Transjugular intrahepatic portosystemic shunts (TIPS) are successfully used in the management of portal hypertension (PH)-related complications. Debate surrounds the diameter of the dilation. The aim was to analyse the outcomes of and complications deriving from TIPS in patients with cirrhosis and identify predictors of survival.
MethodsThis was a retrospective single-centre study, which included patients with cirrhosis who had a TIPS procedure for PH from 2009 to October 2018. Demographic, clinical and radiological data were collected. The Kaplan–Meier method was used to measure survival and predictors of survival were identified with the Cox regression model.
ResultsA total of 98 patients were included (78.6% male), mean age was 58.5 (SD±/−9.9) and the median MELD was 13.3 (IQR 9.5–16). The indications were refractory ascites (RA), variceal bleeding (VB) and hepatic hydrothorax (HH). Median survival was 72 months (RA 46.4, VB 68.5 and HH 64.7) and transplant-free survival was 26 months. Clinical and technical success rates were 70.5% and 92.9% respectively. Age (HR 1.05), clinical success (HR 0.33), sodium (HR 0.92), renal failure (HR 2.46) and albumin (HR 0.35) were predictors of survival. Hepatic encephalopathy occurred in 28.6% of patients and TIPS dysfunction occurred in 16.3%.
ConclusionsTIPS with 10-mm PTFE-covered stent is an effective and safe treatment for PH-related complications in patients with cirrhosis. Age, renal failure, sodium, albumin and clinical success are independent predictors of long-term survival.
Los shunt intrahepático porto-sistémicos (TIPS) son utilizados con éxito en el tratamiento de las complicaciones de la hipertensión portal (HTP). Existe cierta controversia referente al diámetro dilatado. Los objetivos fueron analizar los resultados y las complicaciones derivadas de los TIPS en cirróticos, y determinar los factores predictores de la supervivencia.
MétodosSe trata de un estudio retrospectivo unicéntrico que incluyó pacientes cirróticos que recibieron un TIPS por HTP desde 2009 a octubre-2018. Se recogieron variables clínicas, demográficas y radiológicas. Se determinó la supervivencia mediante el método Kaplan-Meier y se identificaron los predictores de supervivencia con el modelo de regresión de Cox.
ResultadosSe incluyeron 98 pacientes (78,6% varones). La media de edad fue de 58,5 años (DE ±9,9) y mediana de MELD 13,3 (RIC 9,5-16). Las indicaciones fueron ascitis refractaria (AR), hemorragia varicosa (HV) e hidrotórax hepático (HH). La mediana de supervivencia fue de 72 meses (AR 46,4; HV 68,5 y HH 64,7 meses) y la supervivencia libre de trasplante fue de 26 meses. El éxito técnico y clínico fue del 92,9 y 70,5%, respectivamente. La edad (HR 1,05), el éxito clínico (HR 0,33), el sodio (HR 0,92), la disfunción renal (HR 2,46) y la albúmina (HR 0,35) fueron factores predictivos de supervivencia. El 28,6% desarrolló encefalopatía hepática y un 16,3% presentó disfunción del TIPS.
ConclusionesLos TIPS con prótesis recubiertas dilatadas a 10mm son un tratamiento efectivo y seguro de las complicaciones derivadas de HTP en pacientes cirróticos. La edad, la disfunción renal, el sodio, la albúmina y el éxito clínico son factores independientes predictivos de la supervivencia a largo plazo.
Liver cirrhosis may lead to the development of complications of portal hypertension (PH)1 including variceal bleeding (VB) from esophageal or gastric varices and refractory ascites (RA). They represent the main causes of hospital admission in cirrhotic patients.
Transjugular intrahepatic portosystemic shunt (TIPS) has been used for more than 30 years as alternative option to medical and surgical therapy for these PH complications.2 Most clinicians agree that TIPS is an effective option to control refractory VB3,4 and more recently, studies have evaluated the use of early TIPS in high risk patients.5–8 Due to the circulatory mechanism of the PH, TIPS is an interesting option in the management of the RA9,10 and Type 2 hepatorrenal syndrome. On the other hand, TIPS insertion has been used to decompress the portal circulation in patients with vascular disease.11
Currently, the indications of TIPS are well established and therefore, the American Association for the Study of Liver Diseases and European Association for the Study of the Liver guidelines, give definitive recommendations about in whom and when TIPS should be inserted.12–15
Since 2004, Polytetrafluoroethylene (PTFE)-covered stent has become the preferred option for TIPS,16–19 due to a lower rate of stent dysfunction than bare stent.20,21 The elective diameter of the endoprosthesis remains controversial.22,23
In a retrospective single centre study, we evaluated the technical success, efficacy, dysfunction rate and safety of 10-mm covered TIPS in the treatment of portal hypertension related complications in cirrhotic patients. The secondary objectives were to perform subgroup analyses of the survival according to TIPS indications, to determine the predictors of survival and the transplant-free survival (TFS) rate.
Patients and methodsPatients and study designThis retrospective single-center study was conducted at a tertiary-care center. The study was performed according to the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) statement.24
All adult patients (age≥18 years) who underwent a TIPS procedure between 2009 and October-2018 were analyzed. Eligible patients had an established diagnosis of liver cirrhosis and received TIPS as treatment for refractory PH-related complications: RA, refractory hepatic hydrothorax and secondary prophylaxis of VB (after failed combination therapy, pharmacological and endoscopic therapy).
Clinical, laboratory findings and technical data of the TIPS procedure and any re-interventions were collected from the medical histories of the patients. The study protocol was approved by the local Ethic Committee.
Primary end point was to determine the indications, clinical outcomes, rate of complications and clinical and technical success. To identify predictors of survival after TIPS implantation and the TFS rate were considered as secondary end-points.
DefinitionsAccording to Heinzow et al.,25 the following definitions were used:
Refractory ascites (RA): Ascites which cannot be reduced by low-sodium diet and maximal doses of diuretics (400mg spironolactone and 160mg furosemide per day) or treatment-induced complications.
Recurrent VB: VB which did not respond to pharmacological and endoscopy therapy or rebleeding occurred five days after the first VB.
Rescue TIPS: Stent implantation directly associated with the refractory bleeding event (within 3 days) according Bucsics et al.4
Elective TIPS implantation: Stent Implantation occurred after 3 days of bleeding.
Stent Dysfunction was defined as a reduction of diameter of stent ≥50% or reoccurrence of complications of portal hypertension.
Clinical success: Symptom control (Ascites which responds to low doses of pharmacological treatment without paracentesis and lack of VB).
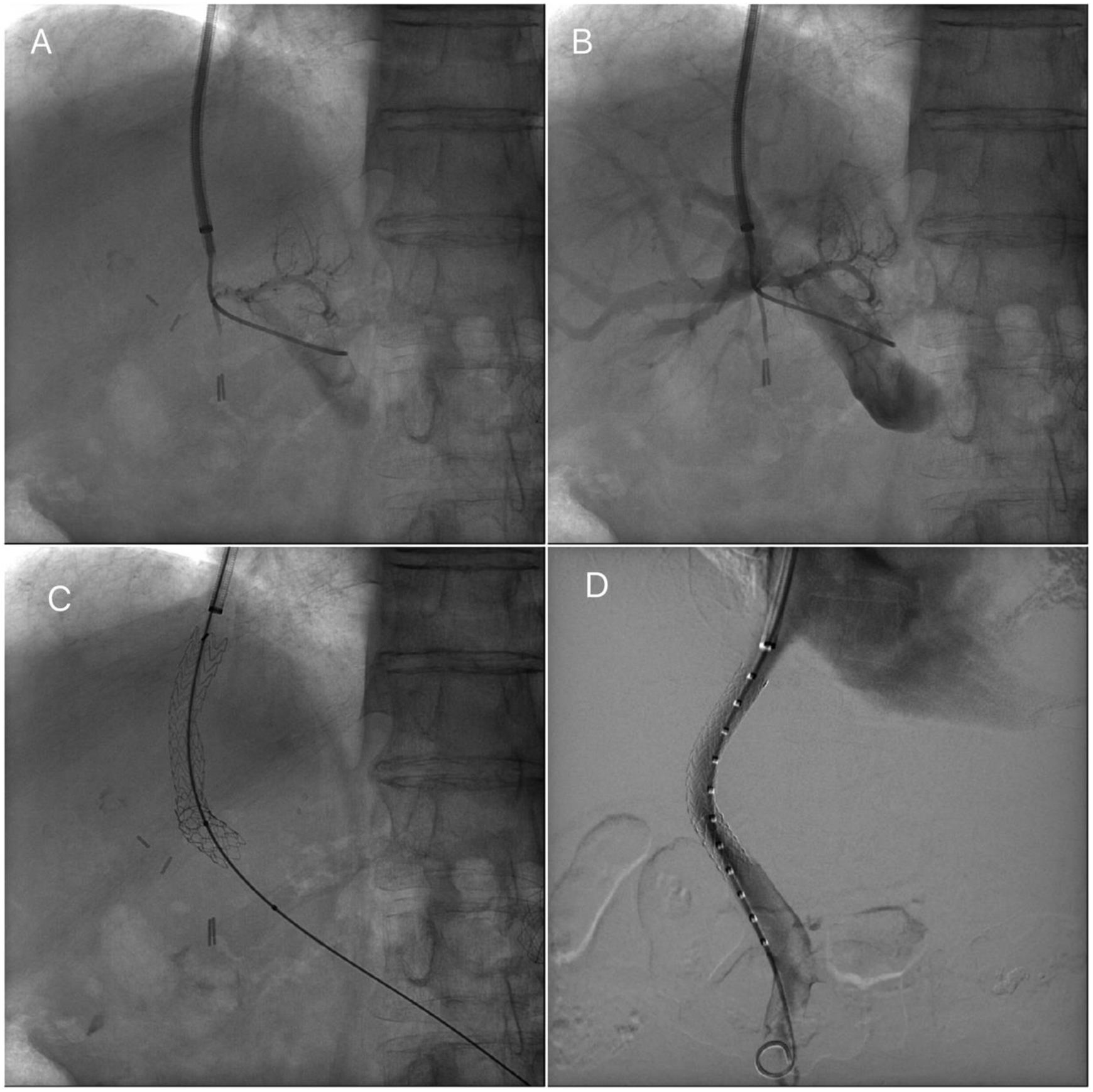
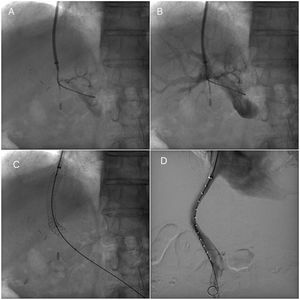
Transjugular intrahepatic portosystemic stent procedureTIPS procedures were conducted by vascular and interventional radiologists and were submitted by the Hepatology unit. The TIPS procedure was performed under sedation controlled by anesthetists. Through a transjugular venous approach, the hepatic vein was punctured and shunt was performed with e-PTFE-covered stents insertion (Viatorr Controlled Expansion Endoprosthesis, Gore) and was dilated to 10mm (Internal diameter was 8–10mm, graft lined length was 4–8cm and graft unlined length was 2cm). In Fig. 1, fluoroscopic image shows TIPS place procedure. Post-intervention Doppler ultrasonography was carried out the day after TIPS implantation to assess stent patency and rule out any complications.
Transjugular intrahepatic portosystemic shunt (TIPS) procedure. (A) Portal venogram after successful puncture from the hepatic vein. (B) Portal venogram after catheter has been advanced into the main portal vein shows normal portal vein bifurcation and intrahepatic branches. (C) Fluoroscopic image demonstrates full deployment of the stent graft. (D) Fluoroscopic image shows flow into the liver and through the stent graft.
Platelets or plasma were administered when platelet counts were below 50×109/L and INR>1.5 (Prothrombin time test<50%) respectively.
All patients were followed up with Doppler ultrasonography at the beginning and every 6 months after the procedure until the OLT, death or the last clinical assistance or recurrence of symptoms of PH was presented (New episodes of VB, moderate ascites or hepatic hydrothorax). If the Doppler ultrasound identified TIPS dysfunction, angioplasty was performed or another stent was inserted.
Primary prophylaxis to prevent the development of hepatic encephalopathy (HE) was done by nonabsorbable disaccharides such as lactulose.
ParametersThe medical records were reviewed by trained physicians. Demographic characteristics included age, date and sex. Comorbidities were recorded as ordinal (American Society of Anesthesiologists physical status classification [ASA]), etiology of liver disease (Alcohol, chronic viral hepatitis B/C, non-alcoholic fibrosis liver disease [NAFLD], or others), hospitalization and medication (Type and doses of diuretics) were collected. TIPS indication included VB, RA and refractory hepatic hydrothorax.
Clinical variables (Grade of ascites and HE according to West Haven criteria), laboratory findings (Creatinine, platelet count, international normalized ratio [INR] and levels of transaminases [AST and ALT], albumin, serum sodium and bilirubin) before TIPS implantation were recorded. Model of End Stage Liver Disease (MELD) and Child–Pugh score evaluated the severity of liver disease. Any complications as cardiac heart failure or HE post-TIPS and new episodes of VB during the follow-up time were documented.
Statistical analysisDescriptive statistics were used to report characteristics of patients. Normally distributed values were shown as mean±standard deviation (SD), otherwise as median and interquartile range (IQR). Quantitative variables were compared using Student's t-test and qualitative variables were compared using the X2 test. All possible independent predictive factors of survival were analyzed using univariate and multivariate analysis with a Cox proportional hazards regression model.
Overall survival after TIPS implantation, transplant-free survival (TFS) rate and development of complications were analyzed using Kaplan–Meier method. Statistical analyses were performed using IBM SPSS Statistic for Windows, (Version 24.0. IBM Corp, NY, USA). P-values<0.05 was considered statistical significance.
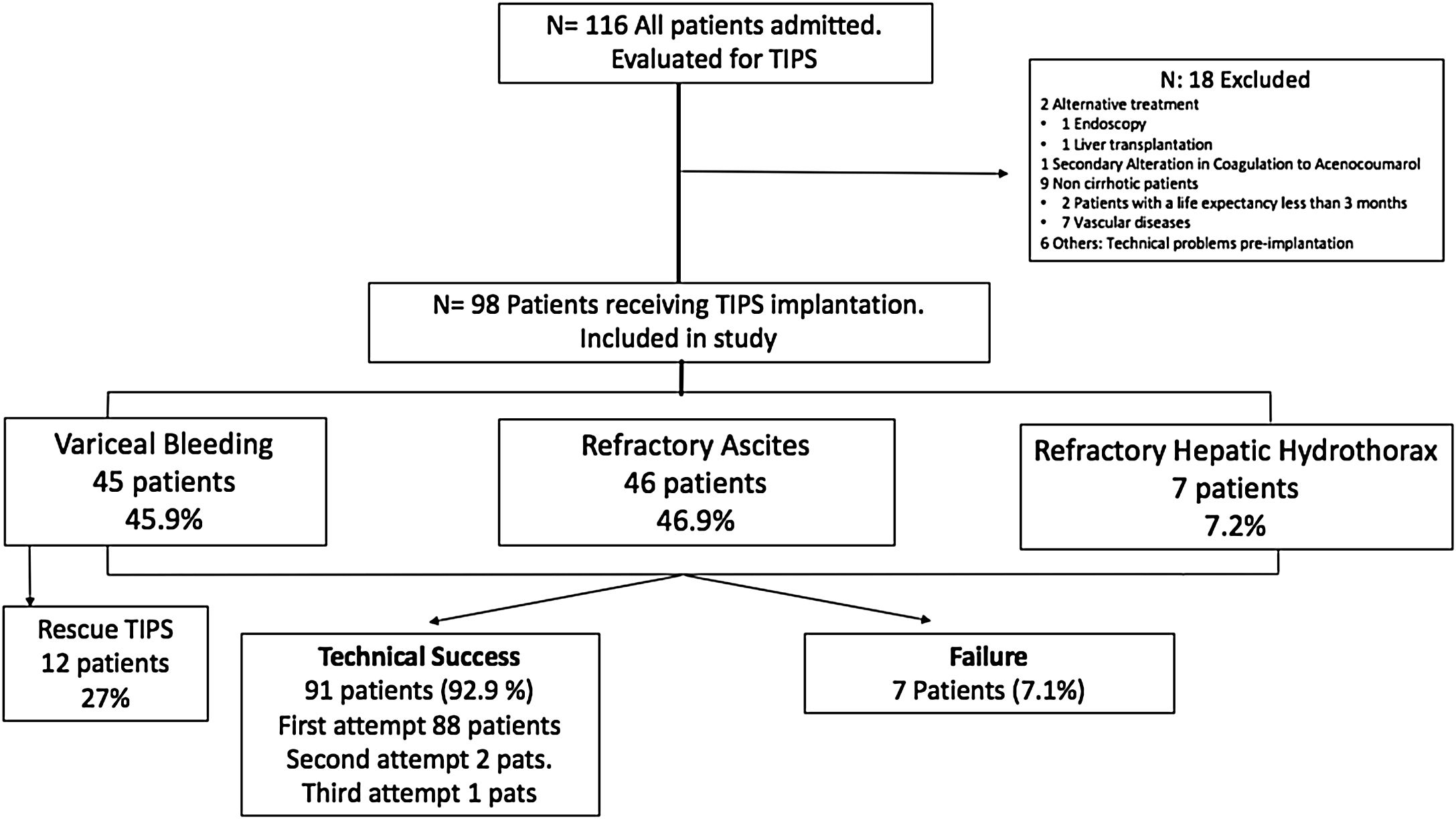
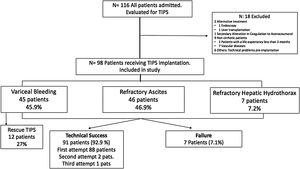
ResultsPatient characteristicsDuring the study period, a total of 116 adult patients were initially scheduled for TIPS implantation. Finally, 98 patients were enrolled from 2009 until October-2018 with a mean age of 58.5±9.9 years (range 32–82 years), 78.6% men. Eighteen patients were excluded of the analysis (Fig. 2).
Since 2009, PTFE-covered stents were implanted and dilated using 10mm diameter balloons. The most frequent etiology of cirrhosis was alcoholic liver disease (ALD) (66.3%), followed by chronic viral hepatitis (VHC and VHB) in 13.3% and NAFLD in 14.3%. Overall, 23.4% of the patients were Child A, 65.3% and 11.2% were Child B and C, respectively. The median MELD score was 13.3 (IQR 9.5–16). The most common indications for TIPS implantation were RA (46.9%), VB (45.9%) and refractory hepatic hydrothorax (7.1%). The baseline characteristics of the study population (total and subgroups according to TIPS indication) are summarized in Table 1.
Baseline patient characteristics.
| Baseline characteristics | Total | Variceal bleeding | Refractory ascites | Hepatic hydrothorax |
|---|---|---|---|---|
| Patients (N) | 98 | 45 | 46 | 7 |
| Age | ||||
| Mean±SD | 58.5±9.9 | 58.7±9.5 | 58.9±11.0 | 57.4 |
| Range | 32–82 | 47–80 | 43–80 | 50–64 |
| Sex (male/female) | 77/21 | 39/6 | 32/14 | 6/1 |
| Admission time period | ||||
| 2009–2013 | 36 (36.7%) | 14 (31.1%) | 17 (36.9%) | 5 (71.4%) |
| 2014–2018 | 62 (63.3%) | 31 (68.9%) | 29 (63.1) | 2 (28.6%) |
| Rescue TIPS | 13 (19.4%) | 9 (20%) | 4 (8.7%) | 0 |
| Child–Pugh score | ||||
| A | 23 (23.4%) | 17 (37.8%) | 3 (6.5%) | 3 (42.9%) |
| B | 64 (65.3%) | 24 (53.3%) | 37 (80.5%) | 3 (42.9%) |
| C | 11 (11.2%) | 4 (8.9%) | 6 (13%) | 1 (14.2%) |
| MELDascore | 13 (IQR 10–16) | 12.5 (IQR 10–15) | 13.2 (IQR 9–17) | 15 (IQR 11–20) |
| Etiology of cirrhosis | ||||
| Alcohol | 65 (66.3%) | 31 (68.9%) | 29 (63%) | 5 (71.4%) |
| Chronic viral hepatitis B/C | 13 (13.3%) | 4 (8.9%) | 7 (15.2%) | 2 (28.6%) |
| NAFLDb | 14 (14.3%) | 6 (1.3%) | 8 (17.4%) | – |
| Others | 6 (6.1%) | 4 (8.9%) | 2 (4.3%) | – |
| Ascites | ||||
| No | 26 (26.5%) | 25 (55.6%) | – | 1(14.2%) |
| Grade I | 6 (6.1%) | 4 (8.9%) | – | 2 (28.6%) |
| Grade II | 20 (20.4%) | 12 (26.7%) | 6 (13%) | 2 (28.6%) |
| Grade III | 46 (46.1%) | 4 (8.9%) | 40 (87%) | 2 (28.6%) |
| Hepatic encephalopathy | ||||
| No | 77 (78.6%) | 39 (86.7%) | 32 (69.6%) | 6 (85.8%) |
| Grade I–II | 21 (21.4%) | 6 (13.3%) | 14 (30.4%) | 1 (14.2%) |
| ASAT (U/L)c | 51.8±41.2 | 55.3±46.3 | 49.2±39.8 | 52.4±35.8 |
| ALAT (U/L)c | 39.8±36.7 | 43.4±37.2 | 37.2±20 | 31.4±23.3 |
| Serum albumin (g/l) | 3.3±0.6 | 3.1±0.6 | 3.2±0.4 | 3.8±0.9 |
| INR (mg/dL)d | 1.3±0.2 | 1.3±0.2 | 1.2±0.2 | 1.4±0.4 |
| Platelets×103/μL | 112.9±90.4 | 92±49.2 | 109.1±7.7 | 160±163 |
| Serum bilirubin (mg/dL) | 1.9±1.9 | 1.8±1.2 | 1.5±1.6 | 1.8±0.7 |
| Creatinine (mg/dL) | 1.1±0.5 | 0.9±0.3 | 1.18±0.6 | 1.1±0.4 |
| ASA score | ||||
| ASA II | 7 (6.7%) | 3 (6.8%) | 2 (4.3%) | 1 (14.2%) |
| ASA III | 73 (69.5%) | 31 (70.5%) | 35 (74.4%) | 3 (42.9%) |
| ASA IV | 25 (23.8%) | 10 (22.7%) | 10 (21.3%) | 3 (42.9%) |
ASA score: American Society of Anesthesiologists score.
Elective TIPS implantation was performed in 85 patients (86.7%) and 13 patients (13.3%) underwent rescue TIPS implantation due to refractory VB. Portal vein thrombosis was presented in 10 (10.2%) patients before TIPS implantation.
Platelets or fresh frozen plasma were transfused previous or during the TIPS procedure in 35 (34.3%; platelets 17.7% and red cell concentrates or fresh frozen plasma 32.3%) patients. Median hospitalization time after TIPS implantation was 2 days (IQR 1–7 days).
Outcome, mortality and transplant-free survivalThe median follow-up was 510 days (IQR 90–1575 days). During this period, 34 patients (34.7%) died, 4 patients (3.8%) were lost and 18 patients (18.4%) underwent OLT. The technical success was achieved in 92.9%; 88 patients reached technical success on first attempt, and 2 and 1 patients on second and third attempt, respectively; due to difficulty to access the portal vein. The most frequent technical failures were portal vein thrombosis (n=5).
During the follow up, TIPS dysfunction occurred in 12 patients (11.4%). The overall clinical success was achieved in 70.5% and the re-bleeding rate was 10.2% in the group of patients who received TIPS for VB. Ascites was moderate in 27.6% of the patients.
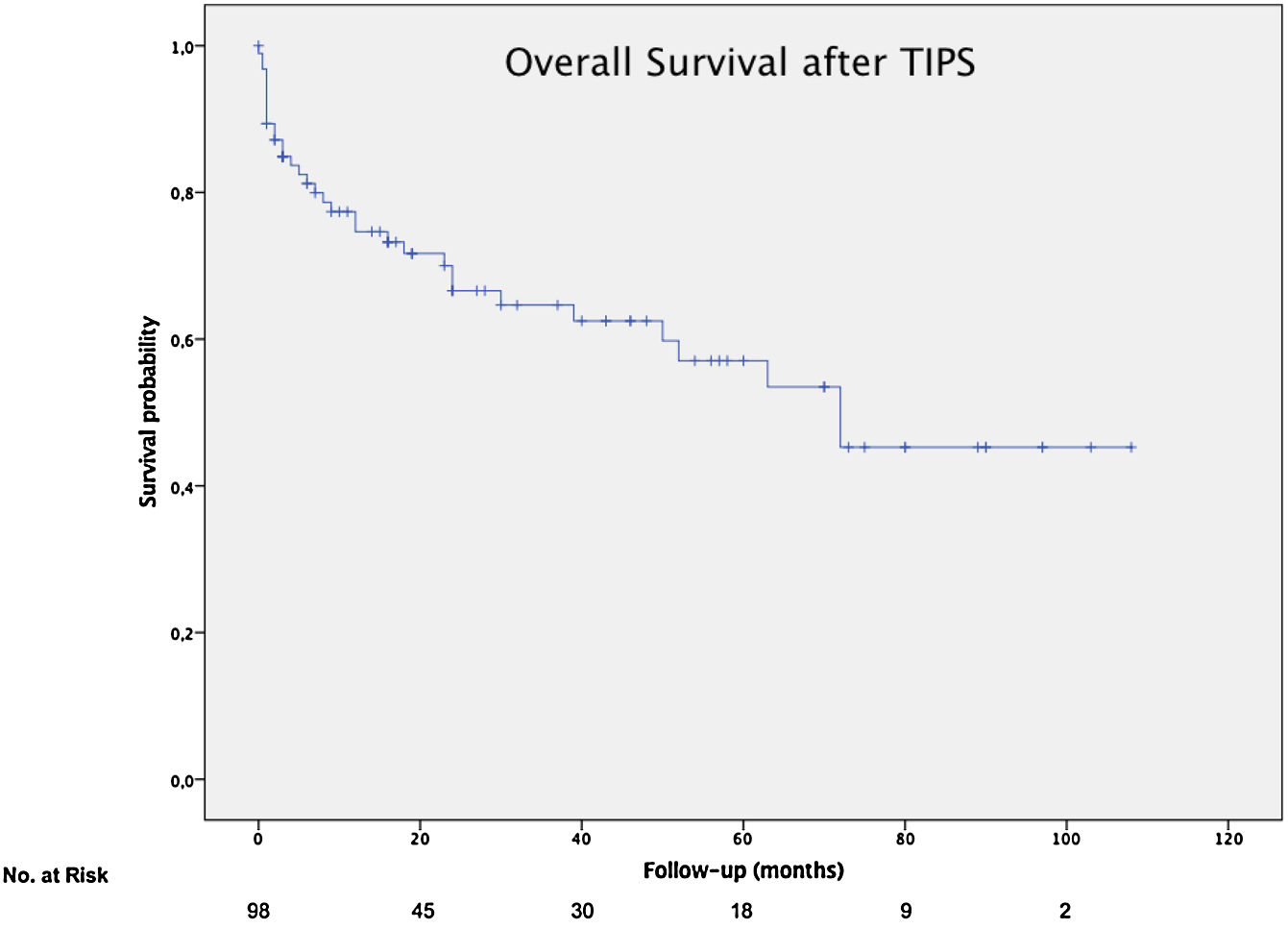
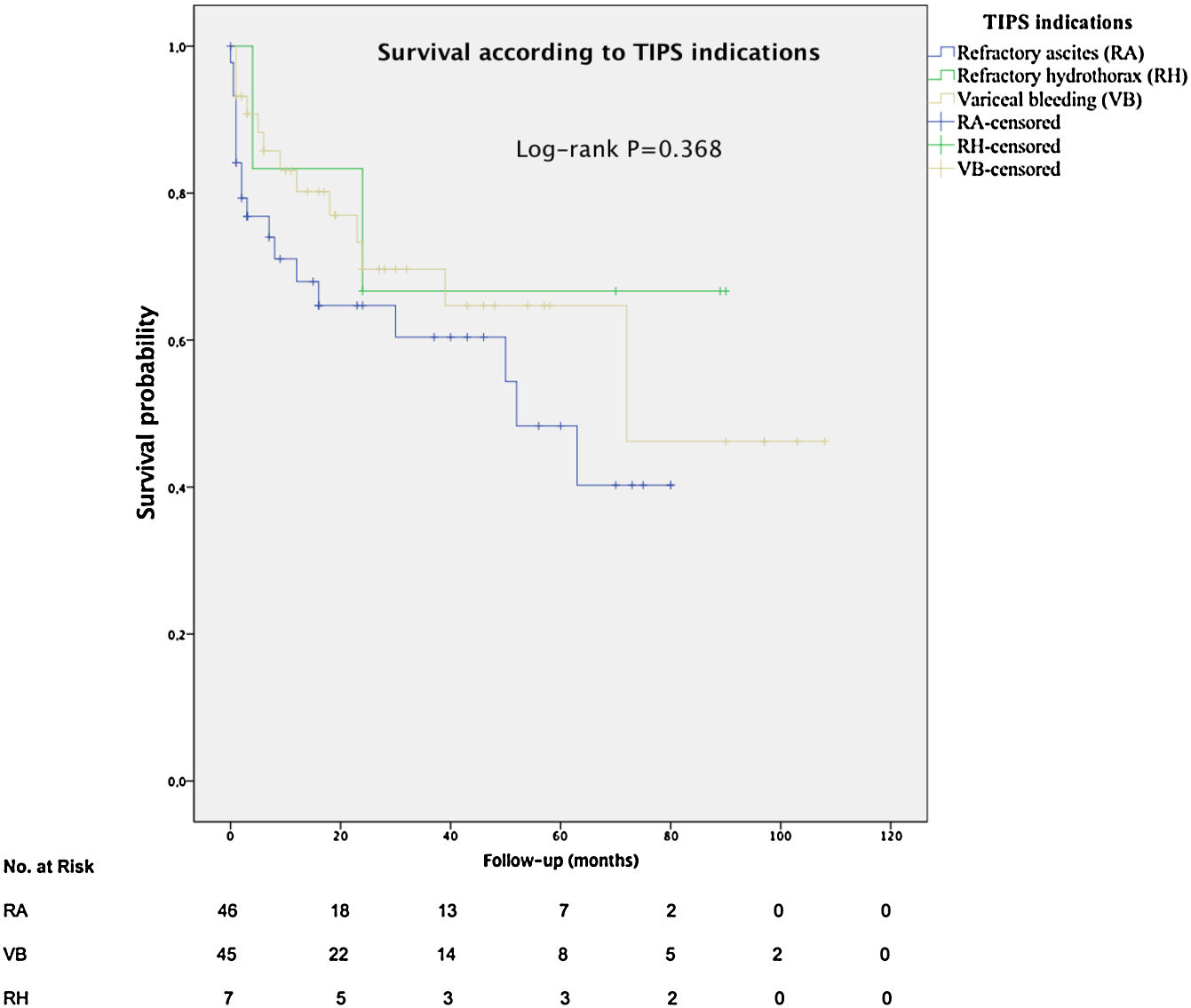
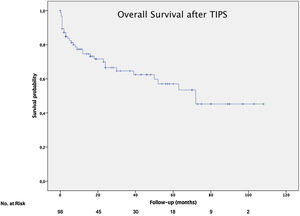
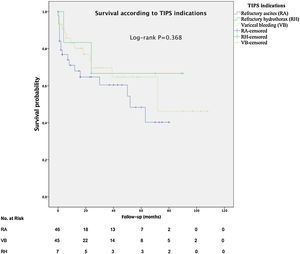
The mean overall survival was 72 months (SD±5.65) (Fig. 3), according to the TIPS indications: 46.4 months (SD±5.78) for RA, 68.5 months (SD±8.1) for VB and 64.7 months (SD±14.82) for refractory hydrothorax (Fig. 4). When patients who underwent OLT were excluded, the median survival rate according to indication was as following: 16 months (IQR 1.5–51) for RA, 18 months (IQR 6–48.5) for VB [16 months (IQR 2–21) for rescue TIPS], and 70 months (IQR 50.4–89) for refractory hydrothorax. The median of TFS rate was 26 months. Furthermore, the median survival rate was longer in patients with VB and refractory hepatic hydrothorax than patients with RA. However, this difference was not statistically significant (68.5mo, 64.7mo and 46.4mo, P=0.368).
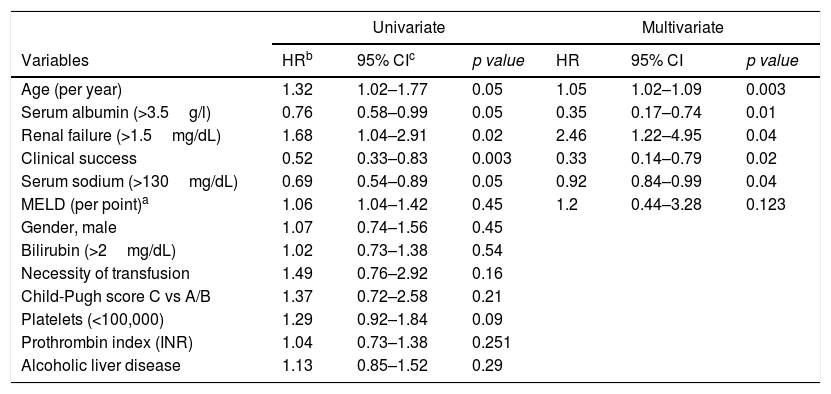
Age, clinical success, serum sodium (>130mg/dL), serum albumin (>3.5g/L) and renal failure (Creatinine>1.5mg/dL) were significantly associated with overall survival in univariable and multivariable analysis (Table 2).
Risk factors for overall survival in patients with TIPS.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Variables | HRb | 95% CIc | p value | HR | 95% CI | p value |
| Age (per year) | 1.32 | 1.02–1.77 | 0.05 | 1.05 | 1.02–1.09 | 0.003 |
| Serum albumin (>3.5g/l) | 0.76 | 0.58–0.99 | 0.05 | 0.35 | 0.17–0.74 | 0.01 |
| Renal failure (>1.5mg/dL) | 1.68 | 1.04–2.91 | 0.02 | 2.46 | 1.22–4.95 | 0.04 |
| Clinical success | 0.52 | 0.33–0.83 | 0.003 | 0.33 | 0.14–0.79 | 0.02 |
| Serum sodium (>130mg/dL) | 0.69 | 0.54–0.89 | 0.05 | 0.92 | 0.84–0.99 | 0.04 |
| MELD (per point)a | 1.06 | 1.04–1.42 | 0.45 | 1.2 | 0.44–3.28 | 0.123 |
| Gender, male | 1.07 | 0.74–1.56 | 0.45 | |||
| Bilirubin (>2mg/dL) | 1.02 | 0.73–1.38 | 0.54 | |||
| Necessity of transfusion | 1.49 | 0.76–2.92 | 0.16 | |||
| Child-Pugh score C vs A/B | 1.37 | 0.72–2.58 | 0.21 | |||
| Platelets (<100,000) | 1.29 | 0.92–1.84 | 0.09 | |||
| Prothrombin index (INR) | 1.04 | 0.73–1.38 | 0.251 | |||
| Alcoholic liver disease | 1.13 | 0.85–1.52 | 0.29 | |||
a MELD: Model for End-Stage Liver Disease score.
The incidence of new episodes or worsening of HE at six months after TIPS implantation was 28.6%. Most of patients who presented HE were grade 1 or 2 and improved with administration of oral lactulose; although, less than 5% patients underwent TIPS reduction to 8-mm in diameter due to refractory or grade≥3 HE. No patients developed symptomatic heart failure after TIPS. Other procedure-related complications were less frequent: Mild intraperitoneal bleeding 8.6%, Technical complications (Portal vein dissection) 1.9%, infection of TIPS 2%, stent migration or placement into inferior vena cava or portal vein 1.8%, acute-on-chronic liver failure 1.8%, hemobilia<1% and others 14.4%.
Thrombosis and TIPS dysfunction. AngioplastyDuring follow up, 16 patients (16.3%) developed TIPS dysfunction due to partial thrombosis or TIPS occlusion/stenosis. The management of these adverse events were anticoagulation therapy in 7 patients, angioplasty or coaxial stent in 3 patients, thrombectomy in 6 patients. Finally, resolution of TIPS dysfunction was reported in 9 (56%) cases.
DiscussionThis is a large study with a real-life cohort of a consecutive series of cirrhotic patients with PH related complications, which evaluates the role of 10-mm ePTFE-covered TIPS in the clinical practice. The baseline characteristics show a heterogeneous population with a typical distribution for western countries, where the most frequent TIPS indications were VB and RA. Nowadays, in the era of self-expanding or controlled expansion covered stents, the TIPS dysfunction is not a big issue. Current challenge aim at further improving the long-term clinical outcomes, at preventing variceal rebleeding/refractory ascites, with low incidence of encephalopathy and liver failure. However, the selection of the stent diameter to correct complications of PH and to avoid HE is controversial. The available evidence is limited.
We confirmed a high technical and clinical success rate in 92.9% and 70.5%, respectively; after a median follow-up of 510 days. According to recent studies,22,26,27 complications related to TIPS insertion were variable; TIPS dysfunction (16.3%) and new or worse HE (28.6%) were the most frequent adverse events. We show that 10-mm stent is not associated with a high overt HE rate and low rate of TIPS dysfunction. Another rare adverse events as hemobilia, TIPS infection, intraperitoneal bleeding, acute-on-chronic liver disease, renal failure and cerebral edema have been reported but any patient did not develop symptomatic heart failure after TIPS. On average, the number of TIPS procedures is increasing in our hospital, between the period of 2009–2018, at 15–20 per year; so this might decrease the risk for complications.28
In contrast to Heinzow et al.25 and Membreno et al.,29 non significant difference was observed in terms of mean survival in patients with TIPS due to VB, refractory hepatic hydrothorax or RA (68.5mo, 64.7mo and 46.7mo, P=0.368). Probably, due to almost 50% of the patients with VB (44.4%) also had ascites. The high survival rate for patients with refractory hydrothorax might be explained by the small number of patients (7/98) in this group with basal MELD >14, so patients with most advanced cirrhosis could have been transplanted. Besides, we detected that age, renal failure, serum sodium, serum albumin and a clinical success were independent predictors of survival in cirrhotic patients after TIPS implantation, suggesting that younger patients with better liver function may respond better to TIPS. This is consistent with Kim et al.30
Recently, García-Pagan et al.6 demonstrated that patients with a high risk of VB had significantly improved short-term and medium-term survival when TIPS was performed early, highlighting the importance of timing in some cases of VB.
This study has some limitations. The retrospective nature of this study limits our data recording to the available medical records and documentation. For instance, portal-pressure gradient was not performed in the routine clinical practice. Despite, database being thoroughly screened, we cannot exclude some degree of underreporting due to inherent limitations of non-standardized clinical documentations. In order to avoid selection bias and represent clinical practice, all the patients who received TIPS were included. Secondly, our study was conducted at a single OLT centre with a short waiting list for OLT (average wait list is less than 3 months) with a relatively small sample size; it should be considered when extrapolating our results to other populations. Thirdly, no patient with early-TIPS were included because this strategy had not been standardized in our center; Probably, these results would have been better if the strategy of early-TIPS had been performed. Future comparative studies using 10-mm PTFE-covered stents may be required to validate our long-term results using a larger multicenter cohort.
In conclusion, the results of our retrospective study confirm the effectiveness and safety of 10-mm PTFE-covered stents for the management of PH related complications of PH, specially RA and variceal re-bleeding. Furthermore, the multivariable analysis identified age, renal failure, serum sodium, serum albumin and a clinical response as independent predictors of survival in patients with cirrhosis who underwent TIPS implantation with 10-mm covered stent.
FundingNo financial support was received.
Conflict of interestThe authors declare that they have no conflict of interest.