Rituximab (RTX) is an anti-CD20 monoclonal antibody that is used as biological therapy in B-cell chronic lymphoproliferative disorders. Most adverse reactions reported are caused by infusion of the medicine and comprise haematological disorders, fever, nausea, asthenia, hypotension and headache. However, the rare cases of rituximab-associated colitis reported in the literature also deserve special mention.1–3
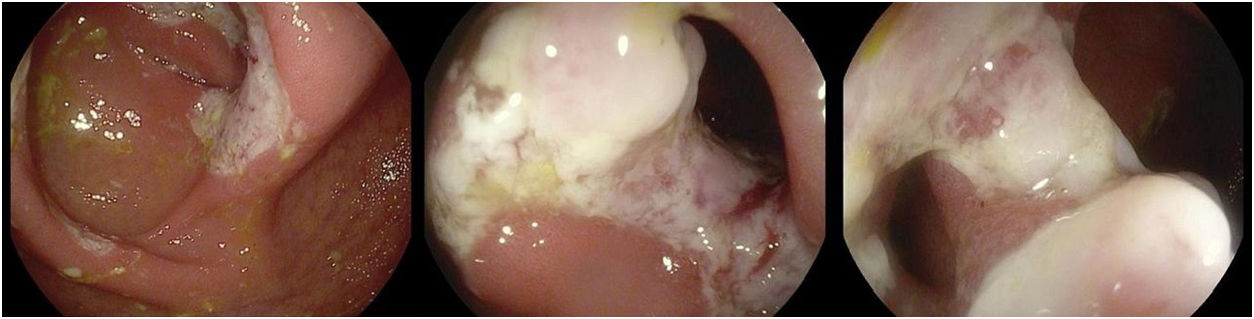
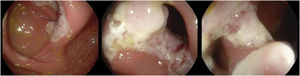
The case of a 55-year-old male diagnosed with advanced stage IV-A mantle cell lymphoma (non-Hodgkin lymphoma) is presented. He received treatment with intensified chemotherapy, autologous haematopoietic stem cell transplantation and conditioning therapy. He then presented with slow progression and relapse, so a new treatment cycle was administered (gemcitabine, oxaliplatin+rituximab for eight cycles), and maintenance therapy with 12 doses of RTX bi-monthly for two years, achieving complete response. He subsequently experienced chronic watery diarrhoea without rectorrhagia or abdominal pain and with negative stool cultures. A colonoscopy was performed that revealed erythematous mucosa of the colon from the rectum to the caecum, with oedema and exudation, as well as erythematous terminal ileum with five isolated ulcerations, the biopsies of which revealed chronic non-specific active colitis with preservation of the glandular architecture, and chronic non-specific ileitis with granulation tissue. Symptomatic treatment with loperamide was prescribed. A colonoscopy was performed 4–5 months after RTX suspension, which still showed segmental colitis, with the caecum and ileocaecal valve showing mucosal ulcers with fibrous stenosis, facilitating the examination of the distal ileum, which was endoscopically normal (Fig. 1). Biopsies of the distal ileum were consistent with mildly-active chronic colitis and necrotic fibrinous-leukocytic fragments suggestive of drug (RTX) induced colitis. With this confirmed diagnosis and given the persistent diarrhoea, albeit to a lesser extent, treatment with mesalazine was initiated, with the patient showing clinical improvement.
This case highlights a rare but potentially serious paradoxical reaction to RTX, which can be particularly acute in immunosuppressed patients. RTX is often used as a potential treatment for patients with ulcerative colitis (UC) who are unresponsive to treatment with corticosteroids. The first clinical trial conducted, which was limited by its small sample size, showed a possible short-term, unsustained response, with no significant effect on the onset of remission in moderately-active UC.4
A review has also been published of the few reported cases of UC onset following treatment with RTX. The symptom described is watery or bloody diarrhoea, with onset several days or even several weeks after starting RTX.5 Treatment consists of withdrawing RTX, standard UC treatment or even surgery.1,3,5
Recently, numerous paradoxical adverse effects consistent with colitis have been reported in patients undergoing treatment with new immunological therapies (immunotherapy) for various conditions, including lung cancer, colon cancer and melanoma, which may represent the negative repercussions of manipulating the immune system with these agents.6 The aetiopathogenesis of these paradoxical processes caused by biological medicinal products like RTX could be because the administration of RTX induces CD20+ B cell depletion. B and T lymphocytes interact in the intestinal wall and are responsible for mucosal immunoregulation, which increases immune tolerance. The histopathological findings suggest that the exacerbation of colitis may be caused by complete CD20+ B cell depletion and the high infiltration of T lymphocytes in the intestinal mucosa.4,5
In conclusion, the purpose of this case study is to highlight this condition that is so rarely reported in the medical literature, encouraging doctors to suspect this complication when colitis-like symptoms manifest in a patient receiving RTX and to promptly administer the appropriate therapeutic measures. RTX-induced colitis is described as a rare RTX-associated complication. It is characterised by symptoms of chronic bloody diarrhoea that causes ulcerations in the colonic mucosa, similar to UC, and which responds favourably after suspending RTX and treating with 5-aminosalicylic acid and/or corticosteroids.1–3
Please cite this article as: Barreiro Alonso E, Álvarez Álvarez A, Tojo González R, de la Coba Ortiz C. Colitis asociada a rituximab. Gastroenterol Hepatol. 2019;42:251–252.