Vitamin D plays a major role in biological processes. Its deficiency is associated with increased morbidity and mortality. Patients who have undergone endoscopic gastrostomy (PEG) present with protein-energy malnutrition, and may be at risk for Vitamin D deficiency, due to their age, less sunlight exposure and lower dietary intake. We aimed to determine the prevalence of hypovitaminosis D in PEG-patients, its change under PEG-feeding, and its relationship with serum proteins and risk factors for Vitamin D deficiency.
MethodsThis was a prospective observational study, over 4 weeks, after gastrostomy. Data were collected at the initial PEG procedure (T0), and after 4 weeks (T1). Initial evaluation included age, gender, underlying disorder, NRS-2002, BMI, serum albumin, transferrin and Vitamin D. At T1 we assessed Vit. D, albumin, and transferrin. Vitamin D was performed with Electrochemiluminescence through Elecsys 2010 assay. Patients were fed with blended homemade meals.
Results200 patients (118 males), 22–92 years of age, were studied. There were initial low values for Vit. D (181), albumin (96), transferrin (121), and BMI (124). A correlation was found between Vit. D and serum albumin (r=0.49, p=0.005) but not with transferrin (r=0.26, p=0.195). At T1 the subgroup who had Vit. D levels assessed (n=48) was part of the initial study group maintained low levels of Vitamin D despite nutritional intervention.
ConclusionWe recommend systematic Vitamin D supplementation of PEG fed patients using homemade meals or at least screening for hypovitaminosis D as a routine part of their care.
La vitamina D desempeña una función muy importante en los procesos biológicos. Su insuficiencia se asocia a una mayor morbimortalidad. Los pacientes que se han sometido a una gastrostomía endoscópica percutánea (GEP) presentan desnutrición proteinicocalórica y pueden correr riesgo de padecer insuficiencia de vitamina D, debido a su edad, menor exposición solar y menor ingestión alimentaria. Nuestro objetivo consistió en determinar la prevalencia de hipovitaminosis D en pacientes sometidos a GEP, su cambio en el contexto de la alimentación a través de GEP y su relación con las proteínas séricas y los factores de riesgo asociados a la insuficiencia de vitamina D.
MétodosSe trató de un estudio observacional y prospectivo de cuatro semanas tras la gastrostomía. Se recogieron datos al inicio de la intervención de GEP (T0) y tras 4 semanas (T1). El análisis inicial incluyó edad, sexo, afecciones preexistentes, NRS-2002, IMC, seroalbúmina, transferrina y vitamina D. En el punto temporal T1, analizamos la vitamina D, albúmina y transferrina. La vitamina D se interpretó utilizando electroquimioluminiscencia mediante Elecsys 2010.
ResultadosSe estudió a 200 pacientes (118 varones) con edades comprendidas entre 22 y 92 años. Al inicio, se detectaron niveles bajos de vitamina D (181), albúmina (96), transferrina (121) y un IMC bajo (124). Se halló una correlación entre la vitamina D y la seroalbúmina (r=0,49; p=0,005), pero no con la transferrina (r=0,26; p=0,195). En el punto temporal T1, el subgrupo en el que ya se habían analizado niveles de vitamina D (n=48), que formó parte del estudio inicial, mantuvo niveles bajos, a pesar de la intervención nutricional.
ConclusiónRecomendamos un suplemento sistemático de vitamina D a los pacientes alimentados a través de GEP con comidas caseras o, al menos, la detección de hipovitaminosis D como parte habitual de su tratamiento.
Vitamin D (Vit. D) plays an important role in most biological processes and affects the expression of more than 200 different genes. Besides regulation of calcium homeostasis and bone metabolism, Vit. D is involved in many other biological processes, including cell differentiation, proliferation and growth of the muscle, skin, parathyroid gland, pancreas and immune system.1–3 Vit. D deficiency is associated with increased morbidity and mortality in the general population. It influences the onset of diverse conditions such as cardiovascular and autoimmune disorders.4 Also, deficiency may constitute a predictor of poor outcome in several diseases.5,6 Data suggest that sufficient levels of Vit. D may have a protective effect against infectious diseases, type 1 and type 2 diabetes, musculoskeletal disorders, cancer, infertility, and adverse pregnancy and birth outcomes.7–10 The protective and/or therapeutic role of Vit. D was also reported in experimental autoimmune encephalomyelitis (EAE).11 The proposed underling mechanisms for this relationship may provide a model for Vit. D protection in several disorders including the promotion of inflammatory cell apoptosis, as well as inhibiting pro-inflammatory cytokine secretion, including IL-12 and IFNγ.12 Sunlight is the necessary source of Vit. D.13 The basic requirement can be satisfied by exposing the skin to the sun. Vit. D deficiency is common in northern countries, and in the past decade has been pointed out as a major public health problem. Only a relatively small number of foods contain substantial amounts of Vit. D, namely animal foods (oily fish, edible fats and fat milk products), which hardly provide a sufficient amount.4 Vit. D deficiency may occur if the diet is poor in the vitamin and sun exposure is scarce.14 Individuals with high risk to develop Vit. D deficiency comprise those with dark skin, old age, obesity, diabetes, and malabsorption or decreased absorption induced by some medications.15,16 Beyond clinically apparent Vit. D deficiencies, subclinical deficiencies may impair metabolism and must be identified, particularly in patients and groups at risk. The prevalence of swallowing impairment is very high in patients with acute or chronic neurological diseases, and head or neck cancer. Whatever the underlying disease, dysphagia reduces oral intake, leading to depletion of macronutrients and micronutrients 17–20 Percutaneous Endoscopic Gastrostomy (PEG) is the gold standard for enteral feeding longer than 3 weeks.21,22 When patients are referred for the PEG procedure for long term enteral feeding they have already had several weeks with low ingestion and frequently present with protein-energy malnutrition and micronutrient deficiency. In some countries, products for enteral nutrition are not reimbursed by the health system and patients are frequently fed with homemade meals after gastrostomy. PEG fed patients may also be at risk for Vit. D deficiency due to their age, less exposure to sunlight, and lower dietary intake.
The main aims of the present were to determine the prevalence of hypovitaminosis D in dysphagic patients who have undergone endoscopic gastrostomy, and to evaluate Vit. D level evolution under PEG feeding using home blended food. The secondary aims of the study were to assess any potential relationship between Vit. D levels and risk factors for Vit. D deficiency, such as age, gender and clinical diagnosis, and to assess the relationship between serum Vit. D and serum albumin, and transferrin concentrations and its evolution in PEG patients.
MethodsWe performed a prospective observational study intended to evaluate serum Vit. D, albumin and transferrin concentration in consecutive adult patients who were referred for and underwent endoscopic gastrostomy to have nutritional support for long term dysphagia (more than 3 weeks). All our patients were fed with enteral homemade meals. After gastrostomy, patients should be fed with blended homemade diets through PEG. The amount of proteins and energy was progressively adjusted according with their needs
Patients were evaluated when the PEG was performed (T0) and after PEG feeding for 4 weeks.
Study populationThe present study intended to evaluate 200 PEG patients. All adult patients who had undergone percutaneous endoscopic gastrostomy (PEG) were invited to participate. The inclusion criteria included clinical indication for endoscopic gastrostomy with dysphagia longer than 3 weeks caused by neurological disorders (ND), or Head and Neck Cancer (HNC) and previous oral intake≤50% of energy needs. Exclusion criteria included age<18 years, refusal to be included in the study, or clinical instability.
All subjects were informed of the purpose and procedures of the study and gave their informed consent. This study was approved by the Hospital Ethics Committee. Collected data included the patient's age, gender, the clinical indication for enteral feeding through PEG, Nutritional Risk Screening, Body Mass Index (BMI), and serum albumin, transferrin and Vit. D serum concentrations.
According to the underlying disease causing dysphagia, the patients were split in two groups: (1) HNC included oral cavity, pharyngeal, laryngeal, oesophageal proximal cancer and cervical cancers arising from other organs or tissues, and (2) ND including acute and chronic neurological disorders.
All these patients were evaluated by the dietitian, gastroenterologist and nurse in the Artificial Feeding Team, using the same protocol, at the time of the PEG procedure, and after 4 weeks.
Baseline evaluation (T0)Dietary recallThe dietitian of the Artificial Feeding Team performed the nutritional protein-energy ingestion using a retrospective 24h dietary recall performed before the gastrostomy. All subjects were in an adjusted progressive diet according to their nutritional needs through PEG.
As many of these patients have significant speech difficulties due to ND or HNC, nutritional assessment tools depending on oral communication were frequently unsuitable. In these cases, a dietary recall was obtained from family or caregivers.
Nutritional risk assessmentThe Nutritional Risk Screening 2002 (NRS-2002) was used for nutritional screening according to ESPEN recommendations.
Global Nutritional AssessmentThe Global Nutritional Assessment relied mostly on objective evaluation, Body Mass Index (BMI) and serum data, including albumin and transferrin. BMI was obtained and expressed as body weight/height squared (kg/m2). If patients were bedridden and could not stand up for weight and height evaluation, BMI was estimated using the Mid Upper Arm Circumference and regression equations described by Powell-Tuck/Hennessy, which were previously used by our group.23,24 Malnutrition was defined as a BMI less than 18.5kg/m2 for adult patients younger than 65 years old and less than 22kg/m2 for patients aged 65 years or older.25 Although serum proteins may be influenced by general non-nutritional factors, albumin<35g/L and transferrin<2g/L were considered suggestive of malnutrition.
Sampling and blood samples assaysA blood sample was obtained from every patient just before the gastrostomy procedure. Blood samples were obtained between 8:00 and 10:00a.m. following at least 12h of fasting. The blood sample from each patient was used for the standard PEG-patient evaluation, including serum proteins and Vit. D. Albumin<35g/L and transferrin<2g/L were considered low serum concentration. Because of its long half-life, plasma 25(OH)D concentration is considered the best measure for assessing Vit. D status.26 The circulating level of 25(OH)D was determined by Electrochemiluminescence through Elecsys 2010 (Roche Diagnostics, Mannheim, Germany) assay. Measurements were based on total 25(OH)D. Patients were stratified as Vit. D sufficient (30–80ng/mL), insufficient (20–29.9ng/mL), deficient 13.1–19.9ng/mL), or severely deficient (≤13ng/mL) based on their 25-OH Vit D concentration.4,27–29
Follow-upPatients were evaluated by the Artificial Feeding Team at 4 weeks after the PEG procedure. Their protein/energy intake was recorded and compliance with the protein-calorie diet prescription assessed. BMI was registered at 4 weeks, and a blood sample was obtained for laboratory assessment, including albumin, transferrin and Vit. D.
All the patients, families and/or caregivers were asked about complications such as vomiting, diet intolerance or diarrhea that could have interfered with prescription diet management.
Statistical analysisThe Statistical Package for Social Sciences (IBM SPSS Statistics), version 22.0 was used. The results were considered significant at a 5% significance level. Descriptive statistics were used to evaluate Vit. D levels in dysphagic patients. We used the Mann Whitney test for the relationship between vitamin D and age, gender and clinical diagnosis. To assess the normality of the data we used the Shapiro–Wilk test. The paired T-test was used to compare proteins (albumin and transferrin) between the two times of testing in the patients who had Vit. D assessed. To determine the difference in Vit. D between the two tests, the Wilcoxon signed-rank test was used. To study the relationship between Vit D level, albumin and transferrin, the Spearman correlation was used. To compare Vit. D levels between BMI categories, the Kruskal–Wallis test was performed, since the assumption of normality of data in BMI categories is not verified.
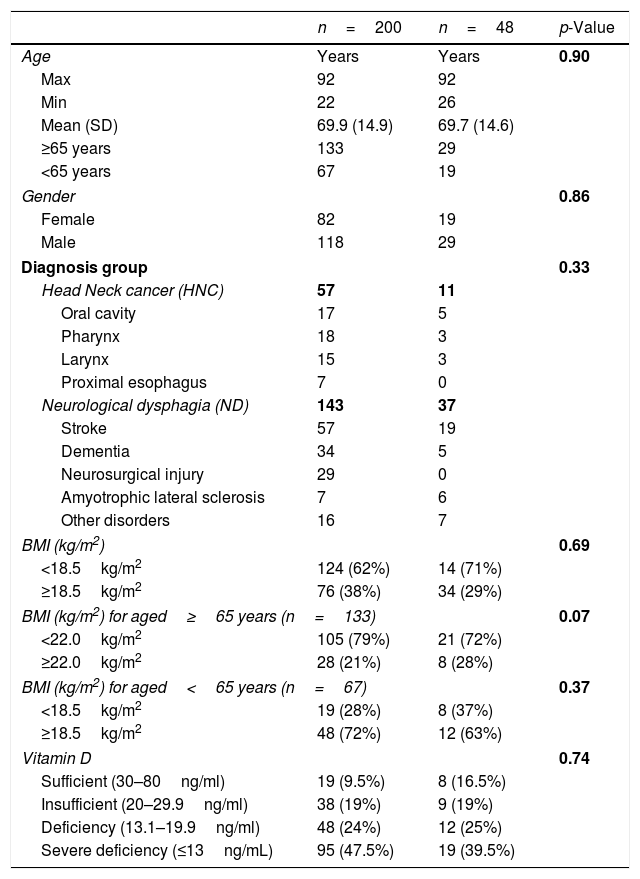
ResultsCharacteristics of the study population at the time of the PEG procedure (Table 1)This study included 200 dysphagic patients who were admitted for PEG, 118 men and 82 women, aged 22–92 years, with a mean age of 69.9 years (SD±14.9), and 133 patients who were 65 years old or older. 181/200 (90.5%) had low values for Vit D. According to their underlying disease, the patients were divided into two groups: 1-HNC group (n=57) and 2-ND group (n=143). In the HNC the disease was in the oral cavity (n=17), larynx (n=15), pharynx (n=18), and proximal esophagus (n=7). The ND patients comprised those suffering from strokes (n=57), dementia (n=34), neurosurgical disorders (n=29), amyotrophic lateral sclerosis (n=7) and other neurological diseases (n=16) causing dysphagia. Before the PEG procedure, oral intake was mostly variable according to the underlying disease which had lasted from a few weeks to several months. Likewise, weight loss previous to the gastrostomy was widely variable. Nevertheless, all patients had prior intake under 50% of their daily protein-energy needs and Nutritional Risk Screening – NRS 2002 – presented a score of ≥3 in every patient, signaling nutritional risk. All patients were clinically stable at the moment of the PEG and sample collection (unstable patients were excluded or the procedure was postponed).
Characteristics of the study population (n=200) and the subgroup of patients with vitamin D levels at 4th week.
| n=200 | n=48 | p-Value | |
|---|---|---|---|
| Age | Years | Years | 0.90 |
| Max | 92 | 92 | |
| Min | 22 | 26 | |
| Mean (SD) | 69.9 (14.9) | 69.7 (14.6) | |
| ≥65 years | 133 | 29 | |
| <65 years | 67 | 19 | |
| Gender | 0.86 | ||
| Female | 82 | 19 | |
| Male | 118 | 29 | |
| Diagnosis group | 0.33 | ||
| Head Neck cancer (HNC) | 57 | 11 | |
| Oral cavity | 17 | 5 | |
| Pharynx | 18 | 3 | |
| Larynx | 15 | 3 | |
| Proximal esophagus | 7 | 0 | |
| Neurological dysphagia (ND) | 143 | 37 | |
| Stroke | 57 | 19 | |
| Dementia | 34 | 5 | |
| Neurosurgical injury | 29 | 0 | |
| Amyotrophic lateral sclerosis | 7 | 6 | |
| Other disorders | 16 | 7 | |
| BMI (kg/m2) | 0.69 | ||
| <18.5kg/m2 | 124 (62%) | 14 (71%) | |
| ≥18.5kg/m2 | 76 (38%) | 34 (29%) | |
| BMI (kg/m2) for aged≥65 years (n=133) | 0.07 | ||
| <22.0kg/m2 | 105 (79%) | 21 (72%) | |
| ≥22.0kg/m2 | 28 (21%) | 8 (28%) | |
| BMI (kg/m2) for aged<65 years (n=67) | 0.37 | ||
| <18.5kg/m2 | 19 (28%) | 8 (37%) | |
| ≥18.5kg/m2 | 48 (72%) | 12 (63%) | |
| Vitamin D | 0.74 | ||
| Sufficient (30–80ng/ml) | 19 (9.5%) | 8 (16.5%) | |
| Insufficient (20–29.9ng/ml) | 38 (19%) | 9 (19%) | |
| Deficiency (13.1–19.9ng/ml) | 48 (24%) | 12 (25%) | |
| Severe deficiency (≤13ng/mL) | 95 (47.5%) | 19 (39.5%) | |
Table 1 shows the characteristics of the study population (n=200) including the demographic data (age and gender), the distribution of underlying diseases, and the subgroup of patients with Vit. D levels at the 4th week.
Body mass indexFrom 200 patients, 124 (62%) showed low BMI. Low BMI was present in 68.5% (n=98) of ND patients and 46% (n=26) of HNC patients. The older group included more malnourished patients, 79% (n=105), than the younger group, 28% (n=19).
Vit. D and proteins serum concentrationVit. DFrom 200 patients the mean for Vit. D was 18.8ng/dL. According to the definition of Vit. D values, we found, 19 patients with values within the sufficient range (mean 37.7±7.1ng/dL) and 181 patients with low values. From these 181 patients, 95 had severe deficiency (mean 8.18±2.72ng/dL), 48 deficiency (mean 16.1±1.91ng/dL), and 38 insufficiency (mean 23.12±1.13ng/dL) of Vit. D.
Serum protein concentrationSerum albumin and transferrin were evaluated in 200 patients. Regarding albumin, we obtained a mean of 35g/L, ranging from 16 to 51g/L. About half, 48% (n=96), presented with low albumin. Regarding serum concentrations of transferrin, we obtained a mean of 1.88g/L, ranging from 0.36 to 3.32g/L. Almost two thirds, 121 patients (61%), presented with low transferrin.
Body mass indexOut of 48 patients, 29 (60%) showed low BMI. Low BMI was present in 64.8% (n=24) of the ND patients and 27% (n=3) of the HNC patients. The older group had more malnourished patients, 65.5% (n=21), than the younger group, 42% (n=8).
No relationship was found between Vit D and age (p=0.198) and gender (p=0.058)
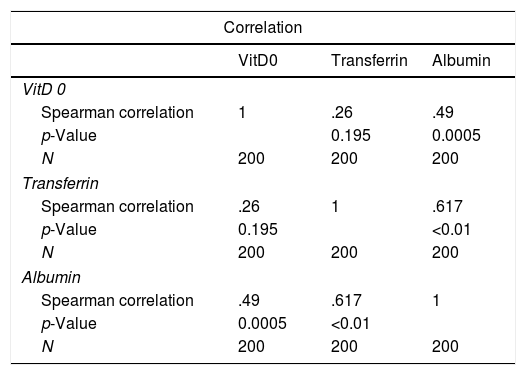
The relationship between albumin, transferrin and Vit D concentrations (Table 2)Regarding the relationship between concentrations of Vit. D and serum albumin and transferrin, a correlation was found between Vit. D and serum albumin (R=0.49, p=0.005) but not with transferrin (R=0.26, p=0.195).
The relationship between Vit. D, albumin and transferrin concentrations at baseline.
| Correlation | |||
|---|---|---|---|
| VitD0 | Transferrin | Albumin | |
| VitD 0 | |||
| Spearman correlation | 1 | .26 | .49 |
| p-Value | 0.195 | 0.0005 | |
| N | 200 | 200 | 200 |
| Transferrin | |||
| Spearman correlation | .26 | 1 | .617 |
| p-Value | 0.195 | <0.01 | |
| N | 200 | 200 | 200 |
| Albumin | |||
| Spearman correlation | .49 | .617 | 1 |
| p-Value | 0.0005 | <0.01 | |
| N | 200 | 200 | 200 |
No statistical correlation was found between serum Vit D, albumin and transferrin and the two groups of underlying diseases (HNC and ND) (p=0.84).
Follow-up after 4 weeksClinical evaluationNo difference was fund between patients with normal or low initial serum Vitamin D regarding first month complications or mortality.
Serum Vit. DAfter 4 weeks, we evaluated 48 patients with the mean for Vit D was 18.8ng/dL. 40/48 (8%) had low values for Vit D. In all cases there was a slight increase in serum Vit. D values. From these results and according to the Vit. D cut-offs value, we found 8 patients with values in the sufficient range, with a mean of 39.9ng/dL, and 40 patients with low values. From these 40 patients with low values of serum 25(OH)D levels, 19 presented with severe deficiency (mean 8.0ng/dL), 12 with deficiency (mean 16.85ng/dL), and 9 with insufficiency (mean 25.9ng/dL).
The subgroup of patients who had Vit D levels assessed after 4 weeks (n=48) was part of the initial study population.
Serum protein concentrationSerum albumin and transferrin were evaluated in 48 patients. Regarding albumin, we obtained a mean of 37g/L ranging from 24 to 48g/L. Twelve of them presented with low albumin. Regarding serum concentrations of transferrin, we obtained a mean of 2.11g/L, ranging from 1.28 to 3.42g/L. More than half of those, 29 patients (60%), presented with normal transferrin.
Protein levels increased in all patients during the first month after gastrostomy with enteral feeding. Most patients reached normal protein levels values. Vit. D had a slower evolution and most patients still displayed low Vit. D after this period with an adjusted diet through tube feeding.
After 4 weeks’ PEG feeding enteral nutrition our preliminary results regarding the goal of improving Vit. D levels were unsuccessful, while serum proteins showed a clear trend towards normalization, as we had observed in previous studies by our team.
In face of this poor result, the investigators had a discussion with the ethical committee and decided to discontinue the study and initiate systematic supplementation with 800IU/day of Vit. D in PEG fed patients.
DiscussionThe serum 25(OH)D levels in our sample indicate a very high prevalence of Vit. D deficiency. The basic requirement for Vit. D can be satisfied by exposing the skin to the sun. Most of our patients were bedridden or had reduced mobility, without sun exposure for a long time. Additionally, the intake of their nutritional needs was less than 50% of the requirements. Since we were aware of their dysphagia-induced low protein-energy intake, it is reasonable to assume that Vit. D deficiency was caused by poor intake and reduced sunlight exposure. These results are consistent with similar studies conducted by Pittas et al, who established a relationship with increased risk for a variety of acute and chronic medical conditions.30 Alongside the outcome of their low protein-energy intake, we obtained low levels of serum proteins. Looking for albumin and transferrin, serum markers of malnutrition and inflammation, we found that half our patients presented with low albumin and almost two thirds with low transferrin. The underlying disease did not significantly influence the prevalence of Vit. D deficiency, namely in HNC and ND patients. We obtained a similar proportion of Vit. D deficiency in the whole sample and in the etiological subgroups. According to the literature, Vit. D deficiency is particularly prevalent in the elderly for many reasons, including the reduction of the capacity of the skin to synthetize Vit. D, the decline in hepatic and renal function, and the lack of sun exposure.31–33 Recent recommendations encourage increasing intake of both Vit. D and protein, for maintaining musculoskeletal health. An adequate Vit. D supply, ranging between 800IU/day and 1000IU/day, is recommended to maintain serum 25(OH)D levels>50nmol/l in older individuals with low physical performance.34–36 Assessing whether age could influence serum Vit. D, we found no significant age influence in the prevalence of Vit. D deficiency. We believe that our results are mainly due to low dietary ingestion and reduced sun exposure, as overlapping other factors.
In the present study, homemade meals, even with protein-energy needs adjusted, were insufficient to normalize serum Vit. D in most patients. Vit. D can be obtained from the diet, enriched food or supplements as Vit. D2 (ergocalciferol) or Vit. D3 (cholecalciferol). However, relatively few foods contain Vit. D, and therefore the dietary intake is considered low. Given the results attained at 4 weeks after gastrostomy in 48 patients, we decided to suspend the study following the advice of the ethical committee of our hospital, and provide all patients with 800IU/day of Vit. D supplementation.
ConclusionFrom the results obtained from this study, we recommend systematic Vit. D supplementation of PEG fed patients using homemade meals or at least screening for hypovitaminosis D with plasma totals of 25(OH)D as a routine part of the care of patients requiring enteral nutrition. Further studies are needed to prepare detailed guidelines for Vit. D supplementation in PEG fed patients.
Authors’ contributionCarla Adriana Santos contributed with conception and designed research and conducted research, wrote the paper and had primary responsibility for final content. Gonçalo Nunes was responsible for data analysis and interpretation and article review. Ana Teresa Barata contributed for data analysis and interpretation. Jorge Fonseca was responsible for article review and approval of the final version.
Conflict of interestThe authors declare that they have no conflict of interest.
All authors are grateful to the patients, families and caregivers.