Patients with coeliac disease have an increased risk of gastrointestinal lymphomas, although the overall incidence is very low. We present the case of a 36-year-old man with a history of Down's syndrome, type 1 diabetes and coeliac disease (CD) diagnosed in childhood, with good adherence to the gluten-free diet, without data to suggest malabsorption and negative coeliac antibodies. In the follow-up biopsy at the age of 19, Marsh 3a villous atrophy persisted, and lymphogram by flow cytometry was compatible with active CD, but without an aberrant lymphocyte population.
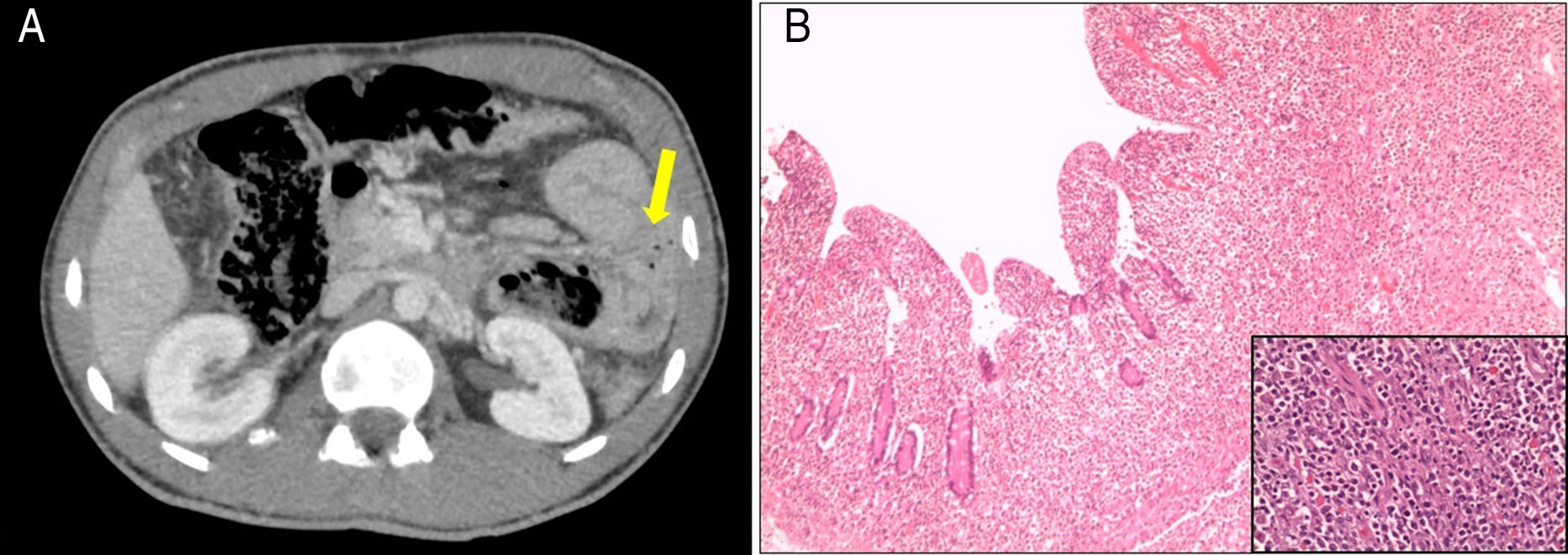
The patient attended the hospital with progressively worsening diffuse abdominal pain associated with nausea and vomiting. Abdominal examination found signs of peritoneal irritation. Blood tests showed glucose 317mg/dl and venous pH 7.32, with no anaemia or leucocytosis. Contrast-enhanced abdominal/pelvic CT scan showed pneumoperitoneum, free intra-peritoneal fluid and a dilated jejunal loop, the wall of which was circumferentially thickened, with a tumour-like appearance and adjacent extraluminal gas bubbles (Fig. 1A). Also detected was lymphadenopathy involving multiple lymph nodes in the root of the mesentery and a thickening of a long segment of sigmoid colon. As perforation was suspected, a laparotomy was performed with resection of the proximal jejunum and primary anastomosis and a sigmoidectomy with terminal colostomy. The histological analysis was compatible with intestinal diffuse large B-cell lymphoma of activated phenotype affecting the mucosa, causing ulceration of the mucosa, the muscle wall and the underlying fat, with no lymph node involvement (Fig. 1B). Bone marrow biopsy ruled out neoplastic infiltration and Epstein Barr virus was negative. The patient received six cycles of CHOP-R (cyclophosphamide, doxorubicin, vincristine, prednisone and rituximab) to treat the lymphoma. Restoration of intestinal transit was subsequently performed with colon-sigmoid anastomosis. Follow-up PET-CT and abdominal CT scans at 18 months detected no evidence of tumour recurrence. The patient is currently well, with good adherence to the gluten-free diet and periodic follow-up of his CD.
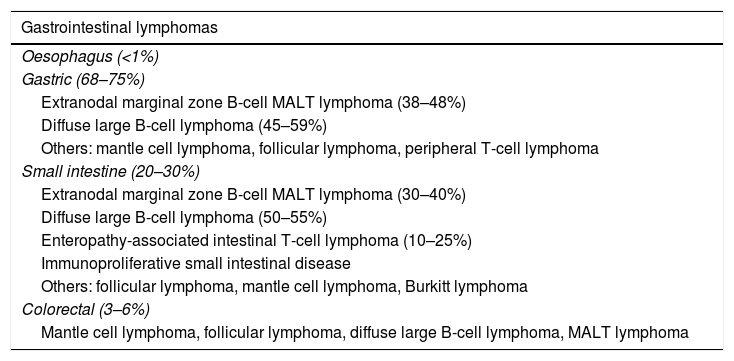
Primary small bowel tumours account for less than 2% of all gastrointestinal cancers, and lymphomas account for 15–20% of them (Table 1). The gastrointestinal tract is the most common site for lymphomas to have extranodal involvement.1,2
Frequency of gastrointestinal lymphomas according to the affected region of the gastrointestinal tract.
| Gastrointestinal lymphomas |
|---|
| Oesophagus (<1%) |
| Gastric (68–75%) |
| Extranodal marginal zone B-cell MALT lymphoma (38–48%) |
| Diffuse large B-cell lymphoma (45–59%) |
| Others: mantle cell lymphoma, follicular lymphoma, peripheral T-cell lymphoma |
| Small intestine (20–30%) |
| Extranodal marginal zone B-cell MALT lymphoma (30–40%) |
| Diffuse large B-cell lymphoma (50–55%) |
| Enteropathy-associated intestinal T-cell lymphoma (10–25%) |
| Immunoproliferative small intestinal disease |
| Others: follicular lymphoma, mantle cell lymphoma, Burkitt lymphoma |
| Colorectal (3–6%) |
| Mantle cell lymphoma, follicular lymphoma, diffuse large B-cell lymphoma, MALT lymphoma |
In primary gastrointestinal lymphomas, the organ most commonly affected is the stomach, followed by the small intestine (60–65% affect the ileum and the rest the jejunum and duodenum).1 Almost 90% are B-cell lymphomas, the most common subtypes being diffuse large B-cell lymphoma and extranodal marginal zone B-cell mucosa-associated lymphoid tissue (MALT) lymphoma.1,2 They may present with abdominal pain, vomiting, weight loss and, rarely, as obstruction, intussusception, perforation (5–15%), gastrointestinal haemorrhage or diarrhoea.1,2 Most lymphomas are treated with a combination of surgery and chemotherapy.3 The tumour stage and the presence of B symptoms are associated with a worse prognosis.3
Patients with CD have a 40% higher risk of developing any cancer compared to the general population,4 and are five times more likely to develop a lymphoma.5 The cancer type most commonly associated with CD is non-Hodgkin's lymphoma, with an incidence of 1.3 per 1000person-years.4 Of these, the most common are diffuse large B-cell lymphoma and enteropathy-associated T-cell lymphoma.2,5 Although no differences have been found in the survival of patients with CD and a lymphoproliferative process with respect to the general population,2 B-cell lymphomas have a better prognosis than T-cell lymphomas.5 The most common gastrointestinal cancers to be associated with CD are adenocarcinoma of the small intestine, cancer of the oesophagus and colorectal cancer.4
Enteropathy-associated T-cell lymphoma is the lymphoma most closely associated with CD. This is a high-grade non-Hodgkin's lymphoma which accounts for less than 5% of gastrointestinal lymphomas and is usually found in primary form in the small intestine.6
The overall risk of malignancy in patients with CD decreases with time after diagnosis and does not increase significantly after 15 years, probably thanks to early diagnosis and early introduction of the gluten-free diet.4,7 Following a gluten-free diet aids healing of the mucosa, but, in almost 20% of cases, villous atrophy is found in the follow-up biopsy.7,9 In cases in which villous atrophy persists, there is twice the risk of developing a lymphoproliferative process than in those with mucosal healing.7
In Down's syndrome there is an increased risk of death compared to the general population, due mainly to cardiac malformations, early-onset dementia and childhood leukaemia. People with Down's syndrome also have a six-fold higher risk of suffering from CD, although the association between the two diseases does not lead to an increase in mortality rates compared to the rest of the population with Down's syndrome.10
In conclusion, properly following a gluten-free diet decreases the risk of developing a lymphoma and any other cancer associated with CD.5,7
Please cite this article as: Ríos León R, Crespo Pérez L, Martínez-Geijo Román C, Barbado Cano A, García-Cosío Piqueras M, Sánchez Rodríguez E, et al. Perforación intestinal secundaria a linfoma intestinal B difuso de células grandes en un paciente con enfermedad celiaca. Gastroenterol Hepatol. 2018;41:503–504.