Neuroendocrine tumors are malignancies that derive from the diffuse endocrine system of the intestine, which secrete up to 40 different cytokines and hormones.1,2 75% manifest in the digestive system, 34% of which in the rectum, where they tend to be asymptomatic and present in the form of sessile masses or thinning of the rectal wall.3 Prognosis will depend on the extent of the vascular or lymphatic invasion. Tumors measuring less than 1cm present metastasis in fewer than 3% of cases, while the rate of metastasis for tumors larger than 2cm increases to up to 70%. Tumor size greater than 2cm, invasion of the muscular layer, mitotic index greater than 2%, the degree of differentiation and positive resection margins4,5 are all predictive factors for poor prognosis.
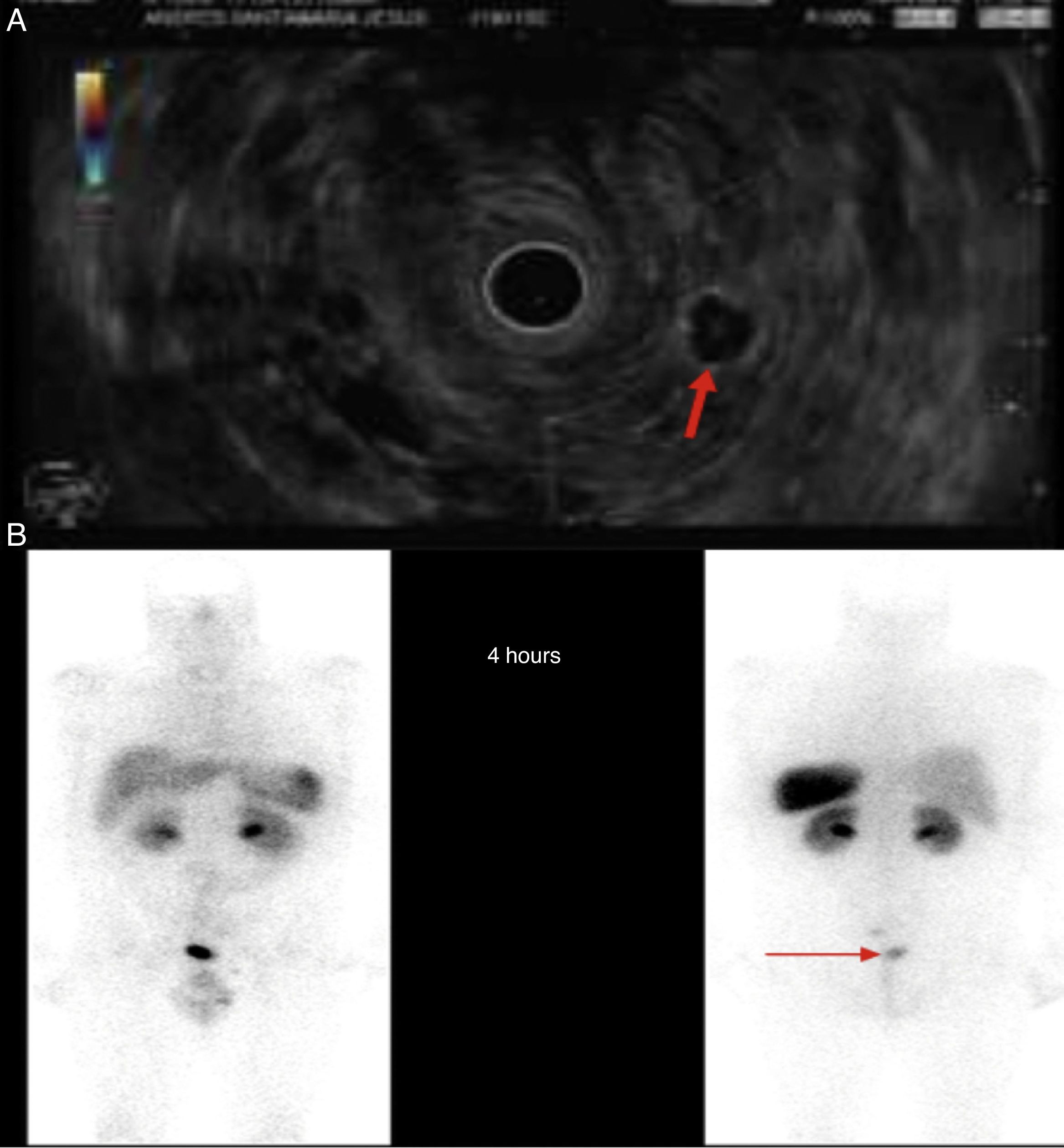
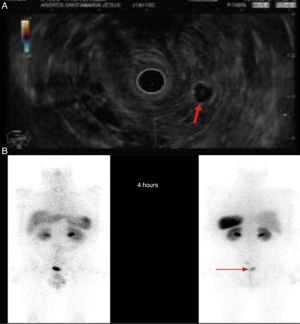
We present the case of a 46-year-old male patient on anticoagulation therapy due to ischaemic stroke. Following episodes of rectorrhagia, a colonoscopy was performed that revealed a 6mm yellowish lesion presenting erosion on its surface, 6cm from the anal margin, consistent with a well-differentiated grade 1 neuroendocrine tumor. The patient was positive for glucagon with a mitotic index (Ki-67) of less than 2% and invasion of the deep border of the submucosal resection. Chromogranin A blood levels were below 100ng/ml. An extension study was conducted that revealed a right lung nodule of less than 1cm and two lymphadenopathies, one of which measuring 18mm in the left pararectal area and the other measuring 9mm in the presacral space. Endoscopic ultrasound-guided fine-needle aspiration of the pararectal lymphadenopathy was performed, confirming the presence of a neuroendocrine tumor (Fig. 1A). Lower anterior resection surgery with total mesorectal excision was performed. The anatomopathological findings of the surgical specimen were without malignancy, with negative edges, without lymphovascular invasion but with metastasis of the grade 1 neuroendocrine tumor in two lymph nodes (T1N1). Adjuvant therapy with somatostatin was started and a whole-body scintigraphy performed after two months, which revealed a pathological accumulation of radioactive material in the left pararectal area (Fig. 1B). It was therefore decided to perform surgery to remove the lymphadenopathy. The anatomopathological study found metastasis of the well-differentiated neuroendocrine tumor. Another scintigraphy was performed after two months, with no evidence of uptake at any level.
In the case that we have presented, the tumor was well-differentiated, Ki-67, less than 2%, less than 1cm, did not extend beyond the submucosa and with negative resection margins in the specimen, but with nodal disease (stage IIIB). Although the treatment indicated for tumors less than 1cm that do not extend beyond the submucosa is endoscopic resection or submucosal dissection, the patient did not undergo either of these as he presented with nodal disease. According to Kasuga et al.,6 the independent risk factor for lymph node metastases in rectal neuroendocrine tumors smaller than 10mm is venous invasion, while Zhou et al.7 also propose tumors with a central depression.
The endoanal ultrasound ascertained the depth of the tumor invasion and the presence of metastasis in the perirectal lymph nodes. The CT scan was performed to forecast involvement of the pelvic lymph nodes prior to surgery, with 95% sensitivity.8 Although the incidence of lymph node metastasis in neuroendocrine tumors smaller than 1cm is low, treatment of this tumor type should not end with endoscopic resection and/or follow-up, as the two procedures referred to above (endoanal ultrasound and CT scan) may detect sub-centimetre lymph node metastasis, which would affect the treatment administered. After this kind of diagnosis, subsequent follow-up is recommended with imaging and marker tests at three and six months. Our case illustrates the need for a personalised approach to these patients given the risk of lymph node metastasis, regardless of phenotypical or molecular characteristics to the contrary. Given its atypical nature, our case was discussed by a specialist tumor committee and it was decided to conduct follow-up at two months. Given the unpredictable biological behaviour of these tumors, follow-up after resection should be a lot more comprehensive than is regularly practised, at least in these types of malignancy.
Please cite this article as: Nutu OA, Brandáriz L, Pérez Carreras M, García-Borda J, Perea J. Tumor neuroendocrino de recto de comportamiento excepcional. Gastroenterol Hepatol. 2017;40:351–352.