Healthcare professionals in endoscopy units have a possible risk of SARS-CoV-2 infection by different routes: inhalation of airborne droplets, aerosols, conjunctival contact and faecal-oral transmission.
ObjectiveTo describe the detection of SARS-CoV-2 in a series of patients scheduled for digestive endoscopy at the Hospital Santa Caterina, Salt, (Girona).
MethodsDescriptive study of a series of cases of patients scheduled for endoscopy during the month of May 2020, when endoscopic activity was resumed after the peak of the pandemic, following SCD, SEED, AEG and ESGE recommendations. We examined nasopharyngeal samples 48−72 h before the appointment, by RT-PCR, in all patients. RNA extraction was performed using the kits: Qiagen®-adapted, BiosSprint®96-DNA-Blood-Kit (384). For amplification-detection of SARS-CoV-2, methods recommended by the WHO and the CDC were followed.
Results110 asymptomatic patients without close contact with a positive case in the previous 14 days were scheduled; 105 (96.4%) were negative and five (4.5%) were positive. Two patients developed respiratory symptoms after diagnosis (presymptomatic) and three remained asymptomatic. Allfive5 patients were autochthonous cases with no history of travel or residence in another city or country associated with high prevalence of infection. Four cases were women aged 60–81 years. The N gene was detected in all cases.
ConclusionsA high prevalence of SARS-CoV-2 infection was detected in patients scheduled for digestive endoscopy. Given the risk of transmission to professionals, we consider it advisable to perform SARS-CoV-2 RT-PCR 48−72 h before the examination in situations of high incidence in the population.
Los profesionales de la salud de las unidades de endoscopia tienen un posible riesgo de infección por SARS-CoV-2 por diferentes vías: inhalación de gotitas en el aire, aerosoles, contacto conjuntival y transmisión fecal-oral.
ObjetivoDescribir la detección del SARS-CoV-2, en una serie de pacientes, programados para endoscopia digestiva en el Hospital Santa Caterina. Salt. (Girona).
MétodosEstudio descriptivo de una serie de casos de pacientes programados para endoscopia durante el mes de mayo de 2020, en el reinicio de la actividad endoscópica después del pico pandémico, siguiendo las recomendaciones de SCD, SEED, AEG y ESGE. Examinamos muestras nasofaríngeas 48−72 horas antes de la cita, mediante RT-PCR a todos los pacientes. La extracción del ARN se hizo mediante kits: Qiagen®-adaptado, BiosSprint®96-DNA-Blood-Kit (384). Para amplificación-detección del SARS-CoV-2 se siguieron métodos recomendados por la OMS y el CDC.
ResultadosSe programaron 110 pacientes asintomáticos sin contacto estrecho con positivo los 14 días previos; 105 (96,4%) fueron negativos y 5 (4,5%) positivos. Dos pacientes desarrollaron clínica respiratoria después del diagnóstico (presintomáticos) y 3 continuaron asintomáticos. Los 5 pacientes eran casos autóctonos y sin antecedentes de viaje o residencia en otra ciudad o país asociado a alta prevalencia de infección. Cuatro casos fueron mujeres entre 60–81 años. El gen N fue detectado en todos los casos.
ConclusionesSe detectó una alta prevalencia de infección por SARS-CoV-2 en pacientes programados por endoscopia digestiva. Dado el riesgo de transmisión a los profesionales, consideramos recomendable realizar RT-PCR del SARS-CoV-2 48−72 horas antes de la exploración en situaciones de alta incidencia poblacional.
In early December, a series of cases of pneumonia of unknown origin were identified in Wuhan, China.1,2 China notified the Office of the World Health Organization (WHO) on 31 December, 2019. On 7 January, 2020, the Chinese health authorities confirmed the identification of a new betacoronavirus (SARS-CoV-2) from the same family as those that caused severe acute respiratory syndrome (SARS) and Middle Eastern respiratory syndrome (MERS). On 30 January, the WHO Director-General declared a Public Health Emergency of International Concern. On 11 March, the WHO declared the outbreak caused by the new betacoronavirus a pandemic.3,4
Between 31 December, 2020 and 28 December, 2021 there were 76,103,424 global cases of COVID-19 reported, with 1,694,717 deaths. In this period, 1,819,249 cases and 49,260 deaths were reported in Spain5 and 341,109 cases and 16,942 deaths in Catalonia.6
The primary route of transmission is the inhalation of droplets, but there may be other mechanisms, such as the conjunctiva: a study showed the detection of viral RNA in the conjunctiva7; faecal: several studies have shown viral RNA in stools and the presence of ACE-2 receptors (the entry point of the virus into the cell) in the gastrointestinal tract8–11; and fomites: viral RNA was isolated from different surfaces in the room of an infected patient.12
In this situation, health professionals who work in Endoscopy Units (PSUE) have an increased risk of infection by SARS-CoV-2 by inhalation of droplets, conjunctival contact and possible faecal-oral transmission.3,13 Performing an upper endoscopy produces aerosols, making it a high-risk procedure.14–20 Viral RNA has also been detected in stools.10,11,13,21,22 The virus's mechanism of entry into the human cell is the angiotensin-converting enzyme 2 (ACE2) receptor,3 which is highly expressed in the gastrointestinal tract23 and makes the risk of transmission in lower endoscopy uncertain. If PSUE acquire the infection, they can subsequently transmit it to their colleagues, families and other patients, generating a hospital outbreak, as has been reported in other European countries.24
The first round of the seroprevalence study in Spain carried out on 13 May, 2020 estimated a prevalence of IgG antibodies against SARS-CoV-2 of 5.9% (CI 95%: 4.9–6.9) in Catalonia and 2.5% (CI 95%: 4.9–6.9) in Girona.25
Our objective was to estimate the prevalence of SARS-CoV-2 infection in a series of asymptomatic patients prior to gastrointestinal endoscopy after the peak of the first epidemic wave in Spain.
MethodsStudy designFollowing recommendations by the Societat Catalana de Digestologia (SCD) [Catalan Society of Digestology],26 the Sociedad Española de Endoscopia Digestiva (SEED) [Spanish Society of Gastrointestinal Endoscopy] and the Asociación Española de Gastroenterología (AEG) [Spanish Association of Gastroenterology],27 as well as the position of the European Society of Gastrointestinal Endoscopy (ESGE) and the Sociedad Europea de Enfermeras y Asociados (ESGENA) [European Society of Nurses and Associates],28 who developed strategies for restarting scheduled endoscopic activity in Gastrointestinal Endoscopy Departments, in May, elective scheduling began at the Endoscopy Department of the Hospital de Santa Caterina [Santa Caterina Hospital] (Salt, Girona).
Once the first phase of the pandemic (pandemic peak) had been overcome, during which only urgent endoscopic procedures were performed (second half of March and April 2020), phase 2 (transition) began in May, with the scheduling of gastrointestinal endoscopies. To this end, we relied on the aforementioned infection prevention and prioritisation recommendations. First, a telephone triage was performed on symptoms (fever, cough, dyspnoea) and/or on contacts with a positive case in the previous 14 days. If the answers were negative, an RT-PCR for SARS-CoV-2 by nasopharyngeal aspirate was carried out 48−72 h before the procedure. If the result was negative, a gastrointestinal endoscopic examination was performed with the recommended prevention measures.
All procedures scheduled between 1 May and 31 May, 2020 were included in the study. A total of 110 patients were recruited.
Patient recruitment, sample collection and laboratory techniqueBiological samples for the diagnosis of SARS-CoV-2 were collected via the nasopharynx according to established guidelines.26,27
Nasopharyngeal exudate samples were taken with a flocked swab that were deposited in a tube with 3 mL of universal transport medium (UTM™), viral transport medium stable at room temperature and specially designed for the collection, transport, maintenance and storage of samples for molecular assays until processing.
The extraction of RNA from the sample was carried out with an adapted Qiagen kit, BiosSprint® 96 DNA Blood Kit (384), which uses magnetic particle technology based on the purification of nucleic acids.
The current “gold standard” for the diagnosis of SARS-CoV-2 infection is reverse transcriptase polymerase chain reaction (RT-PCR). The laboratory used, interchangeably, two assays for the amplification/detection of nCoV SARS-2 with the following methodologies:
- 1
The WHO-recommended method, with three molecular targets, one common to all coronaviruses, the E gene (Envelope gene), and two specific to nCoV SARS-2, the RdRp gene (RNA-dependent RNA polymerase gene) and the N gene (nucleocapsid gene). The Seegene Allplex™ 2019-nCoV Assay kit was used, which detects all three targets simultaneously in a single tube. Amplification and reading were carried out with a CFX96™ system.
- 2
The Center for Prevention and Control Diseases (CDC) method, with three nCoV SARS-2 specific target molecules (N1, N2 and N3) of the N gene. In this method Applied Biosystems™ reagents were used and the QuantStudio™ 7 Flex Real-Time PCR System, by Applied Biosystems™ were used for the amplification and reading of the result.
All measured data are shown in tables and histograms. Descriptive statistics were applied to summarise the data. Results are reported as means and ranges as required. The analysis was carried out with SPSS v24.0.
EthicsThe investigation was conducted in accordance with the General Data Protection Regulation (Regulation (EU) 2016/679 and directive 95/46/EC) and the Acta Española de Datos Personales [Spanish Personal Data Act] (BOE-A-2018-16673. https://www.boe.es/eli/es/lo/2018/12/05/3/con).
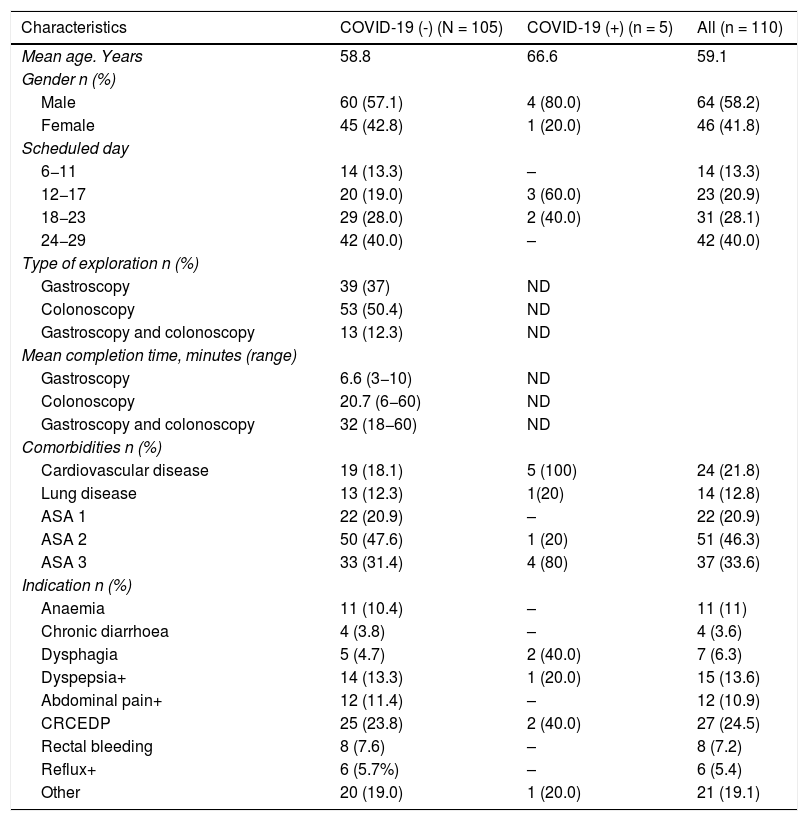
ResultsThe demographic data and baseline characteristics of all asymptomatic patients for SARS-CoV-2 infection without positive contacts in the previous 14 days and who were scheduled for gastrointestinal endoscopy at the Hospital de Santa Caterina Salt (Girona) between the May 1 and 31 are shown in Table 1. 110 patients were scheduled during this period. The presence of SARS-CoV-2 in nasopharyngeal aspirate was detected through RT-PCR in five (4.5%) and was not detected in 105 (96.4%) patients. 39 gastroscopies, 53 colonoscopies and 13 gastroscopies plus colonoscopy were performed. The mean time for the endoscopic examinations was 6.6 min (R: 3−10) in gastroscopy, 20.7 min (R: 6−60) in colonoscopies and 32 min (R: 18−60) in gastroscopy plus colonoscopy.
Demographic and baseline characteristics of all patients scheduled for endoscopy, Santa Caterina Hospital, Girona. May 2020.
| Characteristics | COVID-19 (-) (N = 105) | COVID-19 (+) (n = 5) | All (n = 110) |
|---|---|---|---|
| Mean age. Years | 58.8 | 66.6 | 59.1 |
| Gender n (%) | |||
| Male | 60 (57.1) | 4 (80.0) | 64 (58.2) |
| Female | 45 (42.8) | 1 (20.0) | 46 (41.8) |
| Scheduled day | |||
| 6−11 | 14 (13.3) | – | 14 (13.3) |
| 12−17 | 20 (19.0) | 3 (60.0) | 23 (20.9) |
| 18−23 | 29 (28.0) | 2 (40.0) | 31 (28.1) |
| 24−29 | 42 (40.0) | – | 42 (40.0) |
| Type of exploration n (%) | |||
| Gastroscopy | 39 (37) | ND | |
| Colonoscopy | 53 (50.4) | ND | |
| Gastroscopy and colonoscopy | 13 (12.3) | ND | |
| Mean completion time, minutes (range) | |||
| Gastroscopy | 6.6 (3−10) | ND | |
| Colonoscopy | 20.7 (6−60) | ND | |
| Gastroscopy and colonoscopy | 32 (18−60) | ND | |
| Comorbidities n (%) | |||
| Cardiovascular disease | 19 (18.1) | 5 (100) | 24 (21.8) |
| Lung disease | 13 (12.3) | 1(20) | 14 (12.8) |
| ASA 1 | 22 (20.9) | – | 22 (20.9) |
| ASA 2 | 50 (47.6) | 1 (20) | 51 (46.3) |
| ASA 3 | 33 (31.4) | 4 (80) | 37 (33.6) |
| Indication n (%) | |||
| Anaemia | 11 (10.4) | – | 11 (11) |
| Chronic diarrhoea | 4 (3.8) | – | 4 (3.6) |
| Dysphagia | 5 (4.7) | 2 (40.0) | 7 (6.3) |
| Dyspepsia+ | 14 (13.3) | 1 (20.0) | 15 (13.6) |
| Abdominal pain+ | 12 (11.4) | – | 12 (10.9) |
| CRCEDP | 25 (23.8) | 2 (40.0) | 27 (24.5) |
| Rectal bleeding | 8 (7.6) | – | 8 (7.2) |
| Reflux+ | 6 (5.7%) | – | 6 (5.4) |
| Other | 20 (19.0) | 1 (20.0) | 21 (19.1) |
ASA: American Society of Anesthesiologists Physical Status Classification System, may include anaemia associated with another symptom (FOBT+, melaena, Toxic syndrome); Dyspepsia+: may include dyspepsia associated with toxic syndrome, abdominal pain, may be associated with other symptoms (vomiting, FOBT+, diarrhoea, rectal bleeding); CRCEDP: Colorectal Cancer Early Detection Programme; Reflux+: may include dysphagia; Others: may include other endoscopically controlled diseases.
The patients' clinical characteristics, taking into account their age, sex, scheduled date, presence of comorbidities and indication for the examination, are shown in Table 1.
Five positive cases were detected. Two patients developed mild-moderate symptoms of SARS-CoV-2 infection in the days following diagnosis. Patient three presented cough for two days and patient four dyspnoea and headache for 15 days. The rest of the patients remained asymptomatic throughout the follow-up. They were all isolated for 14 days and an epidemiological follow-up of their contacts was carried out. The endoscopy was postponed in these five cases.
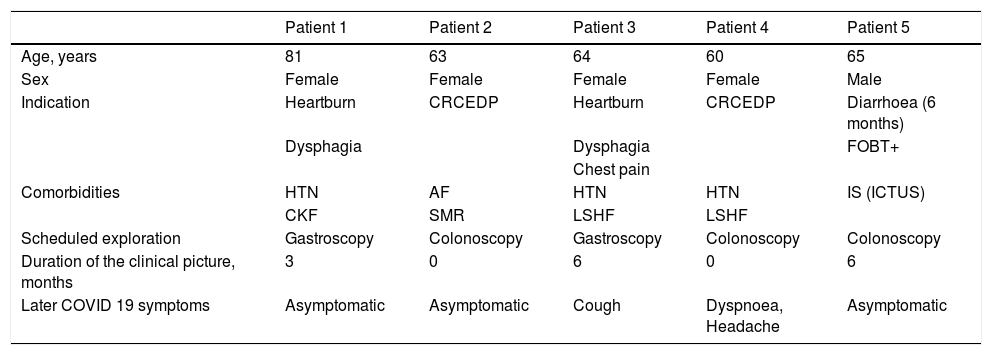
The main epidemiological and clinical characteristics of the five positive patients are shown in Table 2. The N gene was detected in all five positive patients.
Characteristics of patients with SARS-CoV-2 PCR+ in respiratory samples collected 48–72 h before the procedure.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
|---|---|---|---|---|---|
| Age, years | 81 | 63 | 64 | 60 | 65 |
| Sex | Female | Female | Female | Female | Male |
| Indication | Heartburn | CRCEDP | Heartburn | CRCEDP | Diarrhoea (6 months) |
| Dysphagia | Dysphagia | FOBT+ | |||
| Chest pain | |||||
| Comorbidities | HTN | AF | HTN | HTN | IS (ICTUS) |
| CKF | SMR | LSHF | LSHF | ||
| Scheduled exploration | Gastroscopy | Colonoscopy | Gastroscopy | Colonoscopy | Colonoscopy |
| Duration of the clinical picture, months | 3 | 0 | 6 | 0 | 6 |
| Later COVID 19 symptoms | Asymptomatic | Asymptomatic | Cough | Dyspnoea, Headache | Asymptomatic |
GI: gastrointestinal; HTN: Hypertension; CKF: Chronic kidney failure; AF: Atrial fibrillation; SMR: Severe mitral regurgitation; LSHF: Left-sided heart failure; IS: Ischaemic stroke; CRCEDP: Colorectal Cancer Early Detection Programme; FOBT+: Faecal occult blood test positive.
Detection of the genetic material of the SARS-CoV-2 virus in samples from the upper respiratory tract suggest a high risk of transmissibility in asymptomatic and pre-symptomatic subjects during upper gastrointestinal endoscopy due to the generation of aerosols during the procedure or during sample collection29 and potentially in lower endoscopy due to faecal elimination.11 Thus, patients scheduled for gastrointestinal endoscopy may represent a potential route of transmission.
On the other hand, since endoscopic procedures are performed at a short distance from the patient and other PSUEs, it seems logical that patients and PSUEs are exposed to the risk of infection from airborne diseases.29 This fact becomes more relevant at times and in areas of high prevalence.
SARS-CoV-2 (COVID-19) disease can present in a high percentage of cases with gastrointestinal symptoms, the presence of which implies the possibility of a more serious evolution.11 This symptomatology could be a confounding factor in the indication for endoscopy, and we suggest including acute gastrointestinal symptoms as part of COVID-19 telephone screening. We have recently published a systematic review study on the epidemiology of enteric manifestations and the possible faecal-oral transmission of COVID-19.11
The pandemic has severely limited the opportunity to perform endoscopic procedures in an adequate time frame for the early diagnosis of colorectal cancer, and an imaginative redesign of endoscopy services should be carried out using the quantification of occult blood in faeces in the prioritisation of endoscopies as a new method30 and the review of the indication and/or prioritisation of the examinations.
Unlike our study, in which a high prevalence of SARS-CoV-2 infection was obtained by RT-PCR, a recent study obtained a low seroprevalence (1.9%) by determining IgG and IgM antibodies, although it was carried out in a low prevalence area.31
In our study, a high prevalence of infection by SARS-CoV-2 (4.5%) was detected in asymptomatic patients scheduled for elective gastrointestinal endoscopy during May 2020. This fact contrasts with the seroprevalence study carried out in Spain and published on 13 May, which estimated a presence of 2.5% of people with positive IgG in Girona.25
The study demonstrates the importance of screening for SARS-CoV-2 infection prior to the performance of endoscopic studies, especially in areas and times of high prevalence. The negative predictive value of RT-PCR is not 100%, so PSUEs must maintain protective measures, especially in upper endoscopic procedures.
The COVID-19 pandemic has affected routine gastrointestinal endoscopy (both diagnostic and therapeutic) clinical practice and will continue to do so in the foreseeable future. Therefore, endoscopists must adapt, while continuing to provide care and at the same time protect both their patients and themselves by adopting infection prevention and control measures, including prior detection of SARS-CoV-2 infection and the suitable use of personal protective equipment in a pandemic situation.
ConclusionsA high RNA prevalence rate of SARS-CoV-2 tested by RT-PCR has been detected in nasopharyngeal samples of patients electively scheduled, according to prioritisation guidelines, for a gastrointestinal endoscopy.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Pamplona J, Solano R, Ramírez M, Durandez R, Mohamed F, Pardo L, et al. Alta prevalencia de infección por SARS-CoV-2 en pacientes programados para endoscopia digestiva después del pico de la primera onda pandémica. Gastroenterol Hepatol. 2021;44:614–619.