SARS CoV-2 infection has produced a pandemic with serious consequences for our health care system. Although liver transplant patients represent only a minority of the population, the hepatologists who follow these patients have tried to coordinate efforts to produce a protocol the management of immunosuppression during SARS Cov-2 infection. Although there are no solid studies to support general recommendations, experiences with other viral infections (hepatitis C, cytomegalovirus) suggest that management of immunosuppression without mycophenolate mofetil or m-Tor inhibitors (drugs that are also associated with leukopenia and lymphopenia) may be beneficial. It is also important to pay attention to possible drug interactions, especially in the case of tacrolimus, with some of the treatments with antiviral effect given in the context of COVID 19 (lopinavir/ritonavir, azithromycin). Finally, the immunosuppressive effect of immunomodulating drugs (tocilizumab and similar) administered to patients with severe lung disease should be taken into account. The mechanisms of action of the different immunosuppressive drugs are reviewed in this article, as well as their potential effect on Cov-2 SARS infection, and suggests guidelines for the management of immunosuppression.
La infección por el virus SARS CoV-2 ha producido una pandemia con graves consecuencias sobre nuestro sistema sanitario. Aunque el colectivo de pacientes trasplantados hepáticos representa sólo una minoría de la población, los hepatólogos que seguimos a estos pacientes hemos intentado coordinar esfuerzos para protocolizar el manejo de la inmunosupresión durante la infección por SARS Cov-2. Aunque no hay estudios sólidos que avalen recomendaciones generales, las experiencias con otras infecciones víricas (hepatitis C, citomegalovirus) sugieren que el manejo de la inmunosupresión sin micofenolato mofetilo ni inhibidores m-Tor (fármacos que además se asocian a leucopenia y linfopenia) puede resultar beneficiosa. Es importante además prestar atención a las posibles interacciones farmacológicas, especialmente en el caso de tacrolimus, con algunos de los tratamientos con efecto antiviral que se administran en el contexto de la COVID 19 (lopinavir/ritonavir, azitromicina). Finalmente, deberá tenerse en cuenta el efecto inmunosupresor de fármacos inmunomoduladores (tocilizumab y similares) que se administran e pacientes con enfermedad pulmonar severa. En el artículo se revisan los mecanismos de actuación de los diferentes fármacos inmunosupresores, su potencial efecto sobre la infección por SARS Cov-2 y se sugieren unas pautas en el manejo de la inmunosupresión.
Infection with the SARS-CoV-2 virus has caused a pandemic with serious health and economic repercussions. In the fight for the survival of patients, physicians have tried multiple drugs aimed at combating the virus or its effects on the body, with more or less success.1–10 Liver transplant recipients represent a minority of the population, but, in theory, their immunosuppressed state renders them more sensitive to infection. Therefore, hepatologists dedicated to liver transplantation have been very attentive to their patients and have been trying to adjust the immunosuppression to both the seriousness of their disease and the treatments that they have used. This article describes each immunosuppressant's mechanisms of action, explains the balance between its immunosuppressant effect and its antiviral effect in some cases, and sets out a proposal for action in relation to immunosuppressant treatment in patients with COVID-19.
The SARS-CoV-2 virusThe SARS-CoV-2 virus belongs to the Coronaviridae family of viruses, which includes four genera (alpha, beta, gamma and delta). Coronaviruses capable of infecting humans belong to the alpha genus (HCoV-229E and HCoV-NL63) or the beta genus (SARS-CoV, MERS-CoV, HCoV-OC43 and HCoV-HKU1).11,12 The beta genus includes three agents that to date are known to cause lower respiratory tract infections and therefore may be associated with serious clinical conditions (SARS-CoV, MERS-CoV and SARS-CoV-2). Like other agents in its family, SARS-CoV-2 is a positive-sense RNA virus that encodes structural proteins (nucleocapsid, membrane, envelope and spike [S]) and non-structural proteins (RNA polymerase, proteases) in different open reading frames.13–15 It was very recently discovered that the virus uses ACE2 receptors and possibly other additional receptors to enter body cells (as occurs in many other viruses).16–19
The primary mode of SARS-CoV-2 infection is human-to-human transmission through close contact or via fomites infected when a patient coughs. It is highly infectious, primarily in early stages. It colonises the body through the respiratory tract and particularly infects alveolar epithelial cells.19,20 It replicates rapidly, and the infection spreads. In most cases (80%), the infection is asymptomatic or mild.14 Three stages are distinguished: a first asymptomatic stage, which features a high viral load; a second mild symptomatic phase, in which patients experience a dry cough, odynophagia, anosmia, fever, fatigue and sometimes diarrhoea; and a third phase, characteristic of severe cases, which causes pneumonia with respiratory insufficiency and dyspnoea and may lead to adult respiratory distress syndrome (ARDS) and ultimately multiple organ failure and death.14,18,20 In this third stage, viraemia may be low, and impairment of the lungs, endothelium, myocardium, etc. is due to a so-called cytokine storm, resulting from an excessive response on the part of the immune system to SARS-CoV-2 infection.21 The clinical disease corresponding to this viral infection is what we know as COVID-19.
The immune system in the response to SARS-CoV-2Innate responseThe presence of lymphopenia with neutrophilia, increased levels of interleukin 6 (IL-6) C-reactive protein (CRP), and a finding of elevated levels of cytokines associated with innate immunity such as IP-10, MCP-1, MIP-1〈 00A0;and TNF〈 suggest that this plays a very important role in the inflammatory response associated with the infection (which was also seen with SARS-CoV and MERS-CoV) and therefore that it is responsible for progression to severe COVID-19.21–25
The innate immune effector response against viruses is mainly based on interferon (IFN-1).23 To mount this response, innate immune response cells must recognise invasion by the virus through pathogen-associated molecular patterns (PAMPs).23 This recognition leads to activation of IFN-1 and other proinflammatory cytokines.24,25 However, as occurs in other viral infections, SARS-CoV and MERS-CoV contain some proteins that block crucial signalling pathways for interferon activation, such as that associated with the recognition of PAMPs.23 Blockage of interferon pathways facilitates virus replication and the release of more proinflammatory cytokines that would induce an excessive response. Similar inhibition occurs in COVID-19.26
Adaptive responseIn general, the Th1 immune response plays a predominant role in viral infections.22,25,27 Cytokines generated by antigen-presenting cells direct the T-cell response. In general, regulatory T cells coordinate the adaptive response, while cytotoxic T cells are essential for eliminating the virus. The humoral immune response plays a role in limiting infection and preventing reinfection through the production of neutralising antibodies.27–33
Immunosuppression in liver transplantation and COVID-19Although immunosuppression in liver transplantation uses the same drugs as those used in other types of solid organ transplantation, it is characterised by a lesser intensity.34–37 This particularity allows at certain times for the temporary withdrawal of some of the immunosuppressants that are being used, as in the case of a severe infection, kidney failure, neurological disorders, etc.34,35 As seen, the immune response plays a very important role in COVID-19 and, therefore, the immunosuppressants that are regularly used could be determinants both as promoters and as attenuators of the inflammatory response secondary to the disease. The few published studies, some of which are better described as simple case reports, suggest neither an increase in the incidence of cases in transplant recipients nor a higher mortality rate than in the general population.38,39 Preliminary results from a study by the Sociedad Española de Trasplante Hepático [Spanish Liver Transplantation Association] (SETH) suggest a mortality rate of around 15% in infected patients based on data compiled from liver transplant units in Spain.40 However, many of the results presented in the bibliography have the limitation that the actual population of infected individuals is unknown. Therefore, the figures in relation to incidence of infection, mortality, etc., are provided with this caveat. Reasons posited for a lower incidence of infected individuals in the transplant recipient population include: better personal hygiene and habitual hand-washing, more regular use of protective measures, and far greater awareness of the usefulness of social distancing.
In liver transplantation, as in other types of solid organ transplantation, immunosuppressant treatment is based on a calcineurin inhibitor, cyclosporin or tacrolimus. The latter is most commonly used and may or may not be combined with corticosteroids. Furthermore, primarily in cases of kidney failure or efforts to prevent kidney failure, mycophenolate mofetil (MMF) or, less commonly, an mTOR inhibitor such as everolimus is used to decrease necessary calcineurin inhibitor levels.
In the context of COVID-19 infection in a liver transplant recipient, it is very important to review the immunosuppressant regimen. These cases require special attention to potential interactions between immunosuppressants and antiviral or antibacterial drugs used in initial stages of SARS-CoV-2 infection (lopinavir/ritonavir, azithromycin). Likewise, interaction with other immunomodulators that directly target the cytokine response (IL-6 and IL-1 inhibitors), used in the treatment of patients with severe COVID-19, should also be assessed:26,41,42 IL-6 receptor signal inhibitors (tocilizumab and sarilumab), IL-1 receptor signal inhibitors (anakinra) and selective Janus kinase (JAK) inhibitors.42–44 Although the effect that the latter may have in controlling cellular rejection in transplant recipients is not well known, some data do suggest that they may control antibody-mediated rejection.45 In any case, their administration must be considered when adjusting corticosteroid doses and thus avoid the risk of excessive immunosuppression.
Next, the main immunological effects of the most commonly used immunosuppressants in liver transplantation are explained.
GlucocorticoidsGlucocorticoids (GCs) inhibit different aspects of inflammation by stimulating or inhibiting gene transcription and the expression of mediators, receptors, adhesion molecules and cytokines. That is why they have multiple effects, and though they may promote viral replication, they are powerful anti-inflammatory agents that may help to counteract cytokine storm of the disease. GCs may manage inflammation in ARDS and are used in primary and secondary forms of hemophagocytic lymphohistiocytosis.46–48 However, preliminary results of the use of high doses of corticosteroids in SARS and COVID-19 have not demonstrated a beneficial effect on pulmonary lesions.49 Brief regimens of medium-to-low doses of GCs nevertheless proved useful in a study of critically ill COVID-19 patients in China.47 These very limited data with regard to the efficacy and safety of GCs, the absence of controlled studies and the use of GCs in combination with other treatments have made it virtually impossible to come to reliable conclusions. The last recommendation is that high-dose GC boluses should not be used to treat COVID-19.
Their use in liver transplantation is variable. They may or may not be used in the first six months in combination with other immunosuppressants, and they are used in the form of high-dose boluses to treat severe cellular rejection. Starting from this post-transplantation period (which may range from 0 to 6 months), corticosteroids are withdrawn, except in patients who have undergone transplantation due to liver disease of autoimmune aetiology, who usually continue to receive low doses for a very long time. Another use of GCs is to supplement or replace baseline immunosuppression for short periods of time when a patient develops severe complications requiring suspension of their usual immunosuppressant medication, as for example in cases of severe sepsis or kidney failure.32 Depending on the time elapsed since transplantation, corticosteroids at variable doses (in most cases, 20-60 mg/day) are used for periods of 1-2 weeks.
In severe COVID-19 cases, temporary replacement of calcineurin inhibitors and MMF with GCs enables these patients to avoid undesirable effects secondary to the interaction of said immunosuppressants with the multiple medicines that they receive to treat SARS-CoV-2 infection. It also prevents side effects caused by the immunosuppressant themselves (kidney failure, worsening of lymphopenia, etc.). Although it has been indicated that uncontrolled withdrawal of immunosuppression may evidently cause graft rejection,50 the peculiarity of liver transplantation allows for calcineurin inhibitors to be temporarily replaced with GCs with relative safety.51
Calcineurin inhibitorsCyclosporin and tacrolimus are powerful inhibitors of calcineurin phosphatase activity in lymphocytes, and are considered core medicines in immunosuppression in liver transplantation.36 At present, they are usually used jointly with MMF or everolimus so as to maintain lower plasma levels and prevent undesirable effects.34,35 Cyclosporin A binds to cyclophilin (intracellular receptor) and forms an active complex that inhibits calcineurin phosphatase activity. Calcineurin dephosphorylates the cytoplasmic component of nuclear factor of activated T cells (NFATc) so that it may go to the nucleus and activate the genes involved in IL-2 synthesis. IFN-©, IL-4, TNF-®.46 Thus, cyclosporin A, by inhibiting calcineurin, inhibits proliferation of T cells by preventing clonal expansion of helper T cells and cytotoxic T cells.52 Tacrolimus acts in a similar manner, but it binds to a specific immunophilin (FKBP) to block calcineurin phosphatase activity and thus inhibit transcription of genes involved in IL-2 synthesis. Its net effect on COVID-19 and in particular on SARS-CoV-2 is unknown. However, some data suggest that calcineurin inhibitors have a direct antiviral effect. Some years ago, it was demonstrated that overexpression of non-structural proteins of CoVs (Nsp1) and live SARS-CoV infection caused a very significant increase in signalling through the calcineurin/NFAT pathway and an increase in IL-2, consistent with the immunopathogenesis and late deregulation of cytokines seen in patients with severe SARS. By contrast, inhibition of cyclophilins with cyclosporin A blocked replication of all the genera of the virus, including SARS-CoV and human CoV-229E.52 This antiviral effect of calcineurin inhibitors, mainly cyclosporin A, has already been suggested in the case of other viruses such as hepatitis C virus (HCV).53 However, the balance between their antiviral effect and their immunosuppressant effect does not, in most cases, enable observation of a net antiviral effect. With the information currently available it is not possible to affirm that calcineurin inhibitors have a significant (positive or negative) effect on COVID-19
Patients with COVID-19 may be rudimentarily divided into two classifications: 1) patients with mild disease, including asymptomatic patients and patients with symptoms such as fever, dry cough and fatigue, but not respiratory insufficiency; and 2) patients with pneumonia and respiratory insufficiency. This is clearly a very simple classification that does not take into account prognostic factors such as lymphopenia and lactate dehydrogenase (LDH).49 However, it may be useful in making initial treatment decisions and adjusting calcineurin inhibitors. In patients with mild disease, the recommendation is not to modify calcineurin inhibitors, unless antivirals such as ritonavir/lopinavir are used with a high degree of interference, particularly with tacrolimus.54 In this case, the dosage of tacrolimus may be 0.5 mg every 3-5 days. Evidently, this requires intensive monitoring of levels. Interactions with other treatments (azithromycin, hydroxychloroquine, etc.) should also be assessed. Although they do not require an initial dose reduction, they do require monitoring of levels of calcineurin inhibitors. In patients with severe disease, it is better to decrease or withdraw calcineurin inhibitors, as this prevents their undesirable effects, interactions with other (sometimes multiple) drugs and uncontrolled increases and decreases in their levels (which are very difficult to stabilise in these situations).
Mycophenolate mofetilMMF is a pro-drug that is converted to mycophenolic acid (MPA) in the body. MPA metabolism primarily involves glucuronidation by the enzyme uridine 5'-diphosphate-glucuronosyltransferase.37 MPA has enterohepatic recirculation, which lengthens its half-life. MPA is a reversible, non-competitive inhibitor of inosine-5'-monophosphate dehydrogenase (IMPDH). It inhibits proliferation of T and B lymphocytes and production of immunoglobulins by depleting guanosine and deoxyguanosine pools in lymphocytes.37,54 In addition to this powerful immunosuppressant and therefore infection-facilitating action, MPA has shown broad activity in vitro and/or in animal models against different viruses, including West Nile virus,55 Japanese encephalitis virus,56 yellow fever and dengue,57 and Chikungunya virus.58 MPA is also capable of inhibiting HCV replication in vitro and in vivo through increased interferon gene expression and through guanosine depletion.59 In relation to coronaviruses, MPA was not effective against SARS-CoV in an animal model, but it was effective against MERS-CoV.60
Despite these antiviral effects in vitro and in animal models, the reality of transplantation in clinical practice is very different in that there is a clear predominance of the immunosuppressant effect as seen with HCV and cytomegalovirus. Therefore, in our opinion, MMF should be withdrawn if a liver transplant recipient presents SARS-CoV-2 infection in any stage or severity of the disease. Regardless of its reported immunosuppressant effect, it may cause leukopenia, lymphopenia, thrombocytopenia and bone marrow aplasia. These complications may make the resolution of the infection tremendously difficult or endanger the life of the patient.
mTOR inhibitors: sirolimus/everolimusSirolimus is a macrolide from the actinomycete Streptomyces hygroscopicus discovered on Easter Island (Rapa Nui) between December 1964 and February 1965 by the Canadian Medical Research Expedition.61 It is metabolised by cytochrome p450 3A4 and requires adjustment of plasma levels. 90% of its metabolites are eliminated in faeces. To act, mTOR inhibitors must form a complex with an immunophilin. Like tacrolimus, they bind to FKBP-12, but unlike tacrolimus, they do not inhibit calcineurin but the mammalian target of rapamycin, blocking the signal from the IL-2 receptor and inhibiting the proliferation of T and B cells.37 Everolimus is a derivative of sirolimus with a shorter elimination half-life and greater oral bioavailability.
mTOR inhibitors have not only immunosuppressant antiproliferative, etc. effects, but also antiviral properties that have been well demonstrated in transplant recipients in relation to prevention and/or treatment of cytomegalovirus and BK virus infections.62 While it has been suggested that it could have an antiviral effect against coronaviruses through mTOR complex 1- and mTOR complex 2-associated pathways together with activation of AMP-activated protein kinase (AMPK), there is no clinical evidence of this. Just one study in patients with pneumonia and respiratory insufficiency due to influenza virus found a shorter duration of clinical course and a lower incidence of multiple organ failure in patients who received rapamycin [also known as sirolimus].63 There is no clinical evidence to recommend its use in COVID-19. Drug interactions with other medicines and the potential for associated leukopenia and lymphopenia should be assessed.
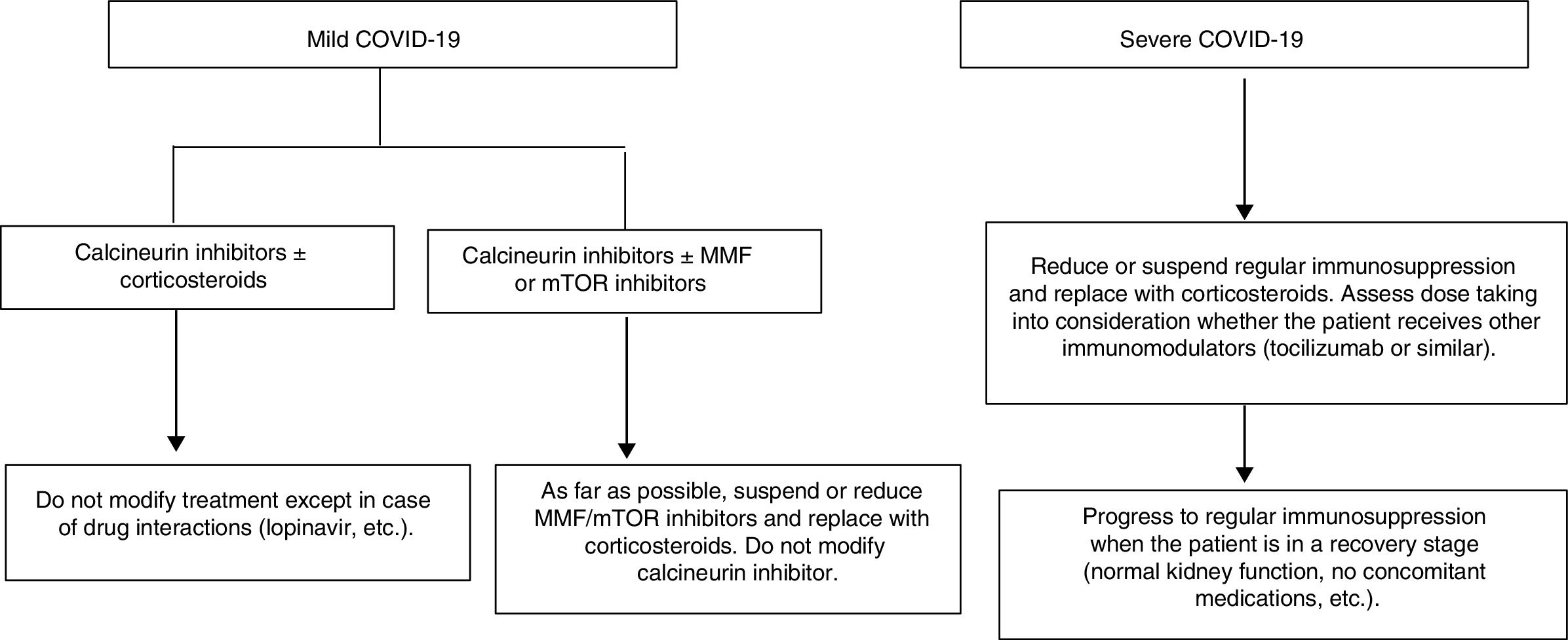
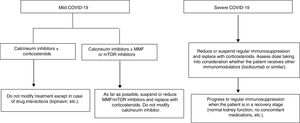
Proposal for action in liver transplant recipients and COVID-19 patientsUndoubtedly, each physician who sees liver transplant patients is capable of formulating a proposal. The following proposal merely aims to set out general guidelines for action to be adapted to patients. A patient who underwent liver transplantation a year ago is not the same as a patient who underwent the same procedure 10 years ago, nor is a patient with many comorbidities the same as a patient with none. These and many other situations require immunosuppression to be adapted to the characteristics of individual patients. As mentioned, the proposal is based on a classification that is very simple yet enables immunosuppressant treatment to be modified before complications associated with said treatment develop (Fig. 1).
- 1
Liver transplant recipients with asymptomatic or mild COVID-19 (dry cough, odynophagia, anosmia, fever, fatigue and sometimes diarrhoea):
- a)
Suspend/reduce MMF or everolimus if included in treatment whenever possible and replace them with low doses of prednisone.
- b)
Carefully monitor drug interactions, especially in patients on calcineurin inhibitors who receive antivirals https://www.hiv-druginteractions.org/checker
- a)
- 2
Liver transplant recipients with severe infection (pneumonia in any stage):
- a)
Suspend MMF or everolimus.
- b)
Reduce or suspend calcineurin inhibitors and replace them with corticosteroids.
- c)
Consider whether the patient has received immunomodulators (IL-6 inhibitors) when deciding on the corticosteroid dose.
- d)
Carefully monitor drug interactions, especially in patients receiving antivirals https://www.hiv-druginteractions.org/checker
- a)
This study did not receive any specific funding from any public- or commercial-sector agencies or non-profit organisations.
AuthorshipAll authors have made substantial contributions to each of the following aspects: (1) review concept and design, data acquisition or data analysis and interpretation; (2) the draft of the article or critical review of the intellectual contents; and (3) final approval of the version submitted.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Forns X, Navasa M. Inmunosupresión en el trasplante hepático en la era covid-19. Gastroenterol Hepatol. 2020. https://doi.org/10.1016/j.gastrohep.2020.06.003