Hepatotoxicity associated with antifungal agents is well known.1 Terbinafine is the only drug in the allylamine family used for the systemic treatment of dermatophytic onychomycosis. Its potential hepatotoxicity has been reported, but only anecdotally,2 with a calculated incidence of 0.5–3 cases/100,000 exposures.
We present the case of a 47-year-old woman with a prior history of HLA B27+ ankylosing spondylitis diagnosed 30 years prior, who was treated for onychomycosis for 28 days with 250mg/day of terbinafine. She had no prior consumption of alcohol or herbal products and her only additional regular treatment was etoricoxib (90mg), which she had taken for years as a fast-acting painkiller, as needed, as a daily dose.
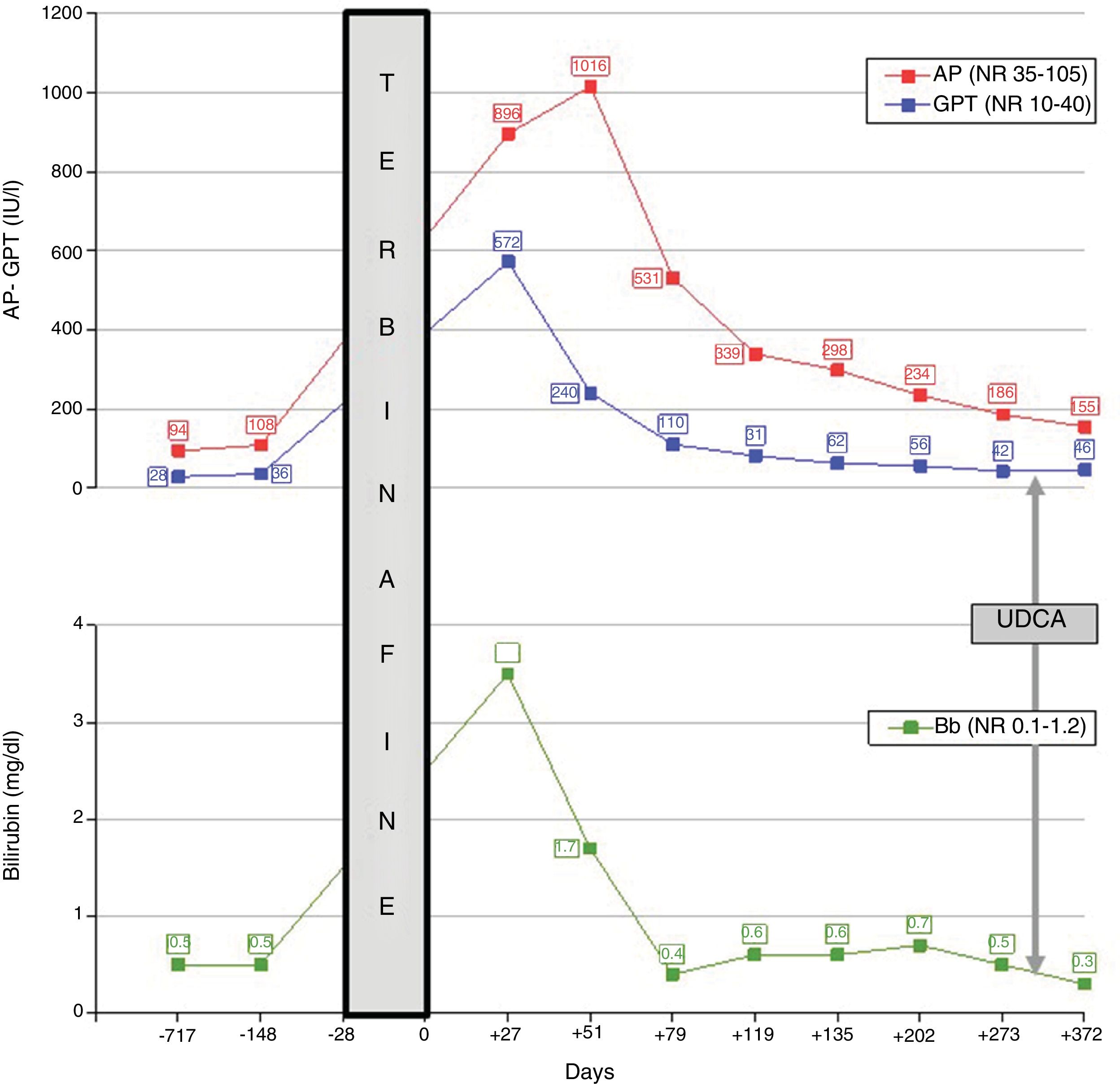
Three weeks after discontinuing the antifungal agent, the patient experienced jaundice, pruritus and asthenia, with no rash or lymph node enlargement. Her body mass index was 28kg/m2 and she exhibited no signs of metabolic syndrome. Blood test results taken two years and five months prior revealed normal hepatic clinical chemistry. Peak bilirubin (3.5mg/dl) and transaminase values (GPT: 572IU/l) were recorded on day +27 after suspension of the medication; peak alkaline phosphatase (1016IU/l) was recorded on day +51. The chronological course of hepatic clinical chemistry abnormalities and their correlation with the use and withdrawal of terbinafine are shown in Fig. 1, with baseline (day 0) defined as the day antifungal treatment was suspended. HBsAg and HCV IgG antibodies were systematically negative. Other causes of viral liver disease were also ruled out (negative anti-HAV and anti-HEV IgM antibodies). Ceruloplasmin (44.5mg/dl), α-1 antitrypsin (185.5mg/dl), transferrin saturation (29) and the complete blood count were normal. An ultrasound ruled out biliary obstruction and hepatic vascular disorders. Antinuclear antibodies were positive—at a 1:160 titre, with speckled cytoplasmic pattern—on day +51 before becoming negative at day +119; antimitochondrial antibodies, anti-LKM antibodies and anti-smooth muscle antibodies were consistently negative. Gamma globulins (1.3g/dl) and IgG (1373.8mg/dl) were normal.
Drug-induced liver injury attributable to terbinafine was identified with a cholestatic pattern: (GPT/40)/(alkaline phosphatase/105)=R 1.7, calculated on day +27. The causality was assessed using the CIOMS/RUCAM scale and it was considered to be probable (8 points): onset of symptoms five to 90 days after the start of treatment (+2); favourable course with >50% fall in alkaline phosphatase levels in the 180 days following treatment withdrawal (+2); complete exclusion of other causes or toxins (+2) and prior known terbinafine hepatotoxicity, reported on the label (+2). The abatement of cholestasis meant a liver biopsy was not required. Re-exposure was also discounted as it was considered disproportionate.
Although progression was favourable, complete resolution was not achieved by month nine, which is why ursodeoxycholic acid 300mg every 12h was then prescribed, with partial response (Fig. 1).
The exact mechanisms by which terbinafine can induce liver injury have still not been fully understood. The association between HLA-A*33:01 and susceptibility to terbinafine-induced cholestatic hepatotoxicity has recently been reported.2 Terbinafine undergoes complex hepatic biotransformation and some of the resulting metabolites are able to bind to hepatobiliary proteins and promote an immune reaction,3 while, in other cases, idiosyncratic hepatotoxicity manifests. Cholestatic toxicity is more typical and tends to be prolonged (2–12 months), which may even progress to ductopenia.4 The latency period (exposure-jaundice) ranges from two to six weeks, while peak bilirubin levels tend to be seen three to five weeks after treatment withdrawal. The condition usually resolves itself after withdrawal. Although there is no specific treatment, ursodeoxycholic acid has been successfully trialled.5
In our case, it is well documented that cholestasis was attributable to terbinafine, prescribed for its standard indication. A high level of clinical suspicion is required to facilitate the early diagnosis of drug-induced liver injury. The aim of publishing cases such as this one is to promote knowledge and clinical awareness of drug-induced hepatotoxicity.
Please cite this article as: Mejías Manzano MÁ, Giráldez Gallego Á, Ontanilla Clavijo G, Sousa Martín JM. Toxicidad hepática inducida por terbinafina. Gastroenterol Hepatol. 2019;42:394–395.