Helicobacter pylori is a spiral Gram-negative bacillus, which colonizes the human stomach and plays a key role in the pathogenesis of a number of gastroduodenal diseases. However, when expose to environmental stressed conditions, such as increased oxygen tension, extended incubation and exposure to antibiotics, H. pylori is able to entering the viable but nonculturable state, in which the bacterium modifies its morphology from a spiral to coccoid form, as a manifestation of cell adaptation to these adverse conditions. In gastric tissues, viable coccoid forms may remain latent for long time and retain virulence factors, so these forms possibly contribute to the treatment failures and recurrence of H. pylori infection and gastroduodenal diseases as well. In this review, we will discuss several aspects of cellular adaptation and survival of H. pylori, antibiotic susceptibility and virulence of coccoid forms and its involvement with recrudescence.
Helicobacter pylori es un bacilo espiral gramnegativo que coloniza el estómago humano y desempeña un papel clave en la patogénesis de diferentes enfermedades gastroduodenales. Sin embargo, cuando se expone a condiciones de estrés ambientales, tales como el aumento de la tensión de oxígeno, la incubación prolongada o la exposición a antibióticos, Helicobacter pylori entra en un estado viable pero no cultivable, en el cual la bacteria modifica su morfología de una forma bacilar a una cocoide como manifestación de adaptación celular a estas condiciones adversas. En el tejido gástrico, las formas cocoides viables pueden permanecer latentes durante mucho tiempo y retener factores de virulencia, por lo que estas formas posiblemente puedan contribuir a los fracasos del tratamiento y la recurrencia de la infección y de las enfermedades gastroduodenales también. En esta revisión se discutirán varios aspectos de la adaptación celular y supervivencia de Helicobacter pylori, la susceptibilidad a los antibióticos y la virulencia de las formas cocoides y su participación en la recrudescencia.
Helicobacter pylori (H. pylori) is a Gram-negative microaerophilic bacterium, whose natural habitat is the stomach. Although it typically has a bacillary form with several flagella at one end, it adopts a coccoid appearance in unfavourable environmental conditions.1,2
H. pylori is a major aetiological factor in active chronic gastritis, peptic ulcer, gastric mucosa-associated lymphoid tissue (MALT) lymphoma and gastric cancer. Although the bacterium is estimated to be present in the gastric mucosa of half the world's population, these diseases only develop in approximately 15–20% of colonized individuals.2,3
The most common treatment regimens have resulted in an eradication rate of around 85% in many geographical areas,4–6 but efficacy has been compromised, especially in recent years, by the rapid emergence of antibiotic-resistant strains and poor treatment adherence.5,7
It is important to consider that the cure (as well as prevention of complications) for active chronic gastritis and peptic ulcer and for some low-grade forms of gastric MALT lymphoma depends on the success of H. pylori eradication. Furthermore, ensuring and sustaining successful eradication of this microorganism in all its biological forms would prevent recrudescence of the infection and, therefore, disease relapses.
The aim of this review is to present a general overview of the coccoid form of H. pylori, highlighting its microbiological profile, antibiotic susceptibility and virulence. Its involvement in gastric disease will also be analyzed, and the extent to which it is associated with infection recrudescence and disease relapse will be examined.
Survival and cellular adaptation of H. pyloriGeneral considerationsAll living organisms are equipped with mechanisms that enable them to survive in adverse environments. For some, their response implies (in addition to metabolic adaptations) changes to cell morphology.8,9 Several microorganisms have the ability to differentiate into a viable but non-culturable (VBNC) state in response to environmental conditions that are unfavourable to survival and growth. This strategy is widely used by microorganisms such as Salmonella, Campylobacter and Escherichia.10,11
In the VBNC state, bacteria generally decrease their size and change shape to become small spherical bodies. They reduce their respiration rate and nutrient transport and change gene expression and molecular synthesis. In this state they cannot be detected using conventional culture techniques. However, changes in environmental conditions may lead these microorganisms to either “revive” (and reconvert to their active state) or degenerate.10–13
Similarly, H. pylori, when it experiences harmful environmental conditions (in or outside its natural habitat), changes its classic bacillary form and enters a VBNC state that leads to morphological and metabolic changes as well as modifications in growth behaviour.14,15
Viable but non-culturable state of H. pyloriAlthough some authors initially tried to demonstrate that the conversion from bacillary to coccoid form was a passive process that resulted in cell death and, therefore, that the coccoid forms were the degenerated remains of dead bacteria,16,17 three forms of H. pylori are presently considered to exist, namely (in order of most to least virulent), the viable, culturable bacillary form, the VBNC coccoid form and the non-viable degenerative form.18–20
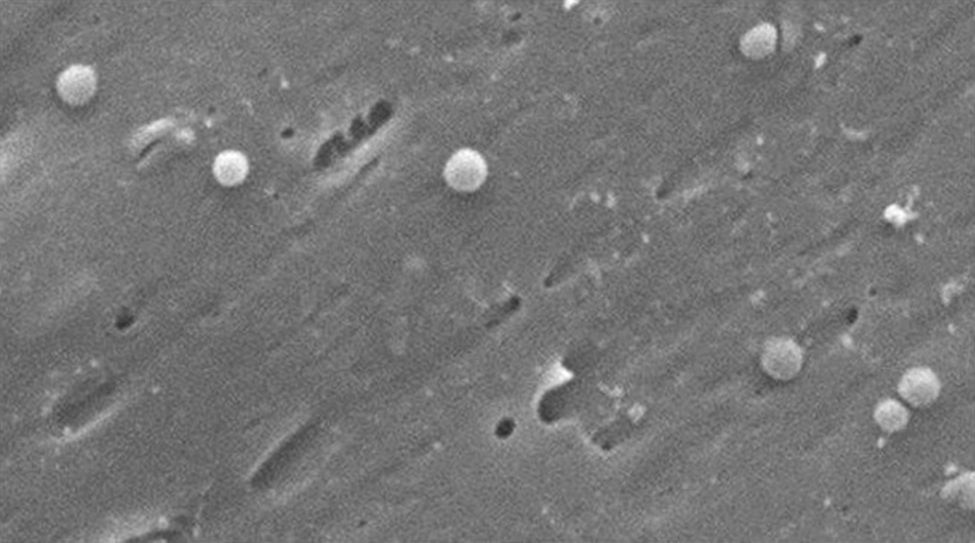
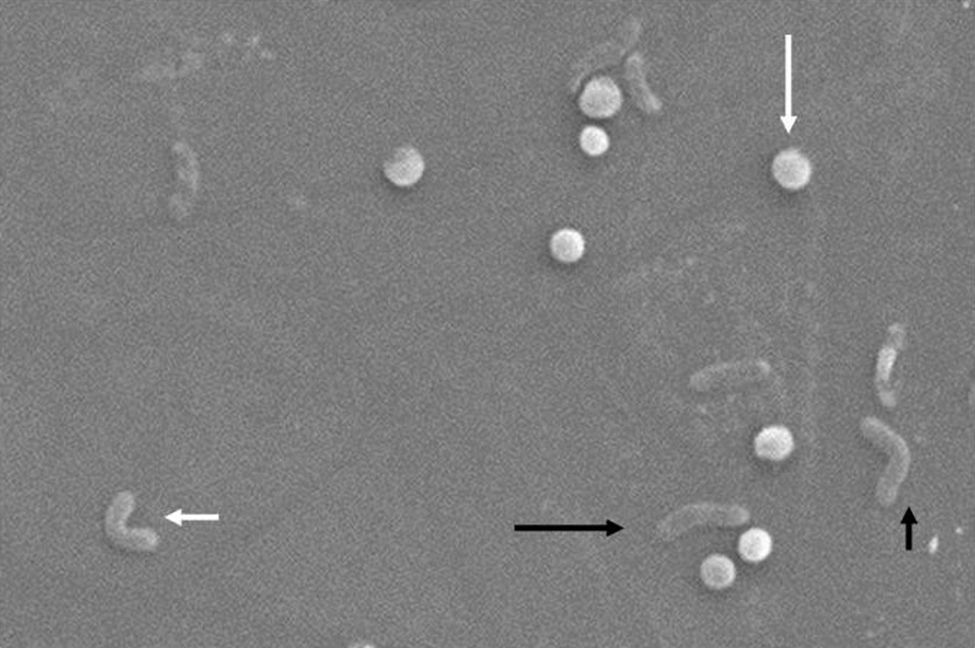
Morphologically, conversion from the bacillary to the coccoid form occurs through intermediate V- and U-forms13,14 (Figs. 1 and 2), in which cell structures like the cytoplasm and cell membrane remain intact,15,21 while the flagella tend to wrap around the coccoid cellular structure, thereby becoming near invisible. Other ultrastructural modifications to the coccoid form result in two sub-types. Type A has irregular edges with a rough surface and is considered to be a dead cell, while type B has a smoother surface, is smaller and is considered to be a living cell.21,22
Morphological changes in Helicobacter pylori. Spiral form (long black arrow), V-form (short black arrow), U-form (short white arrow) and coccoid form (long white arrow). Scanning electron microscope image courtesy of Dr. Nuno F. Azevedo. LEPABE-Chemical Engineering Department of the Faculty of Engineering at the University of Oporto (Portugal).
In scanning electron microscope (SEM) analyses of the surface mucous gel layer of gastric cancer patients infected with H. pylori, Ogata et al.23 observed the co-existence of both bacillary and coccoid forms. Other authors have confirmed the presence of the coccoid form in both the human stomach and duodenum,24–26 although it seems that the percentage of coccoid forms is higher in the duodenum than in the stomach, to the point where only the coccoid form is observed in some duodenal biopsies.26,27 This fact suggests either that H. pylori is forced to adapt to biological conditions in the duodenum or that these conditions are ideal for H. pylori to take refuge there in the VBNC state.
In vitro and in vivo experiments have shown that, in unfavourable conditions like aerobiosis,28,29 alkaline pH,29,30 high temperature,31 lengthy incubation periods,29,32 prolonged incubation in water33 or treatment with proton pump inhibitors32 or antibiotics,34 the coccoid phenotype can maintain a certain level of metabolic activity, as it expresses a wide variety of genes present in the bacillary form,35 maintains detectable levels of urease activity,36 continues synthesizing proteins, albeit in smaller quantities (less than 1% of the quantity of proteins synthesized by the bacillary form)37 and produces small amounts of DNA, maintained even after 3 months storage in physiological saline solution at 4°C.29,38 All these data underline the fact that the biological changes in H. pylori in response to harmful stimuli reflect its powerful ability, during its useful life, to preserve the species.
Biofilm formationAs with other bacteria, the ability of H. pylori to persist as an infectious entity and resist the arsenal of antimicrobial agents aimed at eradicating it is due to the genetic variability that enables it to develop bacterial resistance. Its survival strategy is further enhanced when bacterial cells form what are called biofilms.20 Biofilms are a kind of microbial “community” in which the bacteria adhere firmly to biotic or abiotic surfaces by means of a self-produced matrix. This matrix, composed of extracellular polymeric substances,39,40 creates an environment that is very tolerant of antimicrobial agents and strongly resistant to phagocytosis. Various host defence mechanisms are therefore evaded, making eradication difficult. Biofilms are considered to be truly complex, dynamic systems that facilitate horizontal gene transfer between bacteria. They thus confer bacterial populations with new modified genomes that contribute significantly to bacterial resistance, strain variability and adaptability.41–43
In a study of 21 biopsies from patients in whom the bacterium had previously been eradicated, H. pylori was isolated by culture in 7 of the biopsies, whilst gene expression techniques detected viable H. pylori cells in most of the biopsies. SEM, meanwhile, showed clustered coccoid bacteria arranged in a microbial biofilm, suggesting that the coccoid forms could facilitate bacterial persistence and antibiotic resistance.44
Susceptibility to antibiotics of the coccoid formMany studies have shown that H. pylori can change from the bacillary to coccoid form on exposure (in vitro) to various antimicrobial agents. Different concentrations of amoxicillin, clarithromycin, metronidazole and erythromycin (to mention just a few of the antibiotics available) can induce this morphological transformation.34,35,45 The greatest induction effect has been observed with amoxicillin,27,34,46 known to be highly effective in vitro against H. pylori; however, morphological observations of the cultures show that bacillary forms decrease in number in favour of coccoid forms.34,46 Faghri et al.,47 achieved bactericidal effects for the coccoid forms, at over 60% with metronidazole at twice the minimum inhibitory concentration (MIC), and at 80–90% with clarithromycin at the MIC; however, amoxicillin treatment with MIC and MIC× 2 did not inhibit viable coccoid forms. Similarly, Berry et al.34 observed that while amoxicillin at MIC× 10 eliminated bacillary forms of H. pylori, it also induced the formation of coccoid forms. Perkins et al.48 observed, in a study of cats infected naturally with H. pylori, that 6 weeks after eradication treatment, gastric juices were positive for H. pylori in just one cat, yet polymerase chain reaction (PCR) analysis identified H. pylori genetic material in all the cats in the study. Even though H. pylori was detected in a single cat, the histological lesions were consistent with chronic gastritis and were marked by the presence of lymphoid follicles.
Bearing in mind these microbiological and basic research data, in a previous study conducted in patients infected with H. pylori—where sensitivity of the isolated strains to amoxicillin was previously determined—dual therapy (proton pump inhibitors and amoxicillin) obtained a cure rate for amoxicillin-sensitive strains of only 66%. This outcome demonstrates the presence of important additional independent bacterial resistance factors related to the successful use of this antibiotic.49 This is especially so if we consider that it seems impossible that coccoid forms could be sensitive to β-lactam antibiotics, because coccoid forms have different penicillin-binding protein profiles from bacillary forms.50 It is therefore likely that not all H. pylori organisms are completely eliminated following eradication treatment; rather, some are likely to be converted to coccoid forms and so become resistant to antibacterial drugs. This would explain treatment failure and recrudescence.13,34,45
It is worth highlighting that some very recent studies have demonstrated that free fatty acids, such as linolenic acid and liposomal linolenic acid, have a bactericidal effect on both H. pylori forms, regardless of their resistance to antibiotics.51,52 These molecules could therefore have a potentially effective antimicrobial effect in treating infection with H. pylori, especially in its coccoid form.
Virulence and pathogenicity of the coccoid formThe virulence factors for the bacillary form of H. pylori and the mechanisms by which this bacterium is involved in the development of gastrointestinal diseases have been extensively studied.1,3,4 However, little is known about the virulence and pathogenicity of the coccoid form. Below we review the most relevant findings on this subject.
Like the bacillary form, the coccoid form expresses major virulence genes, such as ureA, ureB, hpaA, vacA and cagA, cagE and BabA.35,53,54 This expression, which occurs over a lengthy period, probably plays an important role in chronic severe stomach disorders.
Adherence of H. pylori to the gastroduodenal epithelium is known to be an important step in the induction of active chronic inflammation of the mucosal layer. SEM studies have found that the coccoid form of H. pylori can present on the plasma membrane surface of the gastric epithelial cells and, like the bacillary form, has the ability to invade these cells.15,55 If cell invasion occurs, the coccoid forms are enclosed in double-layer membrane vesicles and the gastric epithelial cells appear swollen and lytic, showing erosion of the mucosal layer.56 Given that the coccoid form is less susceptible to antibiotics, it is believed that these latent plasma membrane forms can spread and infect other neighbouring epithelial cells in the absence of an effective concentration of antibiotics.57
H. pylori infection is also known to induce a local immune response that fails to eradicate the bacteria, thereby permitting the gastric disease to become chronic. The immune response can be determined by antibody detection using serological methods developed using the bacillary form of H. pylori.58 In fact, the presence of these specific antibodies can be used as an epidemiological indicator of infection and to confirm the success of treatment. There are, however, no serological methods that detect coccoid forms. In order to determine whether coccoid forms had any effect on the immune response in colonized individuals, Figueroa et al.59 devised a specific enzyme-linked immunosorbent assay (ELISA) technique to evaluate and compare the immune response to coccoid and bacillary forms against a panel of sera from symptomatic and asymptomatic infected individuals. The coccoid forms of H. pylori were shown to induce a humoral immune response similar to that induced by the bacillary forms in infected individuals. In another study conducted in children with epigastric pain, the possible role of the coccoid form in H. pylori infection was examined using an ELISA technique and antigens prepared from bacillary and coccoid cell forms. It was found that 13.3% and 55.8% of cells were seropositive for antigens of the bacillary form and the coccoid form, respectively, whereas seropositivity values for asthmatic children were only 7.0% and 26.5%, respectively. This roughly fourfold difference in seropositivity between the coccoid- and bacillary-form antigens in symptomatic and asymptomatic patients could suggest a possible infective role of the coccoid form of H. pylori.60
Cellini et al.61 intragastrically inoculated concentrated suspensions of H. pylori in coccoid form in a BALB/c mouse model. H. pylori was isolated 2 weeks later, histopathological changes occurred 1 month later and all the colonized mice showed a systemic antibody response to H. pylori. In other experiments with BALB/c mice, animals inoculated with coccoid forms developed significant pathological changes in the stomach, including erosive lesions and inflammatory cell infiltration of the gastric mucosa.36 She et al.,53 in order to compare virulence and infectivity, intragastrically inoculated BALB/c mice with H. pylori, one group with the bacillary form and a second group with the coccoid form. In the SEM examination of samples from the 2 groups, they observed adherence of both bacillary and coccoid forms to the epithelial cells of the gastric wall and the presence of flagella in the coccoid forms. Histological examination showed different grades of lesions in the gastric mucosa, from mild inflammatory cell infiltration to erosions and ulcers. The mucosal lesion was milder in the mice infected by the coccoid form, while a positive result was not obtained in the control group that received sterile water.53 Rabelo-Gonçalves et al.62 showed that coccoid forms of H. pylori induced an acute inflammatory response in the stomach of mice from the earliest stages of the infection. The above results highlight the ability of coccoid forms to colonize and infect the gastric mucosa in vivo.
Several studies have revealed the presence of the coccoid form in water.33,63 One such study—by our group—compared 2 groups of weaned Wistar mice, one administered well water and tap water for a prolonged period of time and the other administered sterile distilled water, finding that the study group mice developed a chronic inflammatory process with formation of lymphocytic plaques and the presence of bacilli consistent with H. pylori.64
As previously mentioned, it would be logical to suppose that, in unfavourable conditions, H. pylori enters a “quiescent” state, modifying its classic bacillary form to the coccoid form without producing degenerative changes in its genome and retaining the ability to revert to the bacillary form once environmental or natural habitat conditions improve.
Participation of the coccoid form in recrudescenceRecurrence of H. pylori following successful eradication is rare in developed countries compared to developing countries, with annual recurrence rates of 2.67% and 13%, respectively.65,66
There are two types of recurrence of H. pylori infection: recrudescence, when the bacterial strain responsible for the recurrence is genetically identical to that isolated prior to eradication; and re-infection, when recurrence is caused by a different strain.67 Differentiating recrudescence from re-infection requires PCR or genetic polymorphism analysis to identify bacterial strains.67,68 Since these methods are not routinely applied, it is often impossible to differentiate between recrudescence and re-infection in routine clinical practice.
H. pylori recurrence is clinically relevant, since peptic ulcer relapse can be observed in a considerable proportion of infected patients, whereas the reappearance of microorganisms could explain some MALT lymphoma recurrences following treatment.69 Factors such as drinking tap water, dental and gum disease, recurrent tonsillitis, hospitalization, dental and medical equipment and contact with family members are believed to affect H. pylori recurrence.70–72 Other factors associated with infection recurrence are younger age, diabetes in young patients, low annual income and long-term inhibition of gastric acid following eradication.73–76
Recrudescence is considered the most likely reason for recurrence in the first year following eradication, while re-infection can occur after a longer period.68,77 Many cases of recurrence in developed countries are in reality due to recrudescence. Re-infection is more common in developing countries, since people are apparently constantly exposed to H. pylori.65,78
Using polyacrylamide gel protein electrophoresis techniques, Costas et al.79 found that patients with recurrence 4 weeks after eradication treatment were not in fact infected with another strain of H. pylori; rather, the strain that had caused the original infection had not been completely eradicated by the treatment, leading to recrudescence of the infection. Therefore, it is important to take into account the efficacy of the therapeutic regimen: H. pylori recurrence is frequent in patients treated with low-efficacy therapies, but is rare when high-efficacy therapies are used. This was demonstrated by a study on the incidence of H. pylori recurrence in Spain by Gisbert et al.,80 who found that H. pylori recurrence 6 months after eradication was 8.2% in patients treated with low-efficacy therapies but only 1.7% in patients treated with high-efficacy therapies.
In a study conducted in Korea from 2007 to 2010, H. pylori recurrence rates were analyzed after 6 months of successful first- and second-line eradication therapies, with annual follow-up—to the end of the study period—based on breath tests, stomach biopsy and rapid urease tests.81 It was found that the annual recurrence rates within and after the 2-year follow-up were 9.3% and 2.0%, respectively, after the first-line therapy, and 4.5% and 2.9% respectively, after the second-line therapy. The authors concluded that annual H. pylori recurrence rates for patients who received eradication treatment showed a sharp drop after the 2-year follow-up period. This was considered sufficient time after treatment to confirm eradication, and also sufficient time to enable a distinction to be made between recurrence and recrudescence of H. pylori strains.81
Final considerations and conclusionsGiven the ability of H. pylori to enter a VBNC state when subjected to unfavourable conditions within or outside its habitat, it is reasonable to suppose that antibiotic treatment regimens used to eradicate the bacillary form of H. pylori may induce VBNC coccoid forms capable of surviving for lengthy periods in the gastroduodenal environment. From here they may have direct and indirect pathogenic potential that leads to recrudescence of the infection and, as a result, treatment failures, infection relapses and recurrence of gastroduodenal disease. Successful eradication may therefore require not only eradication of the bacillary forms but also of the coccoid forms, or ensuring that coccoid forms are not induced.
Bearing in mind that, since routine methods currently implemented in clinical practice to confirm H. pylori eradication cannot detect coccoid forms, hosts may be incorrectly diagnosed as free of infection; furthermore, these methods may not be able to provide complete evidence of the clinical potential of the drugs used to eradicate H. pylori. Thus, for eradication to be considered successful, annual follow-up is recommended—using non-invasive techniques or, if available, molecular methods—to determine whether bacteria have been completely eliminated, most especially in high-prevalence areas and in patients at risk of recurrence.
Finally, further studies are necessary of the coccoid VBNC form of H. pylori, its pathogenic potential, its involvement in infection and recrudescence and its role in forming biofilms in the stomach and other locations within the host. Such studies would enable the development both of more effective diagnostic protocols that avoid underestimating colonization by H. pylori and of novel therapeutic strategies aimed at eliminating coccoid forms and “disarming” biofilms.
Conflict of interestsThe authors declare that they have no conflict of interests.
The authors would like to thank Dr. Nuno F. Azevedo of the Chemical Engineering Department of the Faculty of Engineering at the University of Oporto (Portugal) for providing the images published in this article.
Please cite this article as: Sarem M, Corti R. Rol de las formas cocoides de Helicobacter pylori en la infección y la recrudescencia. Gastroenterol Hepatol. 2016;39:28–35.