Sunlight exposure is the main source of vitaminD. Our aim was to describe both sun exposure and sun protection behaviour in a series of patients with inflammatory bowel disease (IBD), and to study their potential association with vitaminD concentration.
Patients and methodsA cross sectional, observational study. The clinical-demographic variables were obtained via clinical interviews and medical history review. The sunlight exposure assessment was carried out using the Sun Exposure Questionnaire and the concentration of 25-hydroxy vitaminD (25OHD) was measured by an electro-chemiluminescence immunoassay. Questionnaires were conducted on quality of life, physical activity, weekly vitaminD intake and sun protection behaviour.
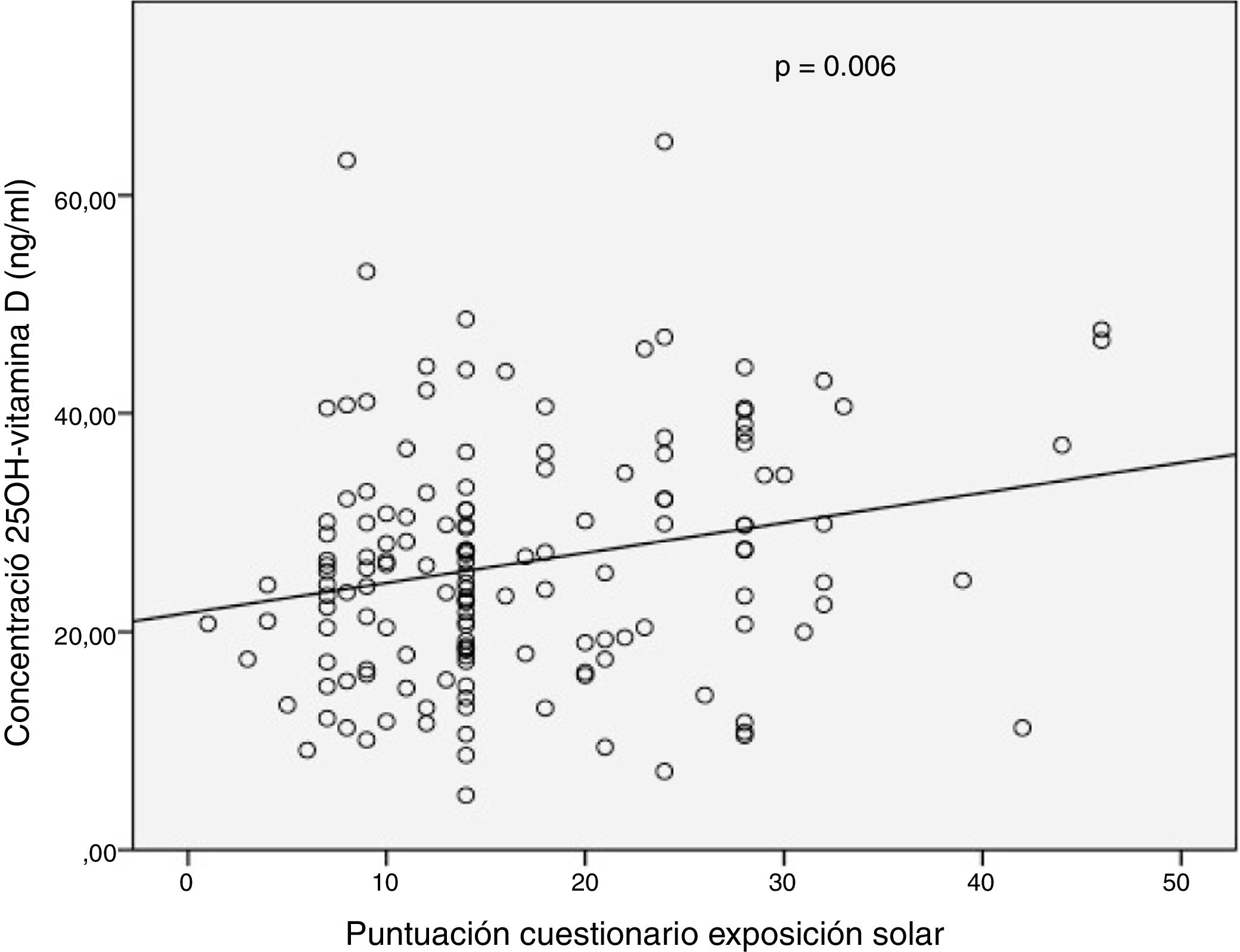
Results149 patients were included. In 69% of patients, deficient or insufficient 25OHD values were recorded. 67% showed low sun exposure. A modest significant correlation was observed between the total score of the solar exposure questionnaire and the 25OHD concentration in the complete series (r=0.226, P=.006) and in the summer (r=0.274, P=.01). The sun protection behaviour questionnaire score did not influence the 25OHD concentration. In the multivariate analysis, only the presence of clinical activity was associated with low sun exposure (OR=3.23).
DiscussionSun exposure according to the questionnaire used was low, was associated with the presence of clinical activity and was weakly correlated with serum 25OHD concentration. More studies are needed to explore the use of individual questionnaires for sun exposure and its relationship with vitaminD in patients with IBD.
La exposición solar es el principal determinante del estado de vitaminaD. Nuestro objetivo fue describir las prácticas de exposición y protección solar de una serie de pacientes con enfermedad inflamatoria intestinal (EII) y evaluar su influencia en la concentración sérica de vitaminaD.
Pacientes y métodosEstudio observacional de tipo transversal. Las variables clínico-demográficas se obtuvieron mediante entrevista clínica y revisión de la historia. La evaluación de la exposición solar se realizó mediante el Sun Exposure Questionnaire. La concentración de 25-hidroxivitaminaD (25OHD) se determinó por electroquimioluminiscencia. Se realizaron cuestionarios de calidad de vida, actividad física, ingesta semanal de vitaminaD y hábitos de protección solar.
ResultadosSe incluyeron 149 pacientes. En el 69% de los pacientes se registraron valores deficientes o insuficientes de 25OHD. El 67% presentaron una baja exposición solar. Se observó una modesta correlación significativa entre la puntuación total del cuestionario de exposición solar y la concentración de 25OHD en la serie completa (r=0,226; p=0,006) y en verano (r=0,274; p=0,01). La puntuación del cuestionario de protección solar no influyó en la concentración de 25OHD. En el análisis multivariado solo la presencia de actividad clínica se asoció a una exposición solar baja (OR=3,23).
DiscusiónLa exposición solar de acuerdo con el cuestionario empleado fue baja, se asoció a la presencia de actividad clínica y se correlacionó débilmente con la concentración de 25OHD sérica. Se necesitan más estudios que exploren el uso de cuestionarios individuales de exposición solar y su correlación con la vitaminaD sérica en la EII.
Vitamin D deficiency is prevalent in patients with inflammatory bowel disease (IBD).1 In addition, percentages of this vitamin deficiency in IBD are higher than those observed in a healthy population.2 Beyond the known classic effects of vitamin D on musculoskeletal health in patients with IBD, a considerable number of studies in the past decade have focused their attention on its extra-skeletal effects.3,4 However, the majority of these studies have a cross-sectional design; therefore, the complex relationship between vitamin D and IBD remains a chicken-and-egg dilemma.5
It has been reported that the greatest relative risk of suffering from Crohn's disease (CD) in countries at more northern latitudes may be related, among other factors, to less sun exposure (north–south gradient).6,7 In addition, some studies conducted in countries in northern Europe have linked sun exposure to clinical recurrences of IBD, development of ulcerative colitis (UC) and a worse clinical course for CD.8–10 Another cross-sectional, multi-centre study in the United States linked lower regional ultraviolet exposure to more serious IBD and a higher rate of surgical procedures.11
Vitamin D is primarily obtained by means of endogenous synthesis based on solar ultraviolet radiation. This converts skin 7-dehydrocholesterol to previtamin D, and subsequent dual hepatic and renal hydroxylation yields the active metabolites of vitamin D.12 Thus, in Mediterranean countries such as Spain, where there is no health policy for fortifying foods with vitamin D, daily dietary intake of vitamin D contributes only marginally to overall vitamin D status in an individual who does not take supplements.13
The main data on sun exposure in patients with IBD come from studies conducted in countries at theoretically unfavourable latitudes (northern and central Europe).14 At moderate latitudes, such as Spain's, sun exposure increases levels of 25-hydroxy vitamin D (25(OH)D) during the summer but not the autumn or winter.15 Other factors, in addition to latitude, that influence skin or endogenous production of vitamin D are solar radiation protection habits, skin phototype, age, ethnicity and cultural determinants (clothing). People with darker phototypes (Fitzpatrick IV-VI) produce a sixth part of vitamin D as opposed to people with lighter skin (Fitzpatrick I-II) despite equal duration of sunlight exposure.16
The development of questionnaires to evaluate sun exposure and sun protection practices among patients has given clinicians new tools to aid in determining who would benefit from closer screening or follow-up of vitamin D status. These questionnaires are meant to be inexpensive, simple ways to estimate quantitative objective determinations of sunlight exposure provided by dosimeters.17
In addition, a significant percentage of patients with IBD are on treatment with thiopurine immunosuppressants. This means that clinicians alert them to the fact that they are at a higher risk of developing skin tumours and advise them to engage in strict sun protection behaviours that may reduce their sun exposure during the periods of the year with the most hours of sunlight.18
Taking all the above into account, given the scant data available on sun exposure in patients with IBD in Spain, the objective of our study was to evaluate sun exposure and sun protection practices in a series of outpatients at a regional reference hospital and to identify predictive factors for these things. We also set out to investigate the relationship between, on the one hand, sun exposure and sun protection practices, and, on the other hand, vitamin D status in these patients.
Patients and methodsThis cross-sectional observational study was conducted at the Hospital Regional Universitario de Málaga [Málaga Regional University Hospital] (Gastrointestinal Clinical Management Unit) from 1 March 2016 to 31 April 2017. The geographic area of the population cared for extends from latitudes 36° 18’ and 37° 17’ North, where the estimated number of hours of sunlight per year is a little more than 3,000 h (data from the Instituto Nacional de Estadística [Spanish National Statistics Institute]).
PatientsThe patients enrolled were over 18 years of age, outpatients and consecutive with a diagnosis of IBD (UC or CD) according to the criteria of the European Crohn's and Colitis Organisation (ECCO), and they were able to understand and fill in the information sheet and the study questionnaires. The exclusion criteria, in addition to refusal to participate in the study or sign the informed consent form, were concomitant coeliac disease, short bowel syndrome, liver and/or kidney failure, pregnancy, breast-feeding, treatment with antiepileptic drugs and taking of vitamin D supplements. Scores for clinical activity were calculated and clinical and demographic variables were recorded at the time of enrolment. The self-administered questionnaires (sunlight exposure, sun protection, dietary vitamin D intake, quality of life and physical activity) were collected a week after enrolment; at the same time, blood was drawn. Laboratory testing was the same as routine outpatient follow-up laboratory testing in an IBD specialist clinic together with determination of 25(OH)D levels. The patients were classified according to the Montreal classification.
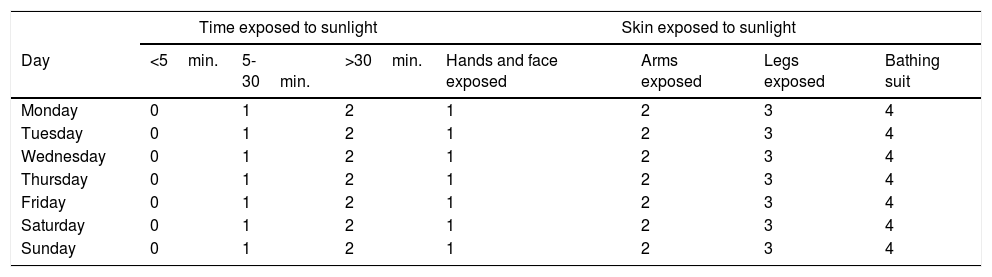
QuestionnairesThe patients’ clinical activity was evaluated using the Partial Mayo Scoring Index in UC and the Harvey–Bradshaw index (HBI) in CD. To measure their quality of life, the Spanish version of the Short Inflammatory Bowel Disease Questionnaire (SIBDQ-9) was used. The abridged Spanish version of the International Physical Activity Questionnaire (IPAQ) was used to evaluate the patients’ level of physical activity, yielding results in terms of multiples of the patients’ baseline metabolic rate, or metabolic equivalents (METs). With the aid of an expert on dietetics, based on the questionnaire designed and used by Vaqueiro et al. in a Spanish population19, the patients filled in a weekly survey on their dietary intake of vitamin D. The results were entered in the Dietstat® v.2.0 software program to determine the patients’ daily intake of vitamin D in micrograms. A version of the Sun Exposure Questionnaire translated into Spanish was used to measure sun exposure20 (Table 1).
Sun Exposure Questionnaire (version translated into Spanish was used).
| Time exposed to sunlight | Skin exposed to sunlight | ||||||
|---|---|---|---|---|---|---|---|
| Day | <5min. | 5-30min. | >30min. | Hands and face exposed | Arms exposed | Legs exposed | Bathing suit |
| Monday | 0 | 1 | 2 | 1 | 2 | 3 | 4 |
| Tuesday | 0 | 1 | 2 | 1 | 2 | 3 | 4 |
| Wednesday | 0 | 1 | 2 | 1 | 2 | 3 | 4 |
| Thursday | 0 | 1 | 2 | 1 | 2 | 3 | 4 |
| Friday | 0 | 1 | 2 | 1 | 2 | 3 | 4 |
| Saturday | 0 | 1 | 2 | 1 | 2 | 3 | 4 |
| Sunday | 0 | 1 | 2 | 1 | 2 | 3 | 4 |
This questionnaire, which showed a good correlation with 25(OH)D levels in a healthy middle-aged Italian population, was a simple and convenient way to obtain a quantitative estimate of the patients’ sun exposure between 9.00 a.m. and 4.00 p.m. based on two simple parameters: duration of sun exposure (0 = ≤5 min., 1 = 5-30 min. and 2 = ≥30 min.) and skin exposed to the sun (1 = face and hands, 2 = arms, 3 = legs and 4 = sunbathing). Daily sun exposure was determined by multiplying time by body surface (range 0-8), and weekly sun exposure was determined by adding up all the days of the week (0-56). Scores on the Sun Exposure Questionnaire were categorised as either low sunlight exposure (≤18) or moderate to high sunlight exposure (19-56). To determine daily sun protection habits, we selected five questions from the consensus questionnaire developed by Glanz et al.21 (use of sunscreen, use of a long-sleeved T-shirt, use of a cap or hat, use of sunglasses and tendency to stay in the shade on a sunny day). Answers to each question were assigned scores ranging from 1 (I don’t use protection at all) to 5 (I always use protection), with total scores of 5 to 25. According to this questionnaire, a score lower than or equal to 12 was considered to correspond to low-protection behaviour.
Evaluation of 25(OH)D levels and faecal calprotectinSerum 25(OH)D was measured using electrochemiluminescence immunoassay (Cobas® e-602, Roche, Switzerland, international standard calibrator NIST SRM 2972). According to the criteria of the Endocrine Society, 25(OH)D levels <20 ng/ml were considered deficient; levels of 20-29.9 ng/ml were considered insufficient and levels ≥30 ng/ml were considered sufficient.22 Given that the season in which the determination was made has been shown to be an essential variable in the assessment of vitamin D status in the majority of studies, a distinction was made between the winter period (November-April) and the summer period (May-October). An ELISA test (Calprest® Eurospital, Trieste, Italy) was used to measure faecal calprotectin (FC).
Statistical analysisQuantitative variables were presented in terms of mean and standard deviation if normal or in terms of median and interquartile range if not normal. Qualitative variables were expressed in terms of their frequency distribution. Normality was confirmed using Kolmogorov–Smirnov and Shapiro–Wilk tests. To analyse associations between qualitative variables, the chi-squared test was used, with Fisher's correction where appropriate. Differences between continuous quantitative variables in two independent groups were analysed using Student's t-test or the corresponding Mann–Whitney test in non-parametric cases. Correlations between quantitative variables were analysed using the Pearson's correlation coefficient test. To determine the variables that were or were not associated with low sun exposure, a multivariate logistic regression was prepared, adjusting for variables that were found to be significant in the bivariate analysis. Analysis was performed with the statistics software program R Project, version 3.4.4.
Ethical considerationsThe study protocol was approved by the Malaga Provincial Independent Ethics Committee. All patients granted their informed consent. Data were recorded anonymously in a codified electronic database. The project complied with the principles of the Declaration of Helsinki and good clinical practice standards.
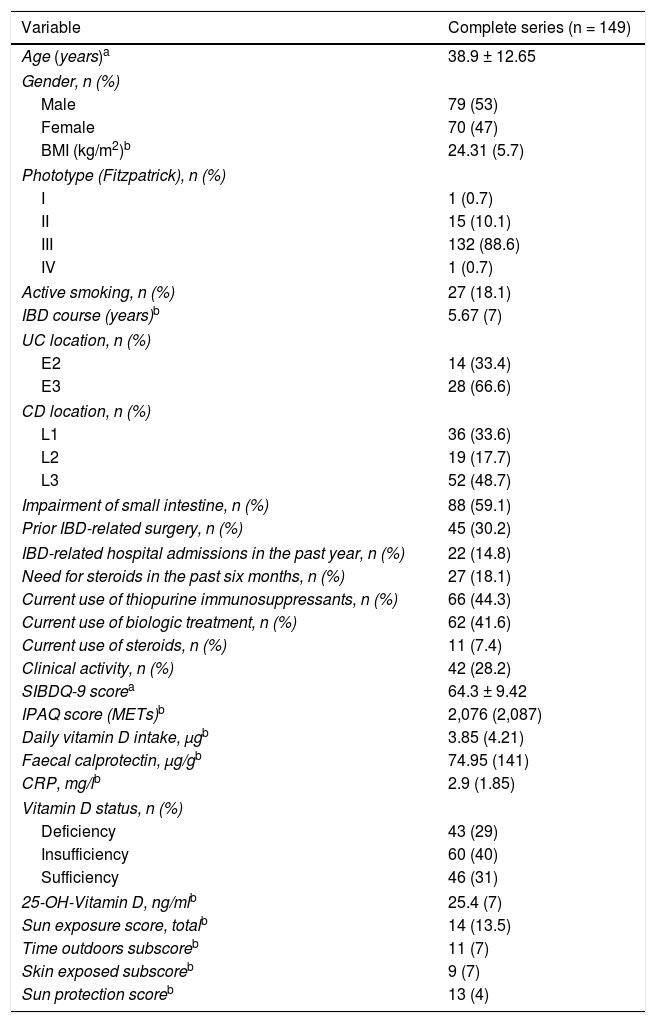
ResultsPatient characteristicsInitially, 178 outpatients and consecutive patients with IBD in regular follow-up were invited to participate. Of them, 15 were excluded for various reasons: language barrier or inability to read/understand questions (n = 9), pregnancy (n = 1), abnormal liver panel (n = 2), decreased glomerular filtration rate (n = 2) and antiepileptic treatment (n = 1). Another 14 patients did not finish filling in at least one of the questionnaires. Therefore, ultimately, 149 patients (42 with UC and 107 with CD) were enrolled for analysis. The general clinical and demographic characteristics, clinical chemistry variables and questionnaire-related variables of the patients enrolled are shown in Table 2.
Clinical and demographic characteristics of the complete series.
| Variable | Complete series (n = 149) |
|---|---|
| Age (years)a | 38.9 ± 12.65 |
| Gender, n (%) | |
| Male | 79 (53) |
| Female | 70 (47) |
| BMI (kg/m2)b | 24.31 (5.7) |
| Phototype (Fitzpatrick), n (%) | |
| I | 1 (0.7) |
| II | 15 (10.1) |
| III | 132 (88.6) |
| IV | 1 (0.7) |
| Active smoking, n (%) | 27 (18.1) |
| IBD course (years)b | 5.67 (7) |
| UC location, n (%) | |
| E2 | 14 (33.4) |
| E3 | 28 (66.6) |
| CD location, n (%) | |
| L1 | 36 (33.6) |
| L2 | 19 (17.7) |
| L3 | 52 (48.7) |
| Impairment of small intestine, n (%) | 88 (59.1) |
| Prior IBD-related surgery, n (%) | 45 (30.2) |
| IBD-related hospital admissions in the past year, n (%) | 22 (14.8) |
| Need for steroids in the past six months, n (%) | 27 (18.1) |
| Current use of thiopurine immunosuppressants, n (%) | 66 (44.3) |
| Current use of biologic treatment, n (%) | 62 (41.6) |
| Current use of steroids, n (%) | 11 (7.4) |
| Clinical activity, n (%) | 42 (28.2) |
| SIBDQ-9 scorea | 64.3 ± 9.42 |
| IPAQ score (METs)b | 2,076 (2,087) |
| Daily vitamin D intake, μgb | 3.85 (4.21) |
| Faecal calprotectin, μg/gb | 74.95 (141) |
| CRP, mg/lb | 2.9 (1.85) |
| Vitamin D status, n (%) | |
| Deficiency | 43 (29) |
| Insufficiency | 60 (40) |
| Sufficiency | 46 (31) |
| 25-OH-Vitamin D, ng/mlb | 25.4 (7) |
| Sun exposure score, totalb | 14 (13.5) |
| Time outdoors subscoreb | 11 (7) |
| Skin exposed subscoreb | 9 (7) |
| Sun protection scoreb | 13 (4) |
BMI: body mass index; CRP: C-reactive protein; SIBDQ: short inflammatory bowel disease questionnaire.
Around 30% of the series had clinically active disease, and around half of them were on immunosuppressant and/or biologic treatment. Approximately a third of the patients with CD had undergone a resective surgery. Phototype III was the predominant phototype (88%).
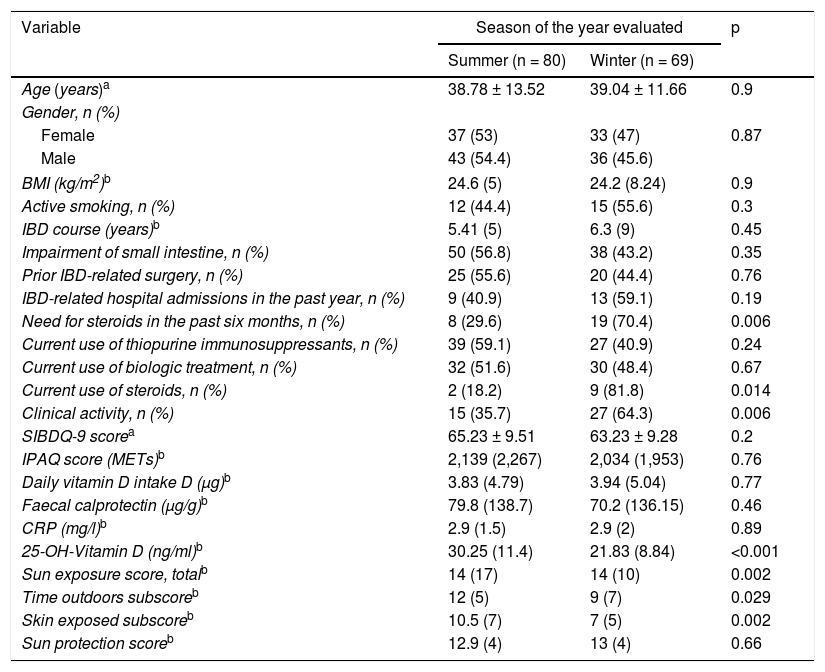
Table 3 shows a comparison of the different variables studied, making a distinction as to the season of the year in which the patients were evaluated. Median vitamin D levels in summer were significantly higher than those seen in the patients in whom vitamin D was measured in winter (p <0.001). Similarly, median sun exposure questionnaire scores and corresponding subscores were higher in the summer period. The highest percentage of patients clinically active in winter (64.3% vs 35.7%; p = 0.006) was not accompanied by differences in median levels of FC and C-reactive protein (CRP) between the two seasons. There were also no differences in sun protection questionnaire scores or greater use of immunosuppressants or biological treatment. A higher percentage of patients analysed in winter were on treatment with steroids or had required them in the past six months.
Comparative study of variables in relation to season of the year evaluated.
| Variable | Season of the year evaluated | p | |
|---|---|---|---|
| Summer (n = 80) | Winter (n = 69) | ||
| Age (years)a | 38.78 ± 13.52 | 39.04 ± 11.66 | 0.9 |
| Gender, n (%) | |||
| Female | 37 (53) | 33 (47) | 0.87 |
| Male | 43 (54.4) | 36 (45.6) | |
| BMI (kg/m2)b | 24.6 (5) | 24.2 (8.24) | 0.9 |
| Active smoking, n (%) | 12 (44.4) | 15 (55.6) | 0.3 |
| IBD course (years)b | 5.41 (5) | 6.3 (9) | 0.45 |
| Impairment of small intestine, n (%) | 50 (56.8) | 38 (43.2) | 0.35 |
| Prior IBD-related surgery, n (%) | 25 (55.6) | 20 (44.4) | 0.76 |
| IBD-related hospital admissions in the past year, n (%) | 9 (40.9) | 13 (59.1) | 0.19 |
| Need for steroids in the past six months, n (%) | 8 (29.6) | 19 (70.4) | 0.006 |
| Current use of thiopurine immunosuppressants, n (%) | 39 (59.1) | 27 (40.9) | 0.24 |
| Current use of biologic treatment, n (%) | 32 (51.6) | 30 (48.4) | 0.67 |
| Current use of steroids, n (%) | 2 (18.2) | 9 (81.8) | 0.014 |
| Clinical activity, n (%) | 15 (35.7) | 27 (64.3) | 0.006 |
| SIBDQ-9 scorea | 65.23 ± 9.51 | 63.23 ± 9.28 | 0.2 |
| IPAQ score (METs)b | 2,139 (2,267) | 2,034 (1,953) | 0.76 |
| Daily vitamin D intake D (μg)b | 3.83 (4.79) | 3.94 (5.04) | 0.77 |
| Faecal calprotectin (μg/g)b | 79.8 (138.7) | 70.2 (136.15) | 0.46 |
| CRP (mg/l)b | 2.9 (1.5) | 2.9 (2) | 0.89 |
| 25-OH-Vitamin D (ng/ml)b | 30.25 (11.4) | 21.83 (8.84) | <0.001 |
| Sun exposure score, totalb | 14 (17) | 14 (10) | 0.002 |
| Time outdoors subscoreb | 12 (5) | 9 (7) | 0.029 |
| Skin exposed subscoreb | 10.5 (7) | 7 (5) | 0.002 |
| Sun protection scoreb | 12.9 (4) | 13 (4) | 0.66 |
BMI: body mass index; CRP: C-reactive protein; SIBDQ: short inflammatory bowel disease questionnaire.
Some 67% (100/149) of patients had low levels of sun exposure (score <18) according to the questionnaire used; the remaining 33% of patients had moderate to high levels of sun exposure. No significant differences were recorded in the median total scores on the sun exposure questionnaire between patients with CD and patients with UC (14 [14] vs 14 [12]; p = 0.29). Median 25(OH)D levels in the low sun exposure group were significantly lower than in the moderate to high sun exposure group (24.23 [12] vs 29.8 [18.5] ng/ml; p = 0.04).
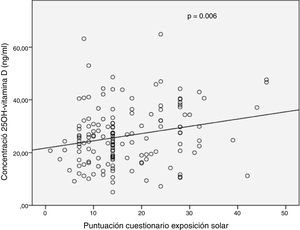
In the complete series (n = 149), a weak significant positive correlation was observed between 25(OH)D levels and total sun exposure score (r = 0.226; p = 0.006) or skin exposed subscore (r = 0.201; p = 0.03) (Fig. 1). No significant correlation was recorded between vitamin D levels and subscore for time outdoors weekly (r = 0.052; p = 0.52). When a distinction was made between seasons in which vitamin D levels were measured, a significant positive correlation was also seen between serum 25(OH)D levels and total score for sun exposure in summer (r = 0.274; p = 0.01).
Sun protection and 25(OH)D levelsIn 58.4% of the patients with IBD, a score on the sun protection questionnaire lower than 12 (low protection) was obtained. No significant correlation was recorded between sun protection questionnaire scores and vitamin D levels either in the complete series or after a distinction was made between summer and winter. Median sun protection questionnaire scores in patients on thiopurine immunosuppressive treatment did not differ from those obtained in patients not on said treatment in the complete series (13 [4] vs 13 [6]; p = 0.43), or following analysis by season of determination.
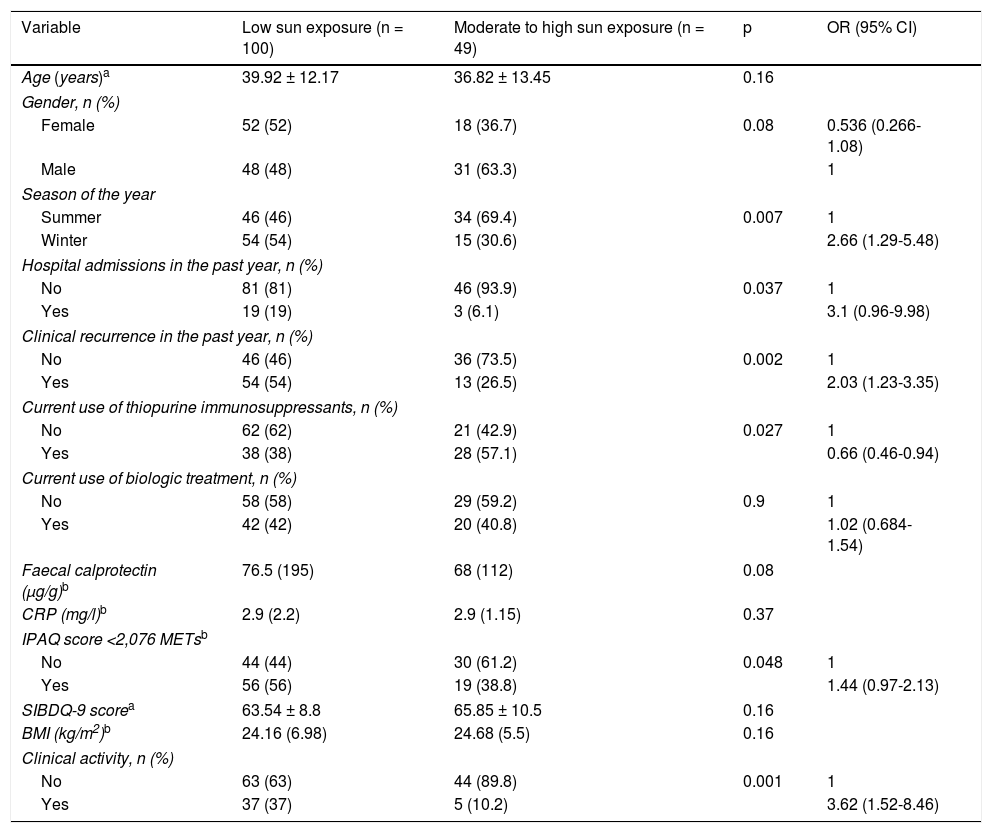
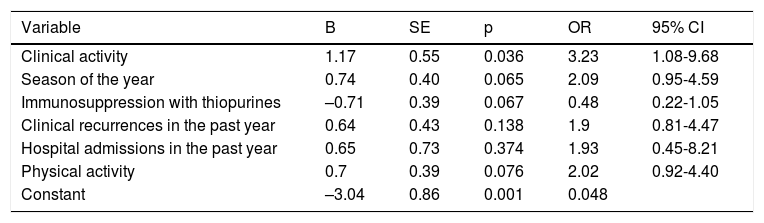
Factors associated with low sun exposureThe presence of clinical activity, evaluated by the Harvey–Bradshaw index or the Partial Mayo Scoring Index, was associated with low sun exposure (37% vs 10.2%; p = 0.001). In addition, the percentage of patients who had experienced at least one episode of clinical recurrence requiring a change in treatment in the previous year was twice as high in the low sun exposure group (54% vs 26.5%; p = 0.002). When biological markers of activity used in regular clinical practice were evaluated, only FC showed a trend towards statistical significance, with higher medians in the low sun exposure group (76.5 [195] vs 68 [112] μg/g; p = 0.08). Of the 22 patients who had been hospitalised for IBD-related reasons in the past year, 19 belonged to the low sun exposure group (p = 0.037). No association was demonstrated between quality of life of the patients in the series and category of sun exposure. By contrast, median scores obtained on the physical activity questionnaire were significantly lower in the group of patients with low sun exposure (1,812.5 [1,863] vs 2820 [2582] METs; p = 0.001). When physical activity was categorised using the median for the complete series (2,076 METs) as a cut-off point, a higher percentage of patients with activity under the median had reported low sun exposure (p = 0.04). The percentage of patients on treatment with thiopurines and with low levels of sun exposure was lower than that recorded in patients on immunosuppressive treatment and moderate to high levels of sun exposure (p = 0.027). This difference was not seen when patients on biologic treatment were evaluated. Table 4 summarises the variables studied in the bivariate analysis. Following preparation of a multivariate logistic regression model (Table 5), adjusting for variables that were found to be significant in the bivariate analysis, only the presence of inflammatory activity measured by clinical indexes was associated with low sun exposure (OR = 3.23).
Bivariate analysis of clinical variables, biological variables and variables related to quality of life in relation to sun exposure.
| Variable | Low sun exposure (n = 100) | Moderate to high sun exposure (n = 49) | p | OR (95% CI) |
|---|---|---|---|---|
| Age (years)a | 39.92 ± 12.17 | 36.82 ± 13.45 | 0.16 | |
| Gender, n (%) | ||||
| Female | 52 (52) | 18 (36.7) | 0.08 | 0.536 (0.266-1.08) |
| Male | 48 (48) | 31 (63.3) | 1 | |
| Season of the year | ||||
| Summer | 46 (46) | 34 (69.4) | 0.007 | 1 |
| Winter | 54 (54) | 15 (30.6) | 2.66 (1.29-5.48) | |
| Hospital admissions in the past year, n (%) | ||||
| No | 81 (81) | 46 (93.9) | 0.037 | 1 |
| Yes | 19 (19) | 3 (6.1) | 3.1 (0.96-9.98) | |
| Clinical recurrence in the past year, n (%) | ||||
| No | 46 (46) | 36 (73.5) | 0.002 | 1 |
| Yes | 54 (54) | 13 (26.5) | 2.03 (1.23-3.35) | |
| Current use of thiopurine immunosuppressants, n (%) | ||||
| No | 62 (62) | 21 (42.9) | 0.027 | 1 |
| Yes | 38 (38) | 28 (57.1) | 0.66 (0.46-0.94) | |
| Current use of biologic treatment, n (%) | ||||
| No | 58 (58) | 29 (59.2) | 0.9 | 1 |
| Yes | 42 (42) | 20 (40.8) | 1.02 (0.684-1.54) | |
| Faecal calprotectin (μg/g)b | 76.5 (195) | 68 (112) | 0.08 | |
| CRP (mg/l)b | 2.9 (2.2) | 2.9 (1.15) | 0.37 | |
| IPAQ score <2,076 METsb | ||||
| No | 44 (44) | 30 (61.2) | 0.048 | 1 |
| Yes | 56 (56) | 19 (38.8) | 1.44 (0.97-2.13) | |
| SIBDQ-9 scorea | 63.54 ± 8.8 | 65.85 ± 10.5 | 0.16 | |
| BMI (kg/m2)b | 24.16 (6.98) | 24.68 (5.5) | 0.16 | |
| Clinical activity, n (%) | ||||
| No | 63 (63) | 44 (89.8) | 0.001 | 1 |
| Yes | 37 (37) | 5 (10.2) | 3.62 (1.52-8.46) | |
BMI: body mass index; CRP: C-reactive protein; SIBDQ: short inflammatory bowel disease questionnaire.
Multiple logistic regression model to predict low sun exposure.
| Variable | B | SE | p | OR | 95% CI |
|---|---|---|---|---|---|
| Clinical activity | 1.17 | 0.55 | 0.036 | 3.23 | 1.08-9.68 |
| Season of the year | 0.74 | 0.40 | 0.065 | 2.09 | 0.95-4.59 |
| Immunosuppression with thiopurines | –0.71 | 0.39 | 0.067 | 0.48 | 0.22-1.05 |
| Clinical recurrences in the past year | 0.64 | 0.43 | 0.138 | 1.9 | 0.81-4.47 |
| Hospital admissions in the past year | 0.65 | 0.73 | 0.374 | 1.93 | 0.45-8.21 |
| Physical activity | 0.7 | 0.39 | 0.076 | 2.02 | 0.92-4.40 |
| Constant | –3.04 | 0.86 | 0.001 | 0.048 |
B: regression coefficient; OR: odds ratio; SE: standard error.
This study has sought to examine the sun exposure and sun protection practices of a series of outpatients with IBD at a moderate latitude with a Mediterranean climate, given the paucity of the data on this subject. Patients with IBD constitute a risk group in whom screening for vitamin D deficiency is recommended. However, there are no guidelines with indications for IBD specialists as to the frequency or greatest level of suspicion with which this parameter should be monitored.23 Therefore, the use of simple questionnaires on sun exposure and sun protection may be an efficient strategy in this regard. There is no questionnaire on sun exposure validated for patients with IBD. The Sun Exposure Questionnaire is a simple tool that measures an individual's weekly levels of sun exposure by evaluating two parameters: time outdoors and skin exposed. It is easy both for the patient to fill in the data and for the clinician to perform the necessary calculations, and consumes neither too much time nor too many resources. This index was validated in a study in a healthy Italian population with a sample size smaller than that in our study, showing a good correlation in summer with serum 25(OH)D levels (rho = 0.58, p = 0.004).20 Our study also found a statistically significant, although modest, correlation between the total score for the version translated into Spanish of this questionnaire and the subscore for skin exposed. When a distinction was made between seasons of the year, a significant association was also observed in summer. Another study, a Canadian study with a multi-ethnic sample of more than 300 healthy patients, found the Sun Exposure Questionnaire to have a weak but significant correlation with 25(OH)D levels.24 In that study, patient ancestry was the risk factor most determinant of 25(OH)D levels. Finally, a Brazilian study in 200 Caucasian patients showed a significant correlation very similar to ours (r = 0.264; p <0.0001) with vitamin D levels measured by electrochemiluminescence.25 The lesser magnitude of correlation demonstrated in our series likely reflects the additive contribution of other variables not taken into account in the questionnaire — both variables related to physiological vitamin D production and variables related IBD itself. Notable among variables related to physiological vitamin D production are patient phototype (in our series, most patients had phototype III), time of day of exposure (lesser capacity for synthesis at 9-10 a.m. and 3-4 p.m.), physical activity and comprehensive collection of sun protection measures (specifically assessed in a separate questionnaire in this study). Notable among variables related to IBD are clinical and biological activity26 and health-related quality of life27.
As in a case–control study conducted in Italian patients with IBD, using a more complex sun exposure questionnaire that included questions on sun protection, we found that 67% of patients had low sun exposure (Sun Exposure Questionnaire score ≤18).28 This figure practically matches the proportion of patients with deficient or insufficient 25(OH)D levels in our series (69%). The low median questionnaire score observed in our study (14 [13.5]) may point to a greater tendency to go about activities with less skin exposed in our health area. In addition, on categorising the sun exposure variable, in accordance with the three scoring sections into which it is divided, median vitamin D levels in the group of patients with a score below 18 was significantly lower than in the group of patients with moderate to high sun exposure (a score of 19-56) (p = 0.04). An Italian study by Vernia et al.28 found that the probability of low levels of sun exposure was higher in subjects with IBD than in control subjects, and among patients with IBD it was higher in CD compared to UC. In our study, sun exposure did not differ between the two diseases. The absence of healthy control subjects in our study made it impossible to determine differences in exposure between patients and a healthy population in our area.
Evaluation of sun protection practices using the other questionnaire based on the set of questions validated by Glanz et al.21 did not show a correlation with vitamin D levels. In this regard, a single Dutch case–control study in patients with CD examined sun protection behaviours and their relationship to vitamin D levels, using the same questionnaire as in our study. No differences were seen between patients and control subjects; a slightly higher median score than ours (15 [3.5]) was obtained.29
Another important consideration related to sun exposure and sun protection in patients with IBD is the use of thiopurine immunosuppressants. This treatment has been associated with an increase in non-melanoma skin tumours; therefore, patients are advised to be extremely careful. In our study, where 44% of the patients were on treatment with thiopurines, no differences were detected with regard to sun protection precautions in patients on active treatment. In addition, sun exposure also did not differ between patients on and patients not on thiopurine treatment. This could reveal a gap in information provided to these patients with respect to potential adverse skin effects of immunosuppression in our setting. Our findings contrast with the above-mentioned Dutch study in which the patients on treatment with thiopurines had a significantly higher score on the protection questionnaire (17 vs 15; p = 0.009).29
As in other studies, dietary intake of vitamin D in our series was much lower than the daily intake recommended in recent clinical practice guidelines from Spain.30 Said intake did not differ by season of the year. That emphasises the repercussions of sun exposure for vitamin D status in these patients.
Low sun exposure was significantly associated in the bivariate analysis with a number of variables. An association was observed with less physical activity, with disease activity measured by clinical indexes and with season of the year in which the study took place. In addition, other variables that involve resource use, such as hospitalisations in the past year and clinical recurrences of IBD, were also associated with lower sun exposure measured by the questionnaire used. However, in the multivariate analysis, only the presence of clinical activity measured by indexes in regular use was significantly associated with less sun exposure (OR = 3.23).
Our study has many limitations. Its lack of a control group of healthy people made it impossible to demonstrate that patients with IBD have less sun exposure than patients with no comorbidities. The single-centre nature of the study, the fact that it was conducted in a tertiary hospital and, therefore, had a selection bias for patients with more complex conditions, and its limited sample size represented other limitations. In addition, the questionnaire used to assess sun exposure, despite having been validated in a healthy population at a similar latitude, has not been previously tested in patients with IBD. Another limitation is the absence of longitudinal follow-up of sun exposure and sun protection practices in a single patient in summer and in winter.
As advantages, determination of 25(OH)D levels in all patients enabled establishment of their correlation with a simple sun exposure test. This factor has been previously explored in very few studies. Other strengths of the study were its prospective data collection by IBD specialists, its inclusion of multiple key variables for explaining vitamin D status and its collection of prognostic variables and use of resources in IBD.
In summary, bearing in mind the multiple limitations of our study, patients with IBD (especially those with clinical activity) presented low sun exposure according to the scores on the questionnaire used. This translated to an insufficient vitamin D status in a high percentage of them. The correlation between the Sun Exposure Questionnaire and serum vitamin D levels was significant but modest. More studies are needed at various latitudes in which sun exposure is estimated by means of more complete questionnaires that incorporate key factors that affect vitamin D levels in patients with IBD. These could guide clinicians in making decisions with respect to screening for and treating vitamin D deficiency, thereby reducing costs in clinical chemistry determinations.
Conflicts of interestThe authors declare that they have no conflicts of interest.
To the ECAI for Methodological and Statistical Advising of the Instituto de Investigación Biomédica de Málaga [Malaga Biomedical Research Institute] (IBIMA) for its contribution to the study design and statistical analysis.
Please cite this article as: Olmedo-Martín RV, González-Molero I, Olveira G, Amo-Trillo V, Jiménez-Pérez M. Exposición solar en la enfermedad inflamatoria intestinal ambulatoria: factores predictivos y correlación con la concentración sérica de vitamina D. Revisión de la enfermedad relacionada con la IgG4. Gastroenterol Hepatol. 2019;42:604–613.