of consciousness.142
The aim of transfusion, in the absence of cardiovascular comorbidities, should be to maintain a haemoglobin value between 7 and 8 g/dlStudies in rats with pre-hepatic portal hypertension almost 40 years ago suggested that non-restrictive transfusion in an experimental haemorrhage model increased portal pressure.143 Subsequently, a randomised study144 demonstrated that a restrictive transfusion policy (transfusion threshold of 7 g/dl haemoglobin) improved survival of patients with variceal bleeding. HVPG increased in patients randomised to receive a non-restrictive transfusion. Therefore, in the absence of cardiovascular comorbidities, the haemoglobin threshold for AVB should be between 7 and 8 g/dl.
Antibiotic treatment is part of AVB therapy and should be adapted to the patient's previous contact with the hospital environment and to the resistance pattern of each hospitalSeveral studies and meta-analyses have suggested that early administration of antibiotics in AVB is associated with a decreased incidence of bacterial infections during the episode and lower overall and infection-induced mortality rates, as well as a shorter hospital stay and reduced likelihood of rebleeding.145,146 However, there is no universal recommendation for determining the type of antibiotic. One controlled study147 identified that IV ceftriaxone (1 g/24 h) was superior to norfloxacin in terms of development of any type of infection, severe infection, SBP or bacteraemia. A recent retrospective observational study148 suggested that the beneficial impact of antibiotic prophylaxis was null in Child-Pugh A patients, although these data require validation. Lastly, the frequent occurrence of infections with multidrug-resistant organisms in patients with cirrhosis and the importance of their early treatment make it advisable to adapt the antibiotic regimen to the local prevalence of resistant microorganisms and to the antibiotic administration policies of each centre.46
There is no current indication for the use of clotting factors, fresh frozen plasma or platelets for the treatment of AVBHaemostasis in cirrhosis is re-balanced by the existence of numerous pro-haemorrhagic and pro-thrombotic changes in its different phases. Classic coagulation assessment parameters (especially INR) do not accurately reflect the haemostatic balance in the cirrhotic patient. Furthermore, the cause of AVB is increased portal pressure and variceal wall tension, not impaired haemostasis.
A recent observational study showed that administration of fresh frozen plasma (adjusted for age, MELD and presence of HCC) in AVB was associated with an increased risk of rebleeding, higher mortality rate and longer hospital stay.149 A recent meta-analysis reported that recombinant Factor VIIa administration, despite improving the likelihood of a combined event (defined as bleeding control, prevention of recurrence at day 5 and mortality at day 5) in patients with AVB at initial endoscopy, was associated with an increased risk of arterial thrombotic events.150 The high cost of the drug has also been seen as a major drawback to its use.151 Lastly, a recent study to assess the efficacy of tranexamic acid in patients with gastrointestinal bleeding did not demonstrate any benefit, either overall or in the subgroup of patients with AVB. In addition, the use of this drug increased the risk of thrombotic events and seizures.152 Therefore, there is currently no indication for systematic correction of coagulation disorders in AVB.
Considering their negative impact on the risk of infection and encephalopathy, it is recommended to discontinue proton pump inhibitors (PPI) as soon as possible after diagnosis and treatment of AVB, unless specifically indicatedPPI are widely used in many different contexts, often without a precise indication. Several studies have identified that PPI use increases the risk of bacterial infection in patients with ascites153 as well as the risk of hepatic encephalopathy and mortality.154,155 In addition, the use of PPI negatively impacts the natural history of SBP, with an increased risk of kidney failure, severe encephalopathy and death.156 Chronic PPI use has also been associated with an increased incidence of fractures in patients with cirrhosis157 and an increased risk of encephalopathy after insertion of a transjugular intrahepatic portosystemic shunt (TIPS).158 Lastly, a large retrospective observational study159 indicated that PPI administration was dose-dependently associated with an increased risk of infection, decompensation and possibly liver disease-related death. However, in patients hospitalised for acute bleeding, the use of PPI had a protective effect. Ultimately, the use of PPI in AVB is justified in the acute phase of the haemorrhage and should only be maintained in the long term in the presence of a recognised indication for their use.
2. Specific treatmentIn all patients with AVB, imaging should be available early to assess vascular anatomy, the presence of portal thrombosis or the existence of HCCIn patients with AVB, it is not uncommon for there to be co-factors affecting the occurrence of bleeding, its severity and its appropriate treatment. These include the presence of portal thrombosis or HCC160,161 (with or without malignant portal vein thrombosis). In addition, the potential need for TIPS in the context of AVB requires an appropriate vascular map to identify the possibility of or potential technical difficulties in performing the procedure. Lastly, an axial imaging test, preferably computed tomography (CT), may be useful in the diagnosis of other possible co-factors that may influence decision-making46 (for example, extrahepatic neoplasms and thoracic pathology).
Routine use of nasogastric tube in AVB is not recommended considering its impact on the risk of respiratory infectionNasogastric tube placement with lavage does not predict the presence of high-risk lesions requiring endoscopic treatment and is not without adverse effects such as pain and epistaxis, in addition to failure of tube placement in up to 34% of cases47 and increased risk of respiratory infections. In addition, a randomised study found no difference in the likelihood of rebleeding or mortality risk and does not recommend its use.162
Diagnostic and therapeutic endoscopy should preferably be performed by expert staff, capable of performing endoscopic treatment, within 12 h of admissionGiven the high mortality rate of AVB, the availability of an endoscopist with expertise in haemostatic techniques is essential at all times. A cohort study showed that in patients with haematemesis on admission, performing endoscopy within 12 h of hospital admission increased the likelihood of rebleeding and mortality at six weeks.163 Another study including 516 patients with gastrointestinal haemorrhage (only 10% with AVB) showed that performing endoscopy very early (before 6 h) offers no survival benefit, emphasising the importance of adequate resuscitation and appropriate medical management prior to endoscopy. However, there is great heterogeneity in the literature regarding the definition of appropriate times for endoscopy, which makes it difficult to analyse. One recent meta-analysis164 involving 2,824 patients with haemorrhage suggests that early endoscopy (before 12 h) can almost halve the overall mortality of cirrhotic patients with AVB. Therefore, in cases of AVB with haemodynamic instability or haematemesis, endoscopy should be performed as early as possible once the patient is stabilised.46
Intubation prior to endoscopy is recommended in patients with vomiting or altered level of consciousnessRoutine intubation prior to diagnostic endoscopy is not recommended in patients with gastrointestinal bleeding. Several meta-analyses show that prophylactic intubation prior to endoscopy in all patients with upper gastrointestinal bleeding may be associated with increased risk of aspiration and pneumonia, longer hospital stay and potentially higher mortality rates,165,166 with similar results in patients with AVB.167 However, patients with haematemesis, agitation or hepatic encephalopathy are at high risk of bronchoaspiration, so prophylactic intubation should be considered in these cases prior to endoscopy to ensure airway protection.46,47
Extubation should be performed as soon as possible after diagnostic endoscopyAirway manipulation is a risk factor for respiratory infection in the context of AVB,168 so early extubation is advisable if the patient's clinical situation permits.46,47
Once AVB is suspected, patients should receive drug treatment as soon as possible and it should be maintained for two to five daysStarting vasoconstrictor drugs before endoscopy decreases the incidence of active bleeding during endoscopy and facilitates endoscopic management.169,170 One meta-analysis, which included 30 studies and 3,111 patients with AVB, shows that patients who received early vasoconstrictive therapy had a lower mortality rate (all-cause), lower transfusion requirements, improved bleeding control and shorter hospital stay.171 The drugs of choice in AVB are somatostatin, terlipressin and octreotide, with no differences in terms of mortality rate, safety or rebleeding rate,172,173 although somatostatin and octreotide have fewer adverse effects. Some studies suggest that a shorter duration of treatment (2−3 days) may not influence the recurrence rate.174,175 However, the limited sample size and the lack of predictive factors for poor prognosis to guide the decision makes it difficult to extrapolate a recommendation. In specific situations, such as in patients with optimal liver function and no risk factors for recurrence, shorter regimens could be considered, pending definitive data.176,177
The endoscopic treatment of choice for AVB is EBLEBL has been shown to be superior to sclerotherapy in the initial control of AVB (90%), also halving the likelihood of rebleeding with fewer adverse effects and lower mortality rates.178,179 No benefit has been found from combined endoscopic therapy of EBL with sclerosis.180 The combination of vasoconstrictor drugs and EBL has demonstrated superiority in terms of efficacy, safety and mortality rates in the treatment of AVB (control of the acute episode 90%, prevention of early recurrence 80%) and is therefore the treatment of choice in patients with AVB.
In cases of refractory AVB, an oesophageal prosthesis should be implanted or oesophageal balloon tamponade should be performed as a bridge to rescue TIPSDespite adequate treatment of AVB, up to 15% of patients experience early rebleeding, often requiring haemostatic treatment as a bridge until rescue TIPS is performed. The same is true in cases of massive bleeding. In these circumstances, both the placement of an oesophageal prosthesis and oesophageal balloon tamponade are appropriate options. There is only one randomised controlled study comparing the two alternatives181; in this study, oesophageal prostheses were shown to be superior in controlling bleeding with less transfusion requirements and fewer adverse effects. However, no difference was found in survival at six weeks and, in addition, 25% of the prostheses migrated. In addition, there is a benefit in terms of insertion time as tamponade balloons must be deflated within 24 h of insertion while the prosthesis can remain in situ without efficacy diminishing for up to 7–14 days, which may be important in patients with a temporary contraindication to TIPS placement (for example, active infection). A recent systematic review and meta-analysis comparing the two alternatives corroborates these results, proposing oesophageal prostheses as an equally effective, but safer, option to tamponade.182
3. Gastric fundus varicesCyanoacrylate glue is the treatment of choice in patients with isolated gastric varices and may be useful in oesophagogastric varices extending beyond the cardiaGastric varices are classified according to the Sarin et al. classification, which divides them into varices located at the oesophagogastric junction and isolated gastric or cardia/fundal varices.183 Cyanoacrylate injection is applied endoscopically to treat isolated gastric AVB. Several systematic reviews with meta-analyses have evaluated the efficacy of cyanoacrylate injection, demonstrating that this technique is superior in terms of efficacy and safety and is therefore first choice.184–186
Embolisation by balloon-occluded retrograde transvenous obliteration (BRTO) is an alternative to endoscopic treatment or TIPS in patients with gastric or ectopic varices. Experience in our setting is limited, but it is an appropriate alternative in centres with experience.187,188 This technique involves occlusion of blood flow by means of a balloon catheter which enables instillation of a sclerosing agent proximal to the occlusion site. The success rates of BRTO and TIPS for bleeding control are similar. However, with BRTO there is a lower likelihood of developing hepatic encephalopathy,189 although it may worsen portal hypertension and aggravate oesophageal varices and/or ascites; these data need to be confirmed in further studies.
In patients with bleeding due to portal hypertensive gastropathy or gastric antral vascular ectasia, endoscopic treatment with argon plasma, ligation or other methods is a reasonable alternativeThe initial treatment for portal hypertensive gastropathy is NSBB. In patients who bleed due to gastropathy despite medical treatment, endoscopic treatment may be considered, although data are limited. Some studies indicate that in this subgroup of patients, endoscopic treatment with argon plasma may reduce transfusion requirements.190,191 The treatment of choice for patients with gastric antral vascular ectasia and chronic anaemia or bleeding is endoscopic. The initial approach is with argon plasma, although recurrence rates are high and patients require several sessions.192 For patients without a good response to argon, EBL of the antrum is recommended. A systematic review with meta-analysis showed that patients treated with ligation had significantly lower post-procedural transfusion requirements than those treated with argon plasma; in addition, EBL was associated with fewer endoscopic sessions and fewer transfusions in the long term.193
In patients with gastric or ectopic AVB receiving TIPS treatment, embolisation of collaterals is a reasonable strategyPatients having TIPS for the treatment of gastric variceal bleeding have a 15% likelihood of re-bleeding.194 TIPS can be combined with collateral bed embolisation to control bleeding or reduce the risk of recurrent variceal bleeding from gastric or ectopic varices (mainly large isolated varices), particularly in cases where, despite a decrease in PPG, portal flow remains diverted to the collaterals.195 Studies with few patients suggest that combination therapy improves eradication of gastric varices with a reduced likelihood of rebleeding, although with no benefit in terms of survival.195,196
4. Gastrointestinal bleeding in patients with acute on chronic liver failureAVB in patients with ACLF has a worse prognosisDespite great advances in the prevention and treatment of gastrointestinal bleeding due to portal hypertension, it remains one of the most lethal complications in patients with cirrhosis.197 ACLF increases the risk of rebleeding and decreases survival.198
ACLF is not a contraindication for TIPS placement, although careful assessment of its potential futility is requiredIn the context of AVB and ACLF, placement of TIPS, both rescue and preventive, is associated with improved bleeding control and a lower mortality rate.199,200 However, there is limited published information on this issue in patients with three or more organ failures (ACLF 3). Studies published to date have failed to establish specific futility criteria in this situation and knowledge about the benefit of TIPS in this subgroup of more severe patients is therefore insufficient.201,202 In such cases, the risk/benefit of TIPS placement must be weighed up on a case-by-case basis.
The existence of clinical HE in patients with AVB is not a contraindication for TIPS placement, especially if there is no history of previous episodes of HEHE is common in the context of AVB. In particular, patients with a high risk of rebleeding (Child-Pugh C 10–13 or Child-Pugh B with active bleeding at the time of endoscopy) have a higher prevalence of HE on admission compared to patients at low risk (39.2% vs. 10.6%).203 The occurrence of clinical HE or worsening of minimal HE after TIPS placement is also a common event, occurring in up to 35% of patients who have preventive TIPS.204,205 For this reason, the risk-benefit ratio of this intervention in patients with gastrointestinal bleeding and a history of HE, or with HE on admission, has been the subject of debate. Recent studies have shown that early TIPS placement increases one-year survival without increasing the risk of HE.203–205 Moreover, a number of HE prevention and treatment measures are now available in this setting which have proven effective, such as the use of covered TIPS206 or smaller diameter TIPS (8 vs. 10 mm)207 and the administration of lactulose and/or prophylactic rifaximin.208,209
Suspected severe alcoholic hepatitis is not a formal contraindication for TIPS, although careful assessment of its potential futility is requiredTo date, the impact of severe alcoholic hepatitis on patients receiving a TIPS for AVB has not been specifically analysed. However, other variables such as MELD score, Child-Pugh, age, serum lactate and other factors have been shown to be useful predictors of survival after TIPS placement.202,210–212 Alcohol consumption was not an important determinant of rebleeding risk or mortality in any of these studies. Therefore, acute alcoholic hepatitis should not be considered a priori as a contraindication for TIPS placement, the relevance of which should be established by current criteria in clinical practice guidelines irrespective of the aetiology of cirrhosis or the origin of decompensation.
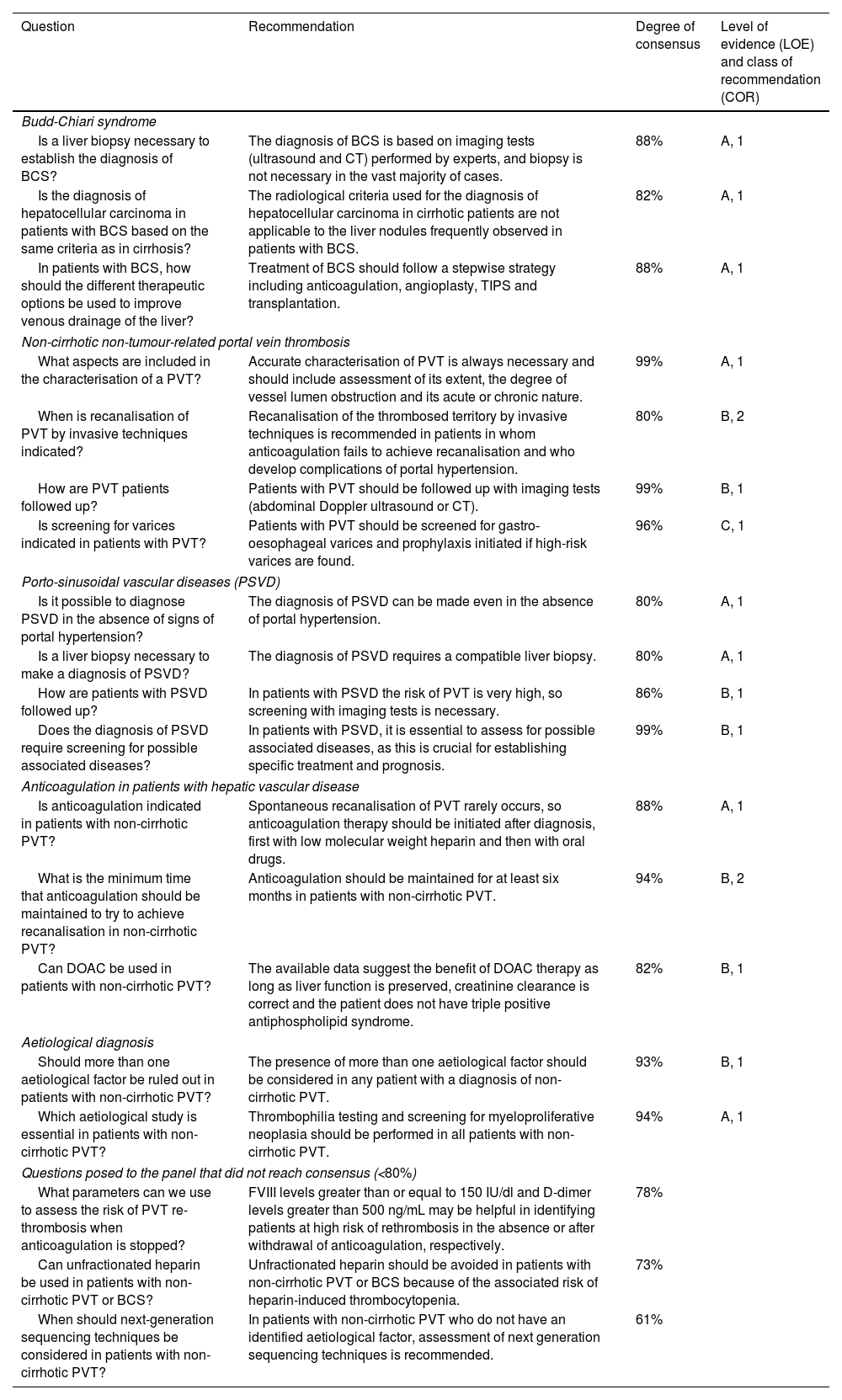
SESSION 4. Other complications of portal hypertension (Table 4)1. Hepatic encephalopathyRifaximin is indicated for secondary HE prophylaxis after a second episode of clinical HE occurring within six months of the initial episodeRifaximin decreased the risk of HE recurrence in a randomised clinical trial in patients with cirrhosis who had had a previous episode in the past six months. Compared to the placebo group, the rifaximin-treated group had fewer episodes of HE (22% vs. 45%) and hospitalisation (13.6% vs. 22.6%).213 In this study, 90% of the patients included were on concomitant treatment with lactulose, which makes it advisable in clinical practice to combine the two drugs. This beneficial effect of combination therapy was confirmed in a meta-analysis.214
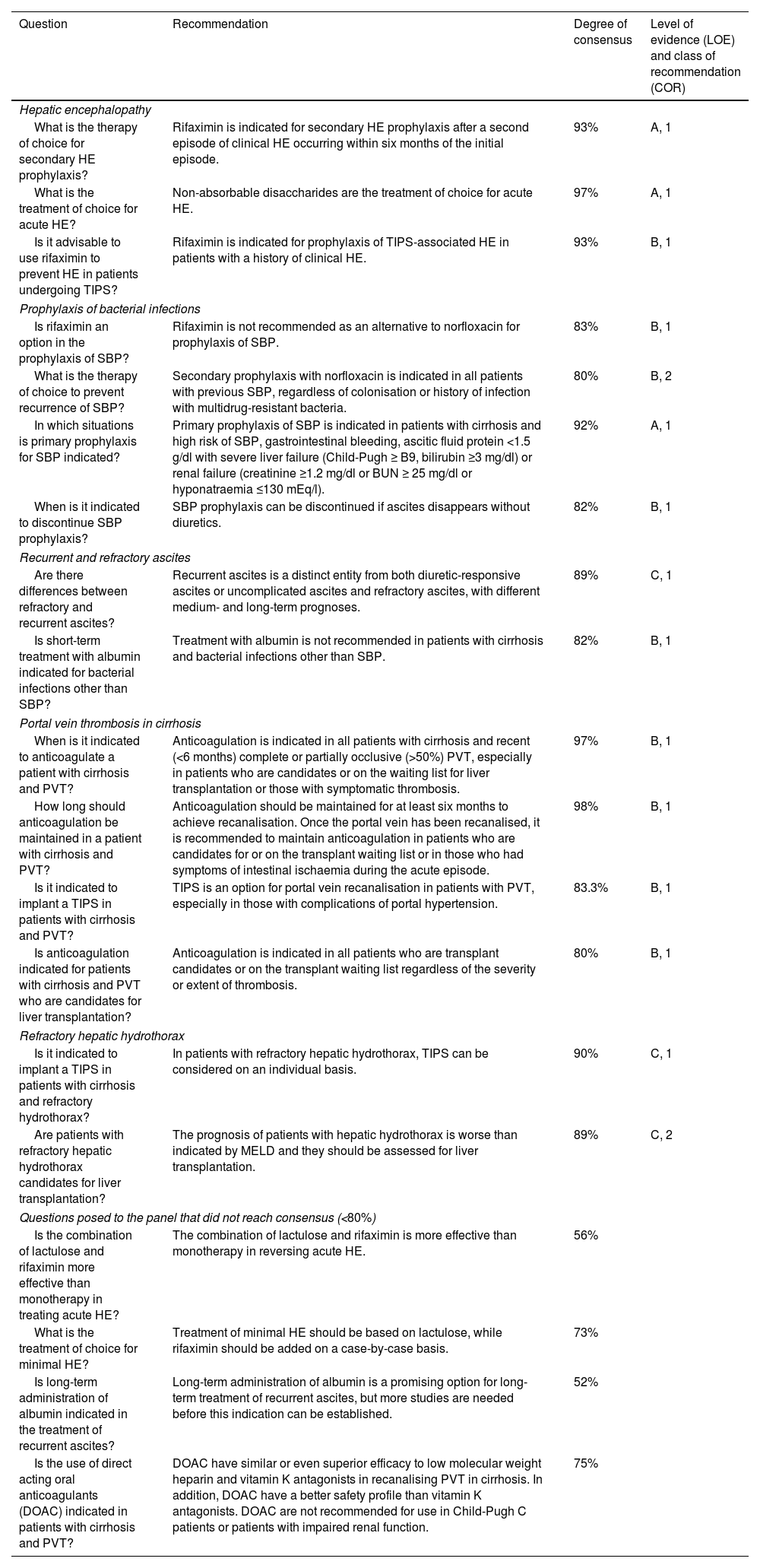
Recommendations on other complications of portal hypertension (session 4).
| Question | Recommendation | Degree of consensus | Level of evidence (LOE) and class of recommendation (COR) |
|---|---|---|---|
| Hepatic encephalopathy | |||
| What is the therapy of choice for secondary HE prophylaxis? | Rifaximin is indicated for secondary HE prophylaxis after a second episode of clinical HE occurring within six months of the initial episode. | 93% | A, 1 |
| What is the treatment of choice for acute HE? | Non-absorbable disaccharides are the treatment of choice for acute HE. | 97% | A, 1 |
| Is it advisable to use rifaximin to prevent HE in patients undergoing TIPS? | Rifaximin is indicated for prophylaxis of TIPS-associated HE in patients with a history of clinical HE. | 93% | B, 1 |
| Prophylaxis of bacterial infections | |||
| Is rifaximin an option in the prophylaxis of SBP? | Rifaximin is not recommended as an alternative to norfloxacin for prophylaxis of SBP. | 83% | B, 1 |
| What is the therapy of choice to prevent recurrence of SBP? | Secondary prophylaxis with norfloxacin is indicated in all patients with previous SBP, regardless of colonisation or history of infection with multidrug-resistant bacteria. | 80% | B, 2 |
| In which situations is primary prophylaxis for SBP indicated? | Primary prophylaxis of SBP is indicated in patients with cirrhosis and high risk of SBP, gastrointestinal bleeding, ascitic fluid protein <1.5 g/dl with severe liver failure (Child-Pugh ≥ B9, bilirubin ≥3 mg/dl) or renal failure (creatinine ≥1.2 mg/dl or BUN ≥ 25 mg/dl or hyponatraemia ≤130 mEq/l). | 92% | A, 1 |
| When is it indicated to discontinue SBP prophylaxis? | SBP prophylaxis can be discontinued if ascites disappears without diuretics. | 82% | B, 1 |
| Recurrent and refractory ascites | |||
| Are there differences between refractory and recurrent ascites? | Recurrent ascites is a distinct entity from both diuretic-responsive ascites or uncomplicated ascites and refractory ascites, with different medium- and long-term prognoses. | 89% | C, 1 |
| Is short-term treatment with albumin indicated for bacterial infections other than SBP? | Treatment with albumin is not recommended in patients with cirrhosis and bacterial infections other than SBP. | 82% | B, 1 |
| Portal vein thrombosis in cirrhosis | |||
| When is it indicated to anticoagulate a patient with cirrhosis and PVT? | Anticoagulation is indicated in all patients with cirrhosis and recent (<6 months) complete or partially occlusive (>50%) PVT, especially in patients who are candidates or on the waiting list for liver transplantation or those with symptomatic thrombosis. | 97% | B, 1 |
| How long should anticoagulation be maintained in a patient with cirrhosis and PVT? | Anticoagulation should be maintained for at least six months to achieve recanalisation. Once the portal vein has been recanalised, it is recommended to maintain anticoagulation in patients who are candidates for or on the transplant waiting list or in those who had symptoms of intestinal ischaemia during the acute episode. | 98% | B, 1 |
| Is it indicated to implant a TIPS in patients with cirrhosis and PVT? | TIPS is an option for portal vein recanalisation in patients with PVT, especially in those with complications of portal hypertension. | 83.3% | B, 1 |
| Is anticoagulation indicated for patients with cirrhosis and PVT who are candidates for liver transplantation? | Anticoagulation is indicated in all patients who are transplant candidates or on the transplant waiting list regardless of the severity or extent of thrombosis. | 80% | B, 1 |
| Refractory hepatic hydrothorax | |||
| Is it indicated to implant a TIPS in patients with cirrhosis and refractory hydrothorax? | In patients with refractory hepatic hydrothorax, TIPS can be considered on an individual basis. | 90% | C, 1 |
| Are patients with refractory hepatic hydrothorax candidates for liver transplantation? | The prognosis of patients with hepatic hydrothorax is worse than indicated by MELD and they should be assessed for liver transplantation. | 89% | C, 2 |
| Questions posed to the panel that did not reach consensus (<80%) | |||
| Is the combination of lactulose and rifaximin more effective than monotherapy in treating acute HE? | The combination of lactulose and rifaximin is more effective than monotherapy in reversing acute HE. | 56% | |
| What is the treatment of choice for minimal HE? | Treatment of minimal HE should be based on lactulose, while rifaximin should be added on a case-by-case basis. | 73% | |
| Is long-term administration of albumin indicated in the treatment of recurrent ascites? | Long-term administration of albumin is a promising option for long-term treatment of recurrent ascites, but more studies are needed before this indication can be established. | 52% | |
| Is the use of direct acting oral anticoagulants (DOAC) indicated in patients with cirrhosis and PVT? | DOAC have similar or even superior efficacy to low molecular weight heparin and vitamin K antagonists in recanalising PVT in cirrhosis. In addition, DOAC have a better safety profile than vitamin K antagonists. DOAC are not recommended for use in Child-Pugh C patients or patients with impaired renal function. | 75% | |
DOAC: direct-acting oral anticoagulants; HE: hepatic encephalopathy; PVT: portal vein thrombosis; SBP: spontaneous bacterial peritonitis; TIPS: transjugular intrahepatic portosystemic shunt.
Non-absorbable disaccharides, such as lactulose, are a first-line treatment for HE. Their efficacy is based on their ability to reduce intestinal production and absorption of ammonium through their effects as a laxative, inhibitor of intestinal glutaminase activity and modulator of intestinal microbiota.215 Multiple studies, including clinical trials, have shown the effectiveness of lactulose in the treatment of an acute episode of HE. An improvement of the acute episode has been demonstrated in 75% of patients receiving lactulose enemas compared to 20% of those treated with non-acidifying enemas.216 In addition, a meta-analysis showed that lactulose treatment reduces mortality associated with the acute episode.217 The route of administration, oral or rectal in enemas, should be individualised according to the patient's condition.
Rifaximin is indicated for prophylaxis of TIPS-associated HE in patients with a history of clinical HETIPS can lead to an increased risk of HE (35–50%)218 especially in patients with previous episodes and with a reduction in PPG below 5 mmHg after the procedure.207,219 A randomised clinical trial involving 197 patients electively implanted with a coated TIPS showed that rifaximin (600 mg/12 h) from 14 days before and up to six months after TIPS placement reduced the likelihood of post-procedural HE compared to placebo (34% vs. 53%).209 A post-hoc analysis of this study showed that the greatest benefit was obtained in patients with a previous episode of HE (n = 24, rifaximin 33.3% vs. placebo 83%) versus those with no previous episode (n = 162, rifaximin 35.5% vs. placebo 51%). At present, we lack robust data to justify the benefit of rifaximin beyond six months after TIPS. Previously, in 2005, the role of rifaximin in this scenario had been evaluated with negative results, probably due to the small sample size (n = 75), the use of non-covered TIPS and the short follow-up of patients (one month).219
2. Prophylaxis of bacterial infectionsRifaximin is not recommended as an alternative to norfloxacin in the prophylaxis of SBPRetrospective studies have suggested that rifaximin may be associated with a lower incidence of SBP and other complications of cirrhosis.220,221 However, these findings have not been confirmed in a prospective observational study.220 Few randomised trials have compared the efficacy of rifaximin versus norfloxacin and these, despite positive results, have methodological limitations.222,223 Lastly, systematic reviews and meta-analyses also fail to provide quality information to support the use of rifaximin as an alternative for secondary prophylaxis of SBP.224,225
Secondary prophylaxis with norfloxacin is indicated in all patients with previous SBP, regardless of colonisation or history of infection with multidrug-resistant bacteriaIn recent years, the prevalence of Gram-positive, quinolone-resistant and multidrug-resistant SBP has increased.30,226 However, a recent study did not find the use of norfloxacin to be associated with an increased prevalence of multidrug-resistant bacteria,227 not even when its use was evaluated in different geographical areas.226 The recurrence rate of SBP after a first episode is 70%,228 which is reduced to 20% with norfloxacin (400 mg/day).229 There is insufficient information to identify groups at risk of recurrence and universal prophylaxis is recommended.
Primary prophylaxis of SBP is indicated in patients with cirrhosis and high risk of SBP; gastrointestinal bleeding or ascitic fluid protein <1.5 g/dl with severe liver failure (Child-Pugh ≥ B9, bilirubin ≥3 mg/dl) or renal failure (creatinine ≥1.2 mg/dl or BUN ≥ 25 mg/dl or hyponatraemia ≤130 mEq/l)In patients with decompensated liver cirrhosis, bacterial infections are significantly associated with higher acute kidney injury, HE and mortality rates,230–233 making it advisable to apply strategies aimed at their prevention. However, the widespread use of antibiotics may lead to an increase in infections with multidrug-resistant bacteria and it is therefore necessary to select individuals at higher risk who will benefit most from primary prophylaxis. The benefit of antibiotic prophylaxis for gastrointestinal bleeding in cirrhosis is well established, reducing bacterial infections, rebleeding and mortality rates.146 Norfloxacin (400 mg/12 h orally) or ceftriaxone (1 g/24 h IV) is recommended, the latter in patients with advanced cirrhosis (ascites, severe malnutrition, HE or bilirubin >3 g/dl).147 Prophylaxis is also indicated in patients with Child-Pugh A cirrhosis because, although the risk of infection in this group is low, the available evidence is still limited in terms of dispensing with prophylaxis.148 Low concentration (<1.5 g/dl) of total protein in ascitic fluid is a risk factor for SBP.234,235 However, recent data question recommending primary prophylaxis for all patients with cirrhosis and low ascitic fluid protein content236,237 limiting it only to those with more severe liver failure (Child-Pugh ≥ B9, bilirubin ≥3 mg/dl) or renal impairment (creatinine ≥1.2 mg/dl or BUN ≥25 mg/dl or hyponatraemia ≤130 mEq/l) in whom norfloxacin reduces the risk of SBP and hepatorenal syndrome and increases survival.238 In any event, taking into account the epidemiological changes of recent years, prophylaxis should always be adjusted to the local resistance pattern.239
SBP prophylaxis can be discontinued if ascites disappears without the need for diureticsConsidering the discrepancies in the effects of norfloxacin in patients with cirrhosis and although it has not been specifically evaluated, it is recommended to discontinue its administration if ascites resolves in patients in whom it is indicated as prophylaxis for SBP.
3. Recurrent and refractory ascitesRecurrent ascites is a different condition from diuretic-responsive ascites or uncomplicated ascites and refractory ascites, with a medium- and long-term prognosis different from bothRecurrent ascites is ascites that partially responds to diuretic treatment and requires occasional (at least three in a year) large volume paracentesis.240 Recurrent ascites has a prognosis at one year similar to that of diuretic-responsive ascites, but better than refractory ascites.60
Treatment with albumin is not recommended in patients with cirrhosis and bacterial infections other than SBPThe potential benefit of albumin in patients with liver cirrhosis and bacterial infections other than SBP has been studied in three clinical trials, which included patients with different severities of cirrhosis and infection.241–243 The use of albumin was not associated in any of them with a survival benefit; in one it significantly improved renal function and in all it caused a larger number of episodes of acute lung oedema. Short-term use of albumin in combination with antibiotic therapy for the treatment of bacterial infections other than SBP is not currently recommended.244
4. Portal vein thrombosis in cirrhosisAnticoagulation is indicated in all patients with cirrhosis and recent (<6 months) complete or partially occlusive (>50%) portal vein thrombosis (PVT), especially in patients who are candidates or on the waiting list for liver transplantation or those with symptomatic thrombosisThe purpose of anticoagulation in patients with cirrhosis and PVT is to recanalise the portal venous axis and prevent thrombus progression. The available information on the efficacy of anticoagulation in this setting is weak, as it is based on meta-analyses of aggregated data, the latter including 33 studies and 1,696 patients, and one of individual cohort data of patients with cirrhosis and PVT treated with traditional anticoagulants.245,246 Based on this information, anticoagulation increases the portal vein recanalisation rate by a factor of 2.6, from 21.3% in the non-anticoagulated group to 56.5%, and the rate of complete recanalisation from 18.3% to 48.8%. Anticoagulation to treat PVT is indicated in all patients with cirrhosis and recent (<6 months), complete or partially occlusive (>50%) thrombosis, especially in transplant candidates or those on a transplant waiting list or those with symptomatic thrombosis, in whom the superior mesenteric vein is often involved. Once the decision has been made, anticoagulant treatment should be started as soon as possible, as its effectiveness decreases if it is delayed for more than six months after diagnosis. Other factors that reduce the efficacy of anticoagulation are complete thrombosis, thrombosis involving the superior mesenteric vein, or thrombosis occurring in the patient with decompensated cirrhosis (Child-Pugh B/C).245,247–249 The decision to anticoagulate patients with minimally occlusive PVT (<50%) should be made on an individual basis, with it being more reasonable to start anticoagulation in patients with PVT who are candidates for transplant or on a transplant waiting list or in those with minimally occlusive thrombosis and documented progression.
Anticoagulation should be maintained for at least six months to achieve recanalisation. Once the portal vein has been recanalised, it is recommended to maintain anticoagulation in patients who are transplant candidates or on the transplant waiting list or in those who had symptoms of intestinal ischaemia during the acute episodePortal recanalisation occurs in most patients during the first five months after starting anticoagulation therapy, so the minimum duration should be six months.249–251 Once recanalisation is achieved, anticoagulation should be maintained for at least another six months. The decision to continue it later depends on the balance between the consequences of possible re-thrombosis and the increased risk of bleeding that any anticoagulation entails. Re-thrombosis occurs in 46.7% of patients after discontinuing anticoagulation,245 and in many cases as early as within the first five months.248,249 Considering the potential risk of re-thrombosis, it is recommended to continue anticoagulation beyond recanalisation in patients who are transplant candidates or on the transplant waiting list or in those in whom PVT was symptomatic. Portal patency should be closely monitored in patients who are withdrawn from anticoagulation, given the high likelihood of re-thrombosis.
TIPS is an option for portal vein recanalisation in patients with PVT, especially in those with complications of portal hypertensionTIPS performed in specialised centres can recanalise PVT in 85–100% of cases.252–254 Importantly, in all studies that have evaluated the efficacy and feasibility of TIPS in patients with PVT, the indication for TIPS implantation was made because the patients had complications of portal hypertension refractory to standard treatment in which the concomitant presence of PVT could aggravate the situation. TIPS is therefore considered the treatment of choice in PVT patients with refractory complications of portal hypertension. It may also be considered, on a case-by-case basis, when thrombosis progresses despite anticoagulation or when anticoagulation is contraindicated.
Anticoagulation is indicated in all patients who are transplant candidates or on the transplant waiting list regardless of the severity or extent of the PVTPVT, especially when complete, can be particularly harmful in liver transplantation, as it involves greater technical difficulties and ischaemia times, reducing survival255,256 especially in cases where a physiological anastomosis cannot be performed.257 Therefore, anticoagulation is recommended for all patients with PVT who are being evaluated for transplantation or are already on the waiting list, regardless of the severity or extent of the thrombosis.
5. Refractory hepatic hydrothoraxIn patients with refractory hepatic hydrothorax, TIPS can be considered on an individual basisThe development of hepatic hydrothorax is associated with a worse prognosis of cirrhosis. Patients with hepatic hydrothorax who, despite optimised medical treatment, require frequent thoracentesis or have significant symptoms, may benefit from TIPS placement, which achieves some degree of improvement in approximately 70% of these patients.258,259 Predictors of worse response to TIPS are similar to those described in refractory ascites, and include older age, severity of cirrhosis and renal failure.258 These parameters can help select patients with refractory hepatic hydrothorax, who may benefit most from TIPS.
The prognosis of patients with hepatic hydrothorax is worse than indicated by MELD and they should be assessed for liver transplantationHepatic hydrothorax is associated in retrospective, single-centre studies with a worse prognosis compared to patients with decompensated cirrhosis or refractory ascites, and has been identified as an independent factor for mortality.260,261 It has also been suggested that MELD is a poor predictor of mortality in these patients.262,263 Since the development of hepatic hydrothorax is a complication of advanced cirrhosis, these patients should be considered for liver transplantation.
6. Questions posed to the panel which only reached a low level of consensusCombination of lactulose and rifaximin is more effective than monotherapy in reversing an acute HE episodeIn a randomised clinical trial comparing rifaximin (1,200 mg/24 h) and lactulose (n = 63) and lactulose monotherapy (n = 57), the combination of the two increased the reversibility of the acute HE episode (76% vs. 50%) and reduced hospital stay (5.8 vs. 8.2 days) and mortality rates (24% vs. 49%).264 A relevant point to note is the severity of the patients included in this study, who had a mean MELD of 24 and half of whom had HE grade IV. These findings have been endorsed in a meta-analysis including seven studies, which found that the combination of rifaximin and lactulose was superior to lactulose monotherapy in both the reversibility of the acute episode and in reducing associated mortality rates.265
Treatment of minimal HE should be based on lactulose, while rifaximin should be added on a case-by-case basisAlthough minimal HE is associated with impaired quality of life, increased incidence of clinical HE and a negative impact on survival,131 studies aimed at answering this question have some shortcomings, such as heterogeneity in the diagnosis of minimal HE, insufficient sample size, short treatment duration and lack of clinically relevant endpoints, which make it difficult to draw robust conclusions. Considering these limitations, rifaximin has been shown to reverse minimal HE and improve quality of life, but its efficacy in reducing clinical HE in patients with baseline minimal HE is less clear.266 In contrast, lactulose has been shown to decrease the risk of clinical HE in patients with minimal baseline HE and to reverse minimal HE.266 Lactulose has also been shown to be more cost-effective than rifaximin, improving quality of life.267
Long-term administration of albumin is a promising option for long-term treatment of recurrent ascites, but more studies are needed before this indication can be establishedAlbumin therapy has been evaluated in patients with cirrhosis in clinical scenarios, such as SBP and hepatorenal syndrome.244 Because of its oncotic, immunomodulatory and plasma-expanding properties, albumin has also been evaluated as a long-term treatment in patients with ascites. Two clinical trials have tested its efficacy; the first one, conducted mostly in patients with refractory ascites and renal failure, did not identify any beneficial effects of albumin.268 In the second trial, with an open-label design, administration of higher doses of albumin and inclusion of patients with recurrent and non-refractory ascites, the results were positive in terms of ascites control, development of complications such as refractory ascites or hyponatraemia and survival.269 Although this appears to be a treatment option associated with significant clinical benefits, confirmatory studies are needed before establishing this new indication for long-term use of albumin in patients with recurrent ascites.
Direct oral anticoagulants (DOAC) have similar or even superior efficacy to low molecular weight heparin and vitamin K antagonists in recanalising PVT in cirrhosis. In addition, DOAC have a better safety profile than vitamin K antagonists. DOAC are not recommended for use in Child-Pugh C patients.
A network meta-analysis has shown that the efficacy of DOAC is similar or even superior to that of traditional anticoagulation in recanalising the portal vein in patients with PVT and cirrhosis.270 A meta-analysis of aggregated data and the analysis of a large cohort of patients with cirrhosis and atrial fibrillation on anticoagulant prophylaxis shows a lower risk of bleeding, usually gastrointestinal, in patients treated with DOAC compared to those receiving vitamin K antagonists.271,272 In these studies the use of DOAC was restricted to Child-Pugh A and some B patients, as these drugs should not be used in Child-Pugh C patients. DOAC are also not recommended in patients with creatinine clearance of less than 30 ml/min. DOAC are therefore as effective and are probably a safer option than vitamin K antagonists for the long-term treatment of PVT in patients with Child-Pugh A and B cirrhosis.
SESSION 5. Transjugular intrahepatic portosystemic shunt (TIPS) (Table 5)1. General considerationsCentres without the capacity to place preventive, rescue or elective TIPS should establish circuits that allow for quick referral to a centre with experience in TIPS insertionTIPS insertion is capable of improving prognosis and increasing survival in numerous clinical scenarios.200,273,274 There are no studies that directly assess the need to transfer patients from centres unable to perform TIPS insertion to centres with experience. However, given the evidence of its benefits,46,244 the healthcare system should be able to guarantee that patients with an indication for TIPS can be treated in centres with the capacity to perform the procedure.275,276 Furthermore, according to data correlating experience in insertion of TIPS, defined as performing 20 procedures a year, with survival,277 the transfer of patients to centres of expertise needs to be clearly established.
Recommendations on transjugular intrahepatic portosystemic shunt, TIPS (session 5).
| Question | Recommendation | Degree of consensus | Level of evidence (LOE) and class of recommendation (COR) |
|---|---|---|---|
| General considerations | |||
| Is it advisable to have referral circuits for patients from hospitals that lack availability for TIPS to other hospitals with access to TIPS? | Centres without the capacity to place preventive, rescue or elective TIPS should establish circuits that allow for quick referral to a centre with experience in TIPS insertion. | 100% | A, 1 |
| Which cardiac function parameters should be measured in a patient who is a candidate for TIPS? | Cardiac evaluation by echocardiogram, electrocardiogram and BNP measurement is recommended in all patients who are candidates for TIPS. | 92% | B, 1 |
| How should patients who have been implanted with a TIPS be followed up? | Follow-up of patients after TIPS placement should be done on a regular basis with ultrasound scans specifically aimed at assessing potential shunt dysfunction by confirming patency and analysing flow velocity. | 96% | D, 2 |
| TIPS in acute variceal bleeding | |||
| What are the parameters used to stratify the risk of death at admission in patients with AVB? | In all patients with AVB, risk stratification for recurrence and death should be performed on admission, taking into account the Child-Pugh classification and the presence of active bleeding on diagnostic endoscopy. | 97% | A, 1 |
| In patients with AVB, when is a rescue TIPS indicated? | The treatment of choice for failure of initial AVB control is placement of a rescue TIPS. | 94% | A, 1 |
| In patients with AVB, what parameters can help define the futility of TIPS placement? | Child-Pugh score, MELD and lactate levels may be useful in considering the futility of TIPS placement. | 87% | B, 1 |
| TIPS in the prevention of recurrence of decompensation | |||
| What is the treatment of choice for recurrent ascites? | The treatment of choice for recurrent ascites is TIPS, as it has demonstrated a survival benefit and prevention of other complications of portal hypertension. | 80% | A, 1 |
| What are the risk factors for poor outcome in patients with refractory ascites undergoing TIPS? | Age, renal function, platelets, sodium and bilirubin may help predict prognosis after TIPS placement in patients with recurrent ascites. | 92% | B, 1 |
| Is TIPS indicated as a first therapeutic option to prevent rebleeding in patients with contraindications or intolerance to NSBB? | TIPS should be considered to prevent rebleeding in patients with contraindications or intolerance to NSBB, especially if they have recurrent ascites. | 85% | B, 1 |
| Other TIPS indications | |||
| What are the treatment options to prevent gastric variceal rebleeding? | TIPS, obliteration by endoscopic cyanoacrylate injection plus NSBB and BRTO are the first-line options in the prevention of rebleeding in patients with gastric varices. | 88% | C, 2 |
| Is it necessary to embolise portosystemic collaterals when a TIPS is implanted to treat or prevent AVB? | Embolisation of portosystemic collaterals should be considered on a case-by-case basis at the time of TIPS placement, as it may help increase efficacy and reduce the risk of encephalopathy if collateral filling persists after prosthesis implantation. | 90% | B, 1 |
| Is it indicated to implant a TIPS in patients with recurrent bleeding due to portal hypertensive gastropathy? | In patients with recurrent bleeding due to portal hypertensive gastropathy, it is recommended that TIPS placement be evaluated. | 82% | C, 2 |
| Is it indicated to implant a TIPS in patients with recurrent bleeding from ectopic varices? | TIPS is an option to consider in any patient with recurrent bleeding from ectopic varices. | 88% | C, 2 |
| Preventive TIPS in surgical patients | |||
| Is there an HVPG value above which the risk of early mortality from non-hepatic abdominal surgery increases? | An HVPG value >16 mmHg increases the risk of early mortality in patients undergoing non-hepatic abdominal surgery. | 92% | C, 2 |
| Is TIPS indicated for patients with cirrhosis who are candidates for major extrahepatic surgery with an increased risk of death? | TIPS placement prior to major surgery should be assessed on an individual basis and may be recommended in high-risk patients identified by an HVPG > 16 mmHg. | 80% | C, 2 |
| Questions posed to the panel that did not reach consensus (<80%) | |||
| Is antibiotic prophylaxis recommended before implanting a TIPS? | It is recommended that antibiotic prophylaxis be considered prior to TIPS. | 55% | |
| Is TIPS indicated as a first therapeutic option to prevent rebleeding in patients who have had variceal bleeding while on NSBB prophylaxis? | TIPS could be considered in the prevention of rebleeding in patients who have had a variceal bleeding episode while on primary prophylaxis with NSBB or endoscopic ligation. | 72% | |
| Is it indicated to implant a TIPS in patients with rebleeding due to enteropathy or hypertensive colopathy? | In patients with rebleeding due to enteropathy or hypertensive colopathy, it is recommended that TIPS placement be evaluated. | 77% | |
AVB: acute variceal bleeding; BNP: brain natriuretic peptide; BRTO: balloon-occluded retrograde transvenous obliteration; HVPG: hepatic venous pressure gradient; NSBB: non-selective beta-adrenergic blockers; TIPS: transjugular intrahepatic portosystemic shunt.
TIPS insertion causes haemodynamic changes which in a limited number of cases could lead to cardiac decompensation, especially in patients with previous heart disease.278 This has been little studied, but may affect up to 20% of patients. The risk factors identified to date are previous heart disease (especially aortic stenosis), elevated BNP and a prolonged QTc interval.279 Therefore, a cardiac assessment prior to TIPS implantation is recommended using accessible tests such as echocardiogram, BNP and electrocardiogram.
Follow-up of patients after TIPS placement should be done on a regular basis with ultrasound scans specifically aimed at assessing potential shunt dysfunction by confirming patency and analysing flow velocitySince the use of coated prostheses, TIPS dysfunction has decreased markedly,206,275,280 especially in the short term. However, long-term dysfunction can occur and ultrasound monitoring is recommended. There are no defined timelines, but many centres perform a check-up one month after implantation, at two to three months and then every six months to coincide with HCC screening,278,281 as ultrasound data on patency, velocity and blood flows are predictive of dysfunction.281
2. TIPS in acute variceal bleedingIn all patients with AVB, risk stratification should be performed on admission, taking into account the Child-Pugh classification and the presence of active bleeding on diagnostic endoscopyMortality in AVB remains high at 15–20%,139 with liver function (Child-Pugh, MELD) being the best predictor of poor prognosis,282 in addition to other factors such as ACLF,200 an HVPG equal to or greater than 20 mmHg,283 active bleeding at initial endoscopy despite treatment with vasoactive drugs,284 bacterial infection on admission285 or other comorbidities. Intensifying treatment in high-risk patients by placing TIPS to reduce portal pressure more aggressively has been shown to improve survival. However, while these factors give an idea of the patient's mortality risk, not all of them have been studied or shown to be useful in guiding treatment. In particular, only HVPG, Child-Pugh classification and active bleeding at initial endoscopy have proven useful in helping select a subgroup of patients who will benefit in terms of survival from treatment with preventive TIPS.274,286–292 The main aim of preventive TIPS is to avoid failure of initial control of the bleeding and it should therefore be placed during the period of highest risk, which is in the first three days, especially in the first 24 h.205,293
The treatment of choice for failure of initial AVB control is placement of a rescue TIPSThe mortality rate during admission in patients with vasoactive drug and EBL treatment failure is very high, making TIPS placement with a coated prosthesis the most effective option to control bleeding and improve survival.200,202
The mortality rate during admission in patients with vasoactive drug and EBL treatment failure is very high, making TIPS placement with a coated prosthesis the most effective option to control bleeding and improve survival.200,202
Child-Pugh score, MELD and lactate levels may be useful when considering the futility of TIPS placementAlthough TIPS is very effective for controlling bleeding in patients with initial treatment failure, the mortality rate in this clinical scenario is very high and it is essential to identify patients in whom the mortality risk is so high in the short term that TIPS is a futile option. A Child-Pugh score above 13 if transplantation is not immediately available, arterial lactate levels >11 mmol/l and MELD >29 are the identifying factors in this population.202,294
3. TIPS in the prevention of decompensation recurrenceThe treatment of choice for recurrent ascites is TIPS, as it has shown a benefit on survival and other complications of portal hypertensionAscites is the most common form of first decompensation of cirrhosis. When it becomes recurrent or refractory, the indication for liver transplantation should be considered. The seven controlled studies that have compared TIPS with conventional treatment (large volume paracentesis with albumin administration) in patients with refractory and/or recurrent ascites have demonstrated better control of ascites after prosthesis insertion295–301 and four of them also demonstrated a survival benefit in favour of TIPS.296–301 It is worth noting that three of the four studies in which TIPS improved survival included patients with recurrent ascites exclusively296 or at a proportion of 30–40%.299,301 In contrast, the two studies in which survival was similar between the two groups included only patients with refractory ascites,297,298 and there was only one study showing a survival benefit with TIPS that included only patients with refractory ascites.300 The one study (the first to be published) that suggested a higher mortality rate in the TIPS group has obvious methodological difficulties which limit, if not invalidate, the applicability of its results.295 The only study using covered prostheses (currently standard of care) only included patients with recurrent ascites301 and demonstrated marked benefits in transplant-free survival and ascites control in patients treated with TIPS. For this reason, the current recommendation is to bring forward the indication for TIPS to the time when ascites becomes recurrent.46
Age, renal function, platelets, sodium and bilirubin may help predict prognosis after TIPS placement in patients with recurrent ascitesThe estimation of the risk of poor outcome after TIPS insertion in patients with recurrent or refractory ascites is a central element of the decision, as it is an elective procedure, unlike the indication for AVB. In this regard, different prognostic factors have been described. The degree of thrombocytopenia and bilirubin concentration have been shown to have marked prognostic value in patients with refractory ascites undergoing TIPS.302 Patients with platelets >75,000/μl and bilirubin <3 mg/dl had a one-year survival rate of 73%, compared to 31% of patients who did not meet either of these two conditions.303 Age, natraemia and MELD score offer adequate predictive ability for risk of death at 6, 12 and 24 months in patients with both recurrent and refractory ascites.304 A Spanish study has also suggested that a model based on age, creatinine and sodium is able to predict the development of recurrent HE or death one year after TIPS in patients with refractory ascites, using a user-friendly nomogram.305
TIPS should be considered to prevent rebleeding in patients with contraindications or intolerance to NSBB, especially if they have recurrent ascitesThe treatment of choice in the prevention of rebleeding is the combination of NSBB (propranolol or carvedilol) and EBL.46 However, in patients with ascites, especially when recurrent or refractory, the tolerability and safety of such drugs may be compromised.99,102–104,306 In addition, data from a pooled analysis of three clinical trials involving a total of 1,198 decompensated patients described a marked increase in mortality associated with AVB, bacterial infection or hepatorenal syndrome307 in patients in whom NSBB was withdrawn (29%). Since TIPS is a very effective alternative in the prevention of complications of portal hypertension, it seems reasonable to indicate it for the prevention of rebleeding in patients with ascites and intolerance or contraindication to NSBB treatment.
4. Other indications for TIPSTIPS, endoscopic obliteration with cyanoacrylate plus NSBB and embolisation by BRTO are first-line options in the prevention of rebleeding in patients with gastric varicesThe low incidence of gastrointestinal bleeding from gastric varices and the absence of methodologically sound studies limit the strength of the recommendations. Most referral centres opt for personalised treatment based on history of previous bleeding, liver function, presence of collaterals and ease of performing TIPS. The combination of cyanoacrylate plus BBNS has been compared with cyanoacrylate monotherapy in two studies,308,309 with no clear survival benefit, although one of them found greater efficacy of the combination therapy in preventing bleeding.308 Data from one clinical trial310 and two observational studies311,312 comparing TIPS (non-covered prostheses) with cyanoacrylate injection show less rebleeding in patients treated with TIPS, but no difference in survival and a higher rate of HE. Four non-randomised studies313–316 and one randomised317 have demonstrated the greater effectiveness of BRTO compared to cyanoacrylate in preventing bleeding, but no survival benefit. No randomised studies comparing BRTO and TIPS are available, but in a meta-analysis of non-randomised studies318 both TIPS and BRTO demonstrated similar efficacy in terms of haemostasis, but no survival benefit. Based on the available data, the choice of these techniques should be made on an individual basis, taking into account the experience of the centre.
Collateral embolisation should be considered on an individual basis at the time of TIPS placement, as it may help increase efficacy and reduce the risk of HE if collateral filling persists after prosthesis implantationThere are several retrospective studies195,196,319 and three randomised studies320–322 evaluating the combination of TIPS plus embolisation of collaterals compared to TIPS alone, with no clear benefit found of the combined treatment either in the prevention of rebleeding or in mortality rates. The studies evaluate very heterogeneous populations and different types of collateral embolisation (antegrade and retrograde) and even the inclusion of oesophageal varices, so the validity of the results is limited. Interestingly, one of the randomised studies reported a beneficial effect on the incidence of HE,321 but no effect on the prevention of rebleeding.
In patients with rebleeding due to portal hypertensive gastropathy, evaluating TIPS placement is recommendedThere are limited data, mostly from case series reports, suggesting that TIPS placement could reduce the severity of bleeding and improve mucosal injury in patients with portal hypertensive gastropathy requiring repeated transfusions and in whom medical treatment with NSBB and endoscopic treatment (argon plasma/EBL thermocoagulation) have failed.278,323
TIPS is an option to consider for all patients with rebleeding from ectopic varicesThe information available in this clinical setting is very limited, but in published studies, TIPS has demonstrated efficacy in terms of controlling the bleeding episode and preventing rebleeding from ectopic varices.324–326 TIPS appears to be useful in the control of bleeding from duodenal varices324 and associated with obliteration in rectal varices325–327 and peristomal varices.328 These patients require a multidisciplinary assessment and planning of the technique based on the expertise of the treating centre.329
5. Preventive TIPS for surgical patientsAn HVPG value higher than 16 mmHg increases the risk of early mortality in patients undergoing non-hepatic abdominal surgerySurgical risk in patients with cirrhosis is determined by several main elements: the degree of liver dysfunction (MELD, Child-Pugh); comorbidities and baseline functional status (ASA class); the type of surgery; the urgent indication; and the degree of portal hypertension.330,331 Studies on surgical risk in patients with cirrhosis have classically assessed the presence of portal hypertension through indirect data (thrombocytopenia, splenomegaly, presence of varices or ascites).332–335 Recently, a study established the association between the degree of portal hypertension and postoperative mortality.336 In this prospective multicentre study, HVPG was measured in 140 cirrhotic patients who underwent major elective surgery, showing that the independent predictors of one-year mortality were high-risk surgery (defined as open abdominal and cardiovascular or thoracic surgery), ASA class and HVPG. An HVPG >16 mmHg was identified as being associated with a higher postoperative mortality rate, with this effect being more marked if the HVPG was ≥20 mmHg. No patient without CSPH developed decompensation.
TIPS placement prior to major surgery should be evaluated on an individual basis and may be recommended in high-risk patients, identified by an HVPG >16 mmHgAccording to the aforementioned study,336 pre-surgical TIPS placement in patients with cirrhosis and high risk of mortality (HVPG >16 mmHg) would seem reasonable, although robust data are lacking. The protective effect of TIPS has not been demonstrated, as all studies are small retrospective series and there are only three case-control series, one of which suggests improved survival.337–339 The most frequently found data regarding preoperative TIPS are better postoperative control of ascites and improved operability.337,338,340 In some of these studies, haemodynamic variables are not available, so potential improvement in these clinical variables cannot be correlated with an improvement in PPG. In fact, in some studies TIPS has been performed with HVPG values <10 mmHg. Therefore, and pending larger and more conclusive studies, preoperative TIPS placement can be considered on an individual basis in patients who are to undergo high-risk surgery (open abdominal, cardiovascular, thoracic) and who have an HVPG >16 mmHg with some additional clinical indication (ascites, previous haemorrhage) or if they have an HVPG >20 mmHg, a value above which postoperative risk increases markedly. These data need to be confirmed in controlled studies with sufficient numbers of patients and with a detailed analysis of pre- and post-TIPS haemodynamic variables.
6. Questions posed to the panel that did not reach consensusIt is recommended that antibiotic prophylaxis be considered prior to performing a TIPSThere are no strong data to support or contraindicate the use of antibiotic prophylaxis prior to TIPS.275,341 In a 1998 randomised study of 105 patients using a second generation cephalosporin, there was no significant difference in the proportion of infections in the antibiotic treatment group (20% vs. 14% in the untreated).342 We should not forget that prophylactic antibiotic treatment in the context of AVB is clearly indicated,145,146 especially in Child-Pugh B and C patients, so when this is the situation that leads to the implantation of a TIPS it is most likely to be performed under antibiotic prophylaxis. Infection of a TIPS is a very serious complication, but indiscriminate use of antibiotic therapy is associated with high rates of microbial resistance. There is therefore no universal recommendation for the use of antibiotic prophylaxis prior to TIPS implantation and its use must be decided on a case-by-case basis. The type of antibiotic therapy should be according to the pattern of multiresistant microorganisms at each centre.
TIPS could be considered in the prevention of rebleeding in patients who have had an episode of AVB while on primary prophylaxis with NSBB or EBLThe treatment of choice in the prevention of rebleeding is the combination of NSBB and EBL.46 However, several studies suggest that TIPS insertion is more effective than combination therapy in preventing rebleeding,254,343–348 but without a beneficial effect in terms of survival and with an increased risk of EH. A recent meta-analysis of individual data including 11 studies comparing TIPS versus conventional therapy in different indications (ascites, preventive therapy in high-risk AVB, prophylaxis after haemorrhage) confirms that there is a lower risk of rebleeding, with no significant survival benefit, when the indication was in prophylaxis after low-risk haemorrhage. However, when the indication for TIPS was for ascites or in the context of AVB, not only was there a lower risk of further decompensation in TIPS patients, but also a significant improvement in survival.273 Consequently, individually indicated TIPS may be a good therapeutic option in patients who have had a haemorrhage while on primary prophylaxis, as it is able to prevent rebleeding as well as other decompensations.
In patients with rebleeding due to enteropathy or hypertensive colopathy, evaluation of TIPS placement is recommendedRebleeding due to enteropathy or hypertensive colopathy is associated with a higher degree of portal hypertension as measured by HVPG.349 It is therefore assumed that pathophysiologically appropriate treatment should be based on portal pressure reduction by NSBB therapy or more aggressive measures, such as TIPS, in patients refractory to medical treatment.350
SESSION 6. Hepatic vascular diseases (Table 6)1. Budd-Chiari syndromeThe diagnosis of Budd-Chiari syndrome (BCS) is based on imaging tests (ultrasound and CT) performed by expert staff, and biopsy is not necessary in the vast majority of casesDoppler ultrasound performed by expert staff is very sensitive and specific for the diagnosis of BCS and is the diagnostic technique of choice. CT and/or MRI support the diagnosis and will enable coexistence of associated thrombosis of the splenoportal axis to be detected.351 Catheterisation of suprahepatic veins is not essential for diagnosis, but if the thrombosed vein can be catheterised (not always possible) the typical "spider web" image can be visualised by injecting iodinated contrast. Liver biopsy is only necessary and essential for the diagnosis of small-vessel BCS.351 Once BCS has been diagnosed, a thorough investigation is required to rule out an underlying prothrombotic state.352
Recommendations on hepatic vascular diseases (session 6).
| Question | Recommendation | Degree of consensus | Level of evidence (LOE) and class of recommendation (COR) |
|---|---|---|---|
| Budd-Chiari syndrome | |||
| Is a liver biopsy necessary to establish the diagnosis of BCS? | The diagnosis of BCS is based on imaging tests (ultrasound and CT) performed by experts, and biopsy is not necessary in the vast majority of cases. | 88% | A, 1 |
| Is the diagnosis of hepatocellular carcinoma in patients with BCS based on the same criteria as in cirrhosis? | The radiological criteria used for the diagnosis of hepatocellular carcinoma in cirrhotic patients are not applicable to the liver nodules frequently observed in patients with BCS. | 82% | A, 1 |
| In patients with BCS, how should the different therapeutic options be used to improve venous drainage of the liver? | Treatment of BCS should follow a stepwise strategy including anticoagulation, angioplasty, TIPS and transplantation. | 88% | A, 1 |
| Non-cirrhotic non-tumour-related portal vein thrombosis | |||
| What aspects are included in the characterisation of a PVT? | Accurate characterisation of PVT is always necessary and should include assessment of its extent, the degree of vessel lumen obstruction and its acute or chronic nature. | 99% | A, 1 |
| When is recanalisation of PVT by invasive techniques indicated? | Recanalisation of the thrombosed territory by invasive techniques is recommended in patients in whom anticoagulation fails to achieve recanalisation and who develop complications of portal hypertension. | 80% | B, 2 |
| How are PVT patients followed up? | Patients with PVT should be followed up with imaging tests (abdominal Doppler ultrasound or CT). | 99% | B, 1 |
| Is screening for varices indicated in patients with PVT? | Patients with PVT should be screened for gastro-oesophageal varices and prophylaxis initiated if high-risk varices are found. | 96% | C, 1 |
| Porto-sinusoidal vascular diseases (PSVD) | |||
| Is it possible to diagnose PSVD in the absence of signs of portal hypertension? | The diagnosis of PSVD can be made even in the absence of portal hypertension. | 80% | A, 1 |
| Is a liver biopsy necessary to make a diagnosis of PSVD? | The diagnosis of PSVD requires a compatible liver biopsy. | 80% | A, 1 |
| How are patients with PSVD followed up? | In patients with PSVD the risk of PVT is very high, so screening with imaging tests is necessary. | 86% | B, 1 |
| Does the diagnosis of PSVD require screening for possible associated diseases? | In patients with PSVD, it is essential to assess for possible associated diseases, as this is crucial for establishing specific treatment and prognosis. | 99% | B, 1 |
| Anticoagulation in patients with hepatic vascular disease | |||
| Is anticoagulation indicated in patients with non-cirrhotic PVT? | Spontaneous recanalisation of PVT rarely occurs, so anticoagulation therapy should be initiated after diagnosis, first with low molecular weight heparin and then with oral drugs. | 88% | A, 1 |
| What is the minimum time that anticoagulation should be maintained to try to achieve recanalisation in non-cirrhotic PVT? | Anticoagulation should be maintained for at least six months in patients with non-cirrhotic PVT. | 94% | B, 2 |
| Can DOAC be used in patients with non-cirrhotic PVT? | The available data suggest the benefit of DOAC therapy as long as liver function is preserved, creatinine clearance is correct and the patient does not have triple positive antiphospholipid syndrome. | 82% | B, 1 |
| Aetiological diagnosis | |||
| Should more than one aetiological factor be ruled out in patients with non-cirrhotic PVT? | The presence of more than one aetiological factor should be considered in any patient with a diagnosis of non-cirrhotic PVT. | 93% | B, 1 |
| Which aetiological study is essential in patients with non-cirrhotic PVT? | Thrombophilia testing and screening for myeloproliferative neoplasia should be performed in all patients with non-cirrhotic PVT. | 94% | A, 1 |
| Questions posed to the panel that did not reach consensus (<80%) | |||
| What parameters can we use to assess the risk of PVT re-thrombosis when anticoagulation is stopped? | FVIII levels greater than or equal to 150 IU/dl and D-dimer levels greater than 500 ng/mL may be helpful in identifying patients at high risk of rethrombosis in the absence or after withdrawal of anticoagulation, respectively. | 78% | |
| Can unfractionated heparin be used in patients with non-cirrhotic PVT or BCS? | Unfractionated heparin should be avoided in patients with non-cirrhotic PVT or BCS because of the associated risk of heparin-induced thrombocytopenia. | 73% | |
| When should next-generation sequencing techniques be considered in patients with non-cirrhotic PVT? | In patients with non-cirrhotic PVT who do not have an identified aetiological factor, assessment of next generation sequencing techniques is recommended. | 61% | |
BCS: Budd-Chiari syndrome; CT: computed tomography; DOAC: direct oral anticoagulants; PSVD: portal-systemic vascular disorders; PVT: portal vein thrombosis; TIPS: transjugular intrahepatic portosystemic shunt.
The radiological criteria used for the diagnosis of HCC in cirrhosis (hypervascularisation in the arterial phase with rapid washout in the portal phase) are not sufficiently sensitive and specific to diagnose this cancer in BCS.353 MRI with liver-specific contrasts could improve diagnostic sensitivity and specificity.354,355 The diagnosis of HCC in patients with BCS therefore requires histological confirmation.351
Treatment of BCS should follow a stepwise strategy including anticoagulation, angioplasty and TIPSTreatment of patients with BCS should be personalised following a tiered approach in a referral centre.356 Some patients will only need anticoagulation. In patients with short stenosis of a suprahepatic vein, angioplasty with or without prosthesis placement is the best option. When medical treatment fails, TIPS is a safe and highly effective procedure. Liver transplantation should be reserved for patients with advanced liver failure, especially those in whom TIPS has failed, provided there is no contraindication due to the disease responsible for the BCS,356 and/or in cases of early liver failure without response to TIPS.356
2. Non-cirrhotic non-tumour-related portal venous thrombosisAccurate characterisation of PVT is always necessary and should include assessment of its extent, the degree of vessel lumen obstruction and its acute or chronic natureRadiological characterisation of PVT (vessels affected; degree of vessel obstruction: <50, 50–100 or 100%; acute or chronic) by CT angiogram or magnetic resonance angiogram is essential, as it determines treatment and the possibility of assessing the response (for example, use of anticoagulation, response, possible progression). In addition, imaging techniques allow local aetiological factors to be identified and help to distinguish between real thromboses and extrinsic venous compression obstructions.46,357–359
Recanalisation of the thrombosed territory by invasive techniques is recommended in patients in whom anticoagulation fails to achieve recanalisation and who develop complications of portal hypertensionExperience with invasive techniques in non-cirrhotic PVT is limited to short series of heterogeneous cases, with a highly variable recanalisation rate (15–85%) and a very high rate of potentially serious complications (7–95%). Therefore, they are currently only recommended when there is an acceptable risk/benefit ratio: acute PVT with intestinal ischaemia that does not respond to anticoagulation therapy46,357,359,360; and selected cases of chronic PVT with refractory or recurrent complications of portal hypertension.361–364 In both situations, these interventions should be carried out in specialised referral centres. Currently, these interventions are not recommended as a preventive measure.46,357,359
Patients with PVT should be followed up with imaging tests (Doppler ultrasound or CT)Patients with PVT should be followed up with imaging tests (Doppler ultrasound or CT) in order to assess response to treatment and to rule out progression.357–359 In acute PVT, contrast-enhanced abdominal CT scanning is advised six months after anticoagulant therapy to assess for possible recanalisation.46 Given the risk of recurrence of splanchnic thrombosis, and irrespective of withdrawal of anticoagulation, follow-up imaging tests are recommended,365,366 although the frequency of such tests has not been established.
Patients with PVT should be screened for gastro-oesophageal varices and prophylaxis initiated if high-risk varices are detectedIn acute PVT, if recanalisation does not occur within approximately six months, and in chronic PVT, screening for gastro-oesophageal varices should be performed by gastroscopy.367 In the absence of varices, repeat screening is recommended in 12 months and every two years thereafter.46 Although there are no controlled studies, extrapolating from current recommendations for cirrhosis, the use of NSBB in primary prophylaxis and combined with EBL in secondary prophylaxis is advised.46,357,359
3. Porto-sinusoidal vascular disordersThe diagnosis of porto-sinusoidal vascular disorder can be made irrespective of the presence of portal hypertensionPorto-sinusoidal vascular disorders (PSVD) are a clinical/pathological concept encompassing a series of histopathological conditions involving liver damage caused by a vascular mechanism.46,368,369 Until the recent update of the diagnostic criteria, the presence of signs which were either unequivocal (oesophageal varices or portosystemic shunts) or suggestive (ascites, splenomegaly or thrombocytopenia) was a prerequisite for diagnosis and the entity was referred to as idiopathic intrahepatic portal hypertension.370 This prevented the diagnosis of the disorder in pre-symptomatic stages. Retrospective studies have shown the presence of histological lesions compatible with PSVD, but without signs of portal hypertension, in liver biopsies performed in patients with different liver function test abnormalities.371 This evidence suggests that there are stages of the disease prior to the development of portal hypertension, hitherto not considered as part of portal hypertension. The description of specific histological signs (obliterative portal venopathy, nodular regenerative hyperplasia and incomplete septal fibrosis/cirrhosis) are considered diagnostic in themselves, even in the absence of signs of portal hypertension. The change will enable a better understanding of the natural history of the disease. It will also help us identify which patients with concordant liver lesions who do not initially have portal hypertension are likely to progress to portal hypertension and what risk factors are involved.372
The diagnosis of PSVD requires a compatible liver biopsyThe diagnosis of PSVD requires a compatible liver biopsy. The biopsy should be more than 20 mm in length and contain at least 10 portal spaces and, ideally, be interpreted by a pathologist with extensive experience in liver histopathology.46 A biopsy that does not fulfil these requirements may occasionally be considered sufficient by the expert pathologist. The biopsy should exclude liver cirrhosis in addition to showing one or more of the three histological lesions considered specific: 1) obliterative portal venopathy (previously known under different names, such as hepatoportal sclerosis, phlebosclerosis or portal venous obliteration); 2) nodular regenerative hyperplasia; and 3) incomplete septal fibrosis/cirrhosis.373–377 In the absence of a specific histological lesion, the diagnosis of PSVD requires data suggestive of portal hypertension and the existence of lesions, which, although not pathognomonic, may be indicative of intrahepatic vascular damage. These include herniation of hepatic venules, sinusoidal dilation, perisinusoidal fibrosis or the presence of aberrant periportal vessels.375
In patients with PSVD, the risk of PVT is very high, so follow-up with imaging tests is necessaryPSVD encompasses a series of hepatic vascular entities which commonly damage intrahepatic vessels at the level of portal veins and/or sinusoids and share a similar clinical phenotype and course, consisting mainly of the development of portal hypertension in the absence of cirrhosis. The presence of PVT was considered an exclusion criterion for the diagnosis of idiopathic intrahepatic portal hypertension. In contrast, the new diagnostic criteria for PSVD do not have this exclusion; the development of PVT should be interpreted as simply another manifestation of the disease, as it occurs in 13–45% of patients during follow-up.376,378–380 There is at present insufficient information to support the use of preventive anticoagulation in these patients, so the most appropriate strategy is to carry out regular follow-up with imaging tests and only treat those patients who develop PVT. The screening interval and method have not been defined, but a six-monthly Doppler ultrasound scan is advised.46
In patients with PSVD, it is essential to assess for possible associated diseases, as this is crucial for establishing both a specific treatment and a prognosisIn 43–58% of patients with PSVD there are one or more associated diseases, including immunological disorders, drug exposure, coagulation disorders and other haematological diseases, infectious diseases and hereditary disorders.380–384 In a patient with PSVD it is important to determine whether there is another associated systemic disease, as this may require specific treatment. In addition, the course of the underlying disease is a very important prognostic element.379 In fact, the severity of the associated disease is one of the most important independent factors related to mortality in patients with PSVD and/or post-TIPS or post-transplant survival.369,385–390
4. Anticoagulation in patients with hepatic vascular diseaseSpontaneous recanalisation of non-cirrhotic PVT rarely occurs, so anticoagulation therapy should be initiated after diagnosis, first with low molecular weight heparin and then with oral drugsAlthough there are no clinical trials, several retrospective studies support the use of anticoagulation. This is based on two retrospective studies showing zero recanalisation without anticoagulation.391,392 Recanalisation is important because lack of patency, with or without maintenance anticoagulation, is associated with the development of complications, the most common being varices formation in 25% of patients at five years. In addition, 30% of cases with varices will suffer bleeding events, as well as other complications, such as recurrent thrombosis and biliary problems.367 A prospective European multicentre study of over 100 patients with acute splenoportal axis thrombosis treated early (within 10 days) with traditional anticoagulants reported 38% with recanalisation of the portal trunk at one year. The presence of ascites and involvement of the splenic vein were associated with a lower likelihood of recanalisation.393 A recent meta-analysis including 7,969 patients (54% of them non-cirrhotic) showed a higher recanalisation rate in anticoagulated patients than in non-anticoagulated patients (57.1 vs. 22.3%), a lower risk of thrombosis progression (3.5 vs. 14.4%) and a lower mortality rate (12.2 vs. 22.6%). There was no difference in the risk of recurrence of thrombotic events or in the risk of major bleeding.394 Initiation with a therapeutic dose of low molecular weight heparin, followed by vitamin K antagonists (with a target INR of 2.0–3.0), is the strategy most recommended.
Anticoagulation should be maintained for at least six months in patients with non-cirrhotic PVTThe indication to anticoagulate for at least six months is based on evidence that vessel recanalisation is possible in this time period, although there may be cases of recanalisation occurring at 9–12 months, especially when the superior mesenteric vein and the splenic vein are involved.393 Permanent anticoagulation is associated with a lower risk of progression or recurrence of thrombosis.395 This is common if there is a known prothrombotic factor, especially if there is an underlying chronic myeloproliferative neoplasm, a history of intestinal ischaemia or previous thrombosis.357 Re-thrombosis can also occur in the absence of these factors, although it is less common.396 Therefore, anticoagulation should be maintained indefinitely if there is an underlying disease with a high thrombotic risk and considered on a case-by-case basis when there is no underlying disease.46,397
The available data suggest the benefit of DOAC treatment as long as liver function is preserved, creatinine clearance is correct and the patient does not have triple-positive antiphospholipid syndromeDOAC have the advantage of being administered orally, many require only one dose per day and they do not require monitoring.397 Retrospective observational studies suggest that DOAC achieve a higher recanalisation rate (80% at two years) than enoxaparin (43%), 4-hydroxycoumarins (28%) or untreated patients (8%), with less than 2% of patients having major bleeding events.398 Similar results were obtained in series of patients with a history of inflammatory bowel disease399 or abdominal surgery.400 A recent study in 100 patients with acute PVT showed a complete recanalisation rate of 47% and a partial recanalisation rate of 36% at three months with early administration of rivaroxaban, associated with a reduced number of bleeding events and re-thrombosis (2.1%).401 The use of DOAC in patients with antiphospholipid syndrome is discouraged, as this medication increases the risk of arterial thrombosis in these patients.
5. Aetiological diagnosisThe presence of more than one aetiological factor should be considered in any patient with a diagnosis of non-cirrhotic PVTStudies have shown that local factors (pancreatitis, intra-abdominal infections, surgery) and/or immunological, haematological or prothrombotic disorders may converge in up to one third of patients with non-cirrhotic PVT, with PVT being the first manifestation of the disease. The combination of several risk factors is more common in these patients than in those who develop venous thrombosis in other locations.402–404
Thrombophilia testing and screening for myeloproliferative neoplasia should be performed in all patients with non-cirrhotic PVTPatients with non-cirrhotic PVT frequently have been associated with infectious or inflammatory abdominal diseases (20−30%), solid abdominal neoplasms (30%) and myeloproliferative neoplasms (40%), as well as other thrombophilic (antiphospholipid syndrome) and systemic diseases (Behçet's disease, paroxysmal nocturnal haemoglobinuria). A European multicentre study in patients with non-cirrhotic VT identified that 42% of patients had a prothrombotic factor at diagnosis. This study should be performed at the time of diagnosis, as proper management of these entities influences the prognosis.393,405,406
6. Questions posed to the panel which only reached a low level of consensusFVIII levels of 150 IU/dl or above and D-dimer levels over 500 ng/mL may be helpful in identifying patients at high risk of re-thrombosis in the absence or after withdrawal of anticoagulation respectivelyIn non-cirrhotic PVT, factor VIII ≥150 IU/dl in non-anticoagulated patients acts as an independent predictor of re-thrombosis.396 In these same patients, a D-dimer ≥500 ng/mL one month after stopping anticoagulation therapy is associated with an increased risk of thrombosis recurrence46 and long-term anticoagulation could be considered in these patients.
Unfractionated heparin should be avoided in patients with non-cirrhotic PVT or BCS because of the associated risk of thrombocytopeniaHeparin-induced thrombocytopenia is a rare condition with an incidence of 5%, but it can have serious consequences, including thrombosis (30–50% of cases) and death. It occurs when platelet factor 4, which is released from the alpha granules of platelets, combines with heparin and produces complexes that activate antibodies, which in turn activate platelets and promote thrombin formation.407 This condition has been observed in subjects with myeloproliferative neoplasms and has been described with all forms of heparin. A meta-analysis of studies on the prevention of thrombotic events in trauma indicates that the risk of thrombocytopenia is 10 times higher with unfractionated heparin than with low molecular weight heparin (2.6 vs. 0.2%).408
In a series of 51 patients with BCS,409 the incidence of heparin-induced thrombocytopenia was found to be 14%. In more recent retrospective studies, an even higher incidence has been observed, especially in patients with BCS, but also in patients with PVT and myeloproliferative neoplasms.410 It is important to be highly suspicious of thrombosis in patients receiving heparin therapy and presenting with symptoms of thrombocytopenia. In such cases, argatroban may be used to treat the condition.
In patients with non-cirrhotic PVT who do not have an identified aetiological factor, assessment of next generation sequencing techniques is recommendedNext-generation sequencing performs massive DNA sequencing, allowing the simultaneous evaluation of multiple genes even at very low mutational levels. In a study of 80 non-cirrhotic PVT patients with no prothrombotic factor identified by conventional techniques (negative for JAK2 [V617F and exon 12], calreticulin gene and thrombopoietin gene), next-generation sequencing identified a mutation in exon 12 of JAK2 in 40% of cases. Some 37.8% of patients with a local aetiological factor had at least one high molecular risk variant, the presence of which was associated with an increased risk of thrombotic recurrence on withdrawal of anticoagulation. Therefore, the application of these techniques makes it possible to identify with greater precision, on the one hand, those patients with a myeloproliferative disorder and, on the other, those with a higher risk of recurrence after discontinuation of anticoagulant therapy.352
Edilmar Alvarado-Tapiasd, Javier Ampueroe, Anna Baigesc, Pablo Bellotf, José Luis Callejag, Andrés Cárdenac, María-Vega Catalinab, Àngels Escorselld, José Ignacio Forteah, Juan Carlos García-Paganc, Juan Genescài, Manuel Hernández-Guerraj, Luis Ibáñez-Samaniegob, Sabela Lensc, Elba Llopg, Macarena Simon-Taleroi, Rosa Martín-Mateosa, Mónica Ponsi, Elisa Posec, Angela Puenteh, Enric Reverterc, Diego Rincónb, Miguel Ángel Rodríguez-Gandíaa, Luis Télleza, Fanny Turónc, Cándido Villanuevad
a Department of Gastroenterology and Hepatology, Hospital Universitario Ramón y Cajal, Instituto Ramón y Cajal de Investigación Sanitaria (IRYCIS) [Ramón y Cajal Institute for Health Research], Universidad de Alcalá, Centro de Investigación Biomédica en Red en Enfermedades Hepáticas y Digestivas (CIBEREHD) [Biomedical Research Networking Centre for Liver and Gastrointestinal Diseases], Madrid, Spain
b Department of Gastrointestinal Medicine, Hospital General Universitario Gregorio Marañón, Instituto de Investigación Sanitaria (IISGM) [Gregorio Marañón Institute for Health Research], Universidad Complutense, Centro de Investigación Biomédica en Red en Enfermedades Hepáticas y Digestivas (CIBEREHD), Madrid, Spain
c Department of Hepatology, Hospital Clínic, Institut de Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS) [August Pi i Sunyer Biomedical Research Institute], Universidad de Barcelona, Centro de Investigación Biomédica en Red en Enfermedades Hepáticas y Digestivas (CIBEREHD), Barcelona, Spain
d Department of Gastrointestinal Diseases, Hospital Santa Cruz y San Pablo, Institut de Recerca Sant Pau (IIB Sant Pau) [Sant Pau Research Institute], Universidad Autónoma de Barcelona, Centro de Investigación Biomédica en Red en Enfermedades Hepáticas y Digestivas (CIBEREHD), Barcelona, Spain
e Department of Gastroenterology, Hospital Universitario Virgen del Rocío, Instituto de Biomedicina de Sevilla (IBIS) [Seville Institute of Biomedicine], Universidad de Sevilla, Centro de Investigación Biomédica en Red en Enfermedades Hepáticas y Digestivas (CIBEREHD), Seville, Spain
f Department of Gastroenterology, Hepatology Unit, Hospital General Universitario de Alicante Dr. Balmis, Alicante, Spain
g Department of Gastroenterology and Hepatology, Hospital Universitario Puerta de Hierro, Instituto de Investigación Sanitaria Puerta de Hierro-Segovia de Arana (IDIPHISA) [Puerta de Hierro-Segovia de Arana Institute for Health Research], Centro de Investigación Biomédica en Red en Enfermedades Hepáticas y Digestivas (CIBEREHD), Madrid, Spain
h Department of Gastroenterology and Hepatology, Hospital Universitario de Valdecilla, Grupo de Investigación Clínica y Traslacional en Enfermedades Digestivas, Instituto de Investigación de Valdecilla (IDIVAL) [Clinical and Translational Research Group in Gastrointestinal Diseases, Valdecilla Research Institute], Santander, Cantabria, Spain
i Department of Hepatology, Gastrointestinal Diseases Area, Hospital Universitari Vall d'Hebron, Vall d'Hebron Institut de Recerca (VHIR) [Vall d'Hebron Research Institute], Universitat Autònoma de Barcelona, Centro de Investigación Biomédica en Red en Enfermedades Hepáticas y Digestivas (CIBEREHD), Barcelona, Spain
j Department of Gastroenterology, Hospital Universitario de Canarias, Instituto Universitario de Tecnologías Biomédicas (ITB) [University Institute of Biomedical Technologies] & Centro de Investigación Biomédica de Canarias (CIBICAN) [Canary Islands Biomedical Research Centre], Universidad de La Laguna, Tenerife, Santa Cruz de Tenerife, Spain