Irritable bowel syndrome (IBS) affects 10–15% of the western population. Drug therapy for this entity has shown limited efficacy. The low Fermentable Oligo-, Di-, Monosaccharides And Polyols (FODMAP) diet has recently emerged as an effective intervention for reducing gastrointestinal symptoms in IBS. Currently, several mechanistic studies have proven the rational basis of carbohydrate restriction. In addition, high-quality evidence (prospective studies and randomized controlled trials) from a variety of countries supports the high effectiveness of a low-FODMAP diet for IBS symptoms (70%), especially abdominal bloating, pain, and diarrhea. Importantly, this diet seems to be superior to a gluten-free diet for patients with non-celiac gluten sensitivity. The most controversial features of the low FODMAP diet are its short- and long-term limitations (a high level of restriction, the need for monitoring by an expert dietitian, potential nutritional deficiencies, significant gut microbiota reduction, lack of predictors of response), as well as the potential lack of advantage over alternative dietary, pharmacological and psychological interventions for IBS. Although liberalization of carbohydrate intake is recommended in the long-term, the reintroduction process remains to be clarified as, theoretically, global carbohydrate restriction is deemed to be necessary to avoid additive effects.
El síndrome de intestino irritable (SII) es una entidad clínica que afecta al 10-15% de la población occidental, para la que los fármacos disponibles han demostrado una eficacia limitada. La dieta con bajo contenido en oligo, di, monosacáridos y polioles (FODMAP) ha surgido recientemente como una medida eficaz para el control de los síntomas gastrointestinales del SII. En la actualidad, los estudios fisiopatológicos han confirmado la base racional de la restricción de carbohidratos en el SII y existe evidencia científica de alta calidad (estudios prospectivos y ensayos clínicos controlados) proveniente de diversos países confirmado la eficacia de la dieta con bajo contenido en FODMAP para el SII (70%), especialmente para la hinchazón y dolor abdominal, así como la diarrea. Cabe destacar que esta dieta parece ser más eficaz que la dieta sin gluten para los pacientes con sensibilidad al gluten no celíaca. Los aspectos más controvertidos de esta dieta son las limitaciones que implica a corto y largo plazo (nivel alto de restricción alimentaria, la necesidad de monitorización por dietistas, riesgo de déficits nutricionales, una descenso marcado de la microbiota intestinal, la ausencia de herramientas predictoras de respuesta), al igual que una eficacia similar a otras intervenciones dietéticas menos restrictivas, farmacológicas y psicológicas en recientes estudios. Pese a que se recomienda liberalizar el consumo de carbohidratos a largo plazo, queda por dilucidar con exactitud la estrategia de reintroducción, ya que teóricamente el éxito de la dieta reside en una restricción global de carbohidratos para evitar efectos aditivos.
Irritable bowel syndrome (IBS) affects 10–20% of individuals worldwide.1 The condition is characterized by chronic abdominal pain associated with disordered defecation or a change in bowel habit.2 IBS has a considerable effect on quality of life and people with IBS spend more days in bed, miss more work days, have more consultations with their primary care physician than those without the condition, besides social functioning is even worse in IBS than in other chronic diseases such as diabetes.3 Furthermore, the chronic nature of IBS, its high prevalence and its associated comorbidities contribute to a considerable economic burden on health-care services.4,5
The pathophysiology of IBS is complex and multifactorial, including altered gastrointestinal motility, increased gastrointestinal fermentation, abnormal gas transit, visceral hypersensitivity, brain – gut axis dysregulation, dysbiosis of the gut microbiota, genetic predisposition and psychosocial aspects.6 Treatment of IBS has historically been symptom-directed (e.g., bulking agents, antispasmodic agents) or centrally acting (e.g., antidepressants, cognitive–behavioral therapy), but the efficacy of these treatments is limited.
Many patients believe that their IBS symptoms are diet-related,7 but evidence supporting the effect of dietary intervention on IBS symptoms has been of limited quality. A controversial study on the efficacy of a tailored therapy for IBS, based on serum IgG levels to foods, definitely set off this line of thinking in 2004.8 The authors suggested a 3-month diet based on IgG results was significantly more effective for IBS symptoms than a sham diet, excluding the same number of foods, but not those to which they had antibodies. This study was much contested due to design and methodological flaws that questioned their conclusion, since the treatment group excluded significantly more different foods than the control group, particularly those foods which appear to exacerbate symptoms of IBS.9,10 As such, differences between diets could largely be explained not by specific identification of food reactions by IgG testing, but rather by the gross differences between the two diets. This questionable study, however, proved dietary restriction was effective for IBS and paved way for a growing interest in dietary approaches for the management of IBS among both clinicians and patients.11
The FODMAP conceptIn parallel with the rising incidence for gastrointestinal diseases (IBS, inflammatory bowel disease or celiac disease) over the past two decades, patterns of food intake and dietary behavior have dramatically changed worldwide. Fructose consumption has increased fourfold in children <10 years old and around 20% in general population. Caloric sweeteners are commonly used for beverages, intake of fast food (pizza, hamburgers, snacks, beverages) and wheat-containing foods (pasta, bread, cakes) continues to grow and away-from-home-foods and snacks account for almost 50% of daily consumed food and energy, respectively.12
Restriction of lactose in those with hypolactasia or fructose with or without sorbitol in IBS patients with fructose malabsorption has been long studied, but this approach has commonly led to little evidence of overall benefit.13,14 An early study first focused on the restriction of fructans in addition to fructose in excess of glucose.15 A pioneering randomized placebo controlled trial in patients with IBS responsive to a restriction of free fructose and fructans diet challenged patients with pure drinks of fructose, fructans or both combined.16 This study first proved symptoms in IBS patients with fructose malabsorption were also triggered by fructans, suggesting additive effects. Dietary restriction was then extended to incorporate all short-chain carbohydrates that were poorly absorbed on the basis of common and additive putative mechanisms of action, and the FODMAP concept was born. It was in 2005 the term FODMAP was first coined in literature by researchers of the Monash University in Melbourne, Australia, to describe a previously unrelated group of short-chain carbohydrates and sugar alcohols (polyols), which all are poorly absorbed in the small intestine, osmotically active molecules (induce increased luminal water content) and rapidly fermented by bacteria.12 High consumption of FODMAP sources, as a reflection of western lifestyle, was initially linked to increased intestinal permeability and susceptibility to Crohn's disease.12 Indeed, up to 50% of patients with either Crohn's disease or ulcerative colitis responded to this dietary intervention, especially in terms of overall abdominal symptoms, abdominal pain, bloating, wind and diarrhoea.17 Until 2010, several nice studies mechanistically proved the hypothetical functional properties described for FODMAP-triggered symptoms, by means of administering diets high and low in FODMAPs: increased luminal water content (diarrhea improvement following low FODMAP diet in patients with colectomy and either ileal pouch formation or ileorectal anastomosis,18 effluent volume increased by a mean of 22% associated to diet high in FODMAP in ileostomate patients19) and bacterial fermentation (increased hydrogen and reduced methane production related to gastrointestinal symptoms and lethargy in IBS patients20).
There are three key components to understand the low FODMAP diet concept21:
- 1.
With the exception of fructose (via GLUT2 or GLUT5 transporters) and lactose (if hydrolyzed by lactase) and polyols (slowly absorbed from the smal bowel), FODMAPs are not absorbed and delivery to the distal small bowel and proximal large intestine is a normal phenomenon, so FODMAPs malabsorption is physiological. Increased flatulence and change of bowel habits after consuming ‘windy vegetables’, such as lentils, chickpeas, baked beans or cauliflower, are common knowledge and related to a high FODMAP content.
- 2.
FODMAPs normally generate gas and bloating in all individuals. Accordingly, FODMAPs themselves are not responsible for symptoms in IBS patients, but rather an anomalous response to FODMAP-induced high osmotic effect and rapid fermentation in IBS patients.
- 3.
The success of the low FODMAP dietary approach relies on global carbohydrate restriction to avoid additive effects. The rationale basis is that restricting one FODMAP in isolation ignores the likelihood that there is potentially a range of FODMAPs in the diet, all of which have similar end-effects in the bowel. Restriction of individual FODMAPs (e.g., lactose, fructose) has been used with varying success in the management of functional gut symptoms for a long time. Such a global approach to restricting carbohydrates that have similar actions should optimize symptom control in patients with IBS. As aforementioned, a pioneering study from the Australian group showed 77% of IBS patients with documented fructose malabsorption receiving a low free fructose diet with increasing fructan content had symptoms inadequately controlled.16
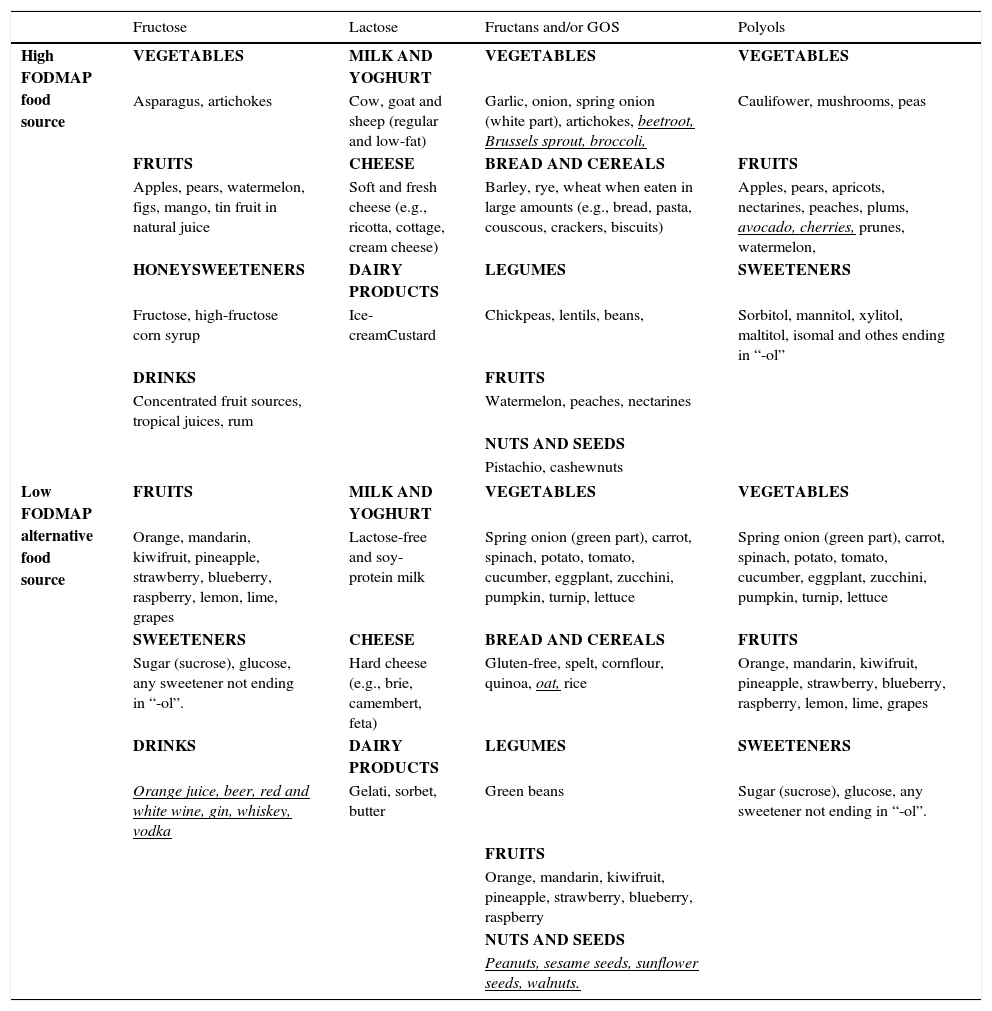
The content of FODMAPs in the diet varies across geographical areas due to variable doses delivered in the diet, the most common being fructose and fructans.22 For instance, consumption of fructans/galacto-oligosaccharides (GOS) is higher in the Mediterranean countries due to increased bread and legume intake. High FODMAP food sources (where FODMAPs are problematic based on standard serving size) and suitable low FODMAP alternatives, according to food analysis conducted in Australian foods,23–25 are displayed in Table 1.
Food sources where FODMAP content is problematic on standard serving size and alternative low FODMAP food sources.
| Fructose | Lactose | Fructans and/or GOS | Polyols | |
|---|---|---|---|---|
| High FODMAP food source | VEGETABLES | MILK AND YOGHURT | VEGETABLES | VEGETABLES |
| Asparagus, artichokes | Cow, goat and sheep (regular and low-fat) | Garlic, onion, spring onion (white part), artichokes, beetroot, Brussels sprout, broccoli, | Caulifower, mushrooms, peas | |
| FRUITS | CHEESE | BREAD AND CEREALS | FRUITS | |
| Apples, pears, watermelon, figs, mango, tin fruit in natural juice | Soft and fresh cheese (e.g., ricotta, cottage, cream cheese) | Barley, rye, wheat when eaten in large amounts (e.g., bread, pasta, couscous, crackers, biscuits) | Apples, pears, apricots, nectarines, peaches, plums, avocado, cherries, prunes, watermelon, | |
| HONEYSWEETENERS | DAIRY PRODUCTS | LEGUMES | SWEETENERS | |
| Fructose, high-fructose corn syrup | Ice-creamCustard | Chickpeas, lentils, beans, | Sorbitol, mannitol, xylitol, maltitol, isomal and othes ending in “-ol” | |
| DRINKS | FRUITS | |||
| Concentrated fruit sources, tropical juices, rum | Watermelon, peaches, nectarines | |||
| NUTS AND SEEDS | ||||
| Pistachio, cashewnuts | ||||
| Low FODMAP alternative food source | FRUITS | MILK AND YOGHURT | VEGETABLES | VEGETABLES |
| Orange, mandarin, kiwifruit, pineapple, strawberry, blueberry, raspberry, lemon, lime, grapes | Lactose-free and soy-protein milk | Spring onion (green part), carrot, spinach, potato, tomato, cucumber, eggplant, zucchini, pumpkin, turnip, lettuce | Spring onion (green part), carrot, spinach, potato, tomato, cucumber, eggplant, zucchini, pumpkin, turnip, lettuce | |
| SWEETENERS | CHEESE | BREAD AND CEREALS | FRUITS | |
| Sugar (sucrose), glucose, any sweetener not ending in “-ol”. | Hard cheese (e.g., brie, camembert, feta) | Gluten-free, spelt, cornflour, quinoa, oat, rice | Orange, mandarin, kiwifruit, pineapple, strawberry, blueberry, raspberry, lemon, lime, grapes | |
| DRINKS | DAIRY PRODUCTS | LEGUMES | SWEETENERS | |
| Orange juice, beer, red and white wine, gin, whiskey, vodka | Gelati, sorbet, butter | Green beans | Sugar (sucrose), glucose, any sweetener not ending in “-ol”. | |
| FRUITS | ||||
| Orange, mandarin, kiwifruit, pineapple, strawberry, blueberry, raspberry | ||||
| NUTS AND SEEDS | ||||
| Peanuts, sesame seeds, sunflower seeds, walnuts. | ||||
Underlined foods in italics should not be restricted, but specific limited intake daily is recommended.
Fructose is a 6-carbon monosaccharide that is dose-dependently and variably absorbed. Fructose absorption can occur through a number of routes of facilitated transport in the intestinal epithelium. When fructose is present in excess of glucose (also termed ‘free fructose’), free fructose is taken up by a low-capacity facultative transporter (GLUT5). Since free fructose is absorbed largely via low capacity transporter-mediated mechanisms, the greater the load, the more likely that malabsorption will occur. For example, in a study of 17 healthy volunteers, 53% of participants malabsorbed a 50g dose of fructose, but this proportion fell to 12.5% when the ingested dose was 25g.26 When fructose is present with glucose, fructose is taken up more efficiently through the GLUT-2 transporter (glucose:fructose co-transport).27 As for this latter carrier, the fructose:glucose ratio is key for adequate fructose absorption. A 1:1 ratio is optimal for fructose absorption to occur, but excess of fructose over glucose will lead to fructose malabsorption.28 In patients with IBS, consumption of a 35g dose of fructose alone is incompletely absorbed in 30–60% of patients, a similar proportion to that of healthy controls.29 Fructose is mostly present in fruit, fruit products and products sweetened with high-fructose sweeteners.
LactoseThe disaccharide lactose requires hydrolysis by the brushborder enzyme lactase, to the monosaccharides glucose and galactose, prior to intestinal absorption. However, up to 70% of humans exhibit hypolactasia, which may result in lactose malabsorption. The prevalence of lactose malabsorption in IBS patients (20–85%) is similar than that in the general population.29,30 As such, diagnosis of lactose malabsorption is not clinically meaningful unless lactose consumption exacerbates gastrointestinal symptoms, which is termed lactose intolerance. Lactose is naturally present in mammalian milk (e.g., cow, sheep and goat) and dairy products, but might also be added to commercial foods such as breads, cakes and slimming products.
Fructans and galacto-oligosaccharides (GOS) (prebiotic effect)The human digestive tract lacks enzymes to digest oligosaccharides as fructans and galacto-oligosaccharides.22 As a result, undigested oligosaccharides continue to travel through the gastrointestinal tract to reach the large intestine and are available for fermentation to gases and short-chain fatty acids. Fructans and galacto-oligosaccharides meet the criteria to be termed ‘prebiotics’ due to their ability to selectively stimulate the growth and activity of putatively beneficial colonic bacteria, specifically Bifidobacteria and Lactobacilli, distinguishing them from most other fermentable substrates such as non-starch polysaccharides.31,32 The inulin-type fructans are a major dietary source of fermentable carbohydrates and are present as storage carbohydrates in plants. Most dietary fructans are obtained from wheat, rye, onion and garlic, which are fairly low in fructans but are consumed in large quantities.22 Galacto-oligosaccharides are usually present in legumes and some grains, nuts and seeds.
PolyolsThere are several types of polyols (sugar alcohols) in the diet including sorbitol, mannitol, lactitol, xylitol, maltitol and isomalt. Sorbitol and mannitol are the major type in food and they found naturally in fruits and vegetables or as added sweeteners in low-calorie food products. Of note, sugar-free chewing gum is a major source, containing at least 10 times the amount of sorbitol per gram compared with many fruits and vegetables.33 Their rates of absorption depend largely on molecular size. Their absorption is passive, variable between individuals, although 60–70% of healthy people and those with IBS incompletely absorb a 10g dose of sorbitol.33
Pathophysiological rationale for low FODMAP dietThe rationale behind using low FODMAP diet is that a reduction of the detrimental gastrointestinal effects of non-absorbed carbohydrates, mainly increase in luminal water content and bacterial fermentation, will likely improve symptoms in IBS patients.21,22 After FODMAP ingestion, increased delivery of water to the small intestine (measured by magnetic resonance imaging)34,35 and the proximal colon (ileal effluent in ileostomates patients),19 due to their osmotic effects, have been consistently shown. These luminal changes may led to distension and symptoms even without altering the gas production pattern, underscoring a normal breath test may not rule a carbohydrate as the potential trigger of symptoms. Of note, lactulose34 and fructose35 have been shown to increase the small intestine water content, but not inulin, a fructan-type carbohydrate which, in turn, caused more colonic fermentation than fructose.35 These findings prove FODMAPs may have overlapping but heterogeneous effects on the gastrointestinal tract.
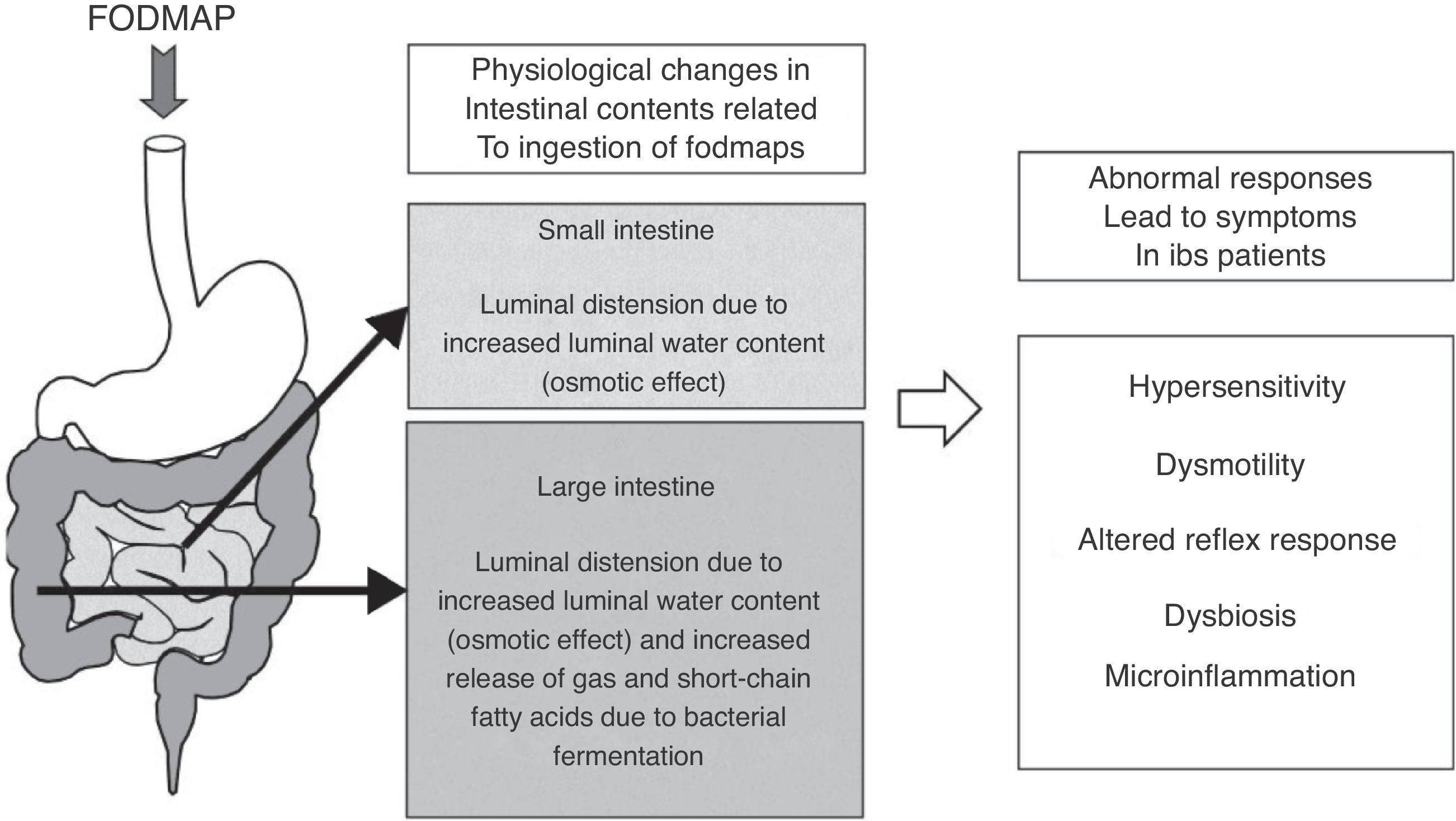
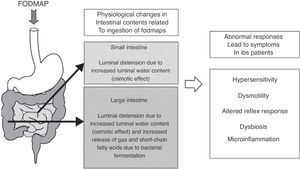
As for fermentation, this is a normal phenomenon in the human colon. When carbohydrates reach the colon, colonic bacteria ferment these sugars and, as a result, gases and short-chain fatty acids are released.36 The main gases produced during colonic fermentation are carbon dioxide (CO2), hydrogen (H2) and methane (CH4). Under normal circumstances, this process occurs virtually unperceived or produces mild abdominal sensations, not considered unpleasant by individuals. Why then normal intestinal phenomena as bacterial fermentation and increment of water luminal content due to FODMAP ingestion can induce symptoms in IBS patients? There is not a single answer to this question; symptoms in IBS rather develop as the final result of the interaction of several factors leading to abnormal accommodative responses. These factors may include alterations in sensitivity (increased visceral sensitivity with decreased tolerance to intestinal gas),37,38 dysmotility (excess of gas retained due to altered intestinal transit and evacuation),39,40 abnormal viscero-somatic reflex responses (after increase in colonic volume, impaired normal reflex contraction of the muscles of the abdominal wall and reflex relaxation of the diaphragm produce an abnormal reconfiguration of the abdomen leading to visible abdominal distension)41,42 and either increased small intestine bacterial overgrowth43 or different gut microbiota composition44,45 in IBS patients. Mechanisms of FODMAP-induced abdominal symptoms in IBS patients are summarized in Fig. 1.
Schematic representation of the mechanisms of FODMAP-induced abdominal symptoms. Ingestion of FODMAP leads to physiological intestinal phenomena as increased water content and fermentation, which abnormally induce symptoms in IBS patients, likely as a result of the interaction of different altered accommodative responses.
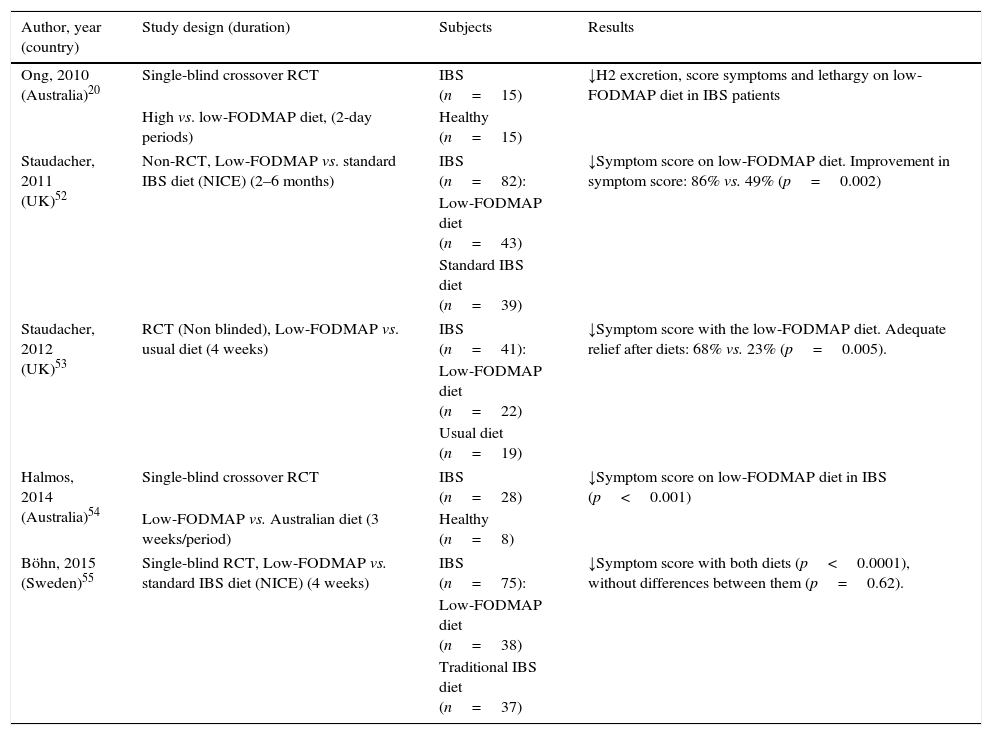
A number of clinical uncontrolled studies from Australia, New Zealand, Norway, United Kingdom, Denmark and Spain15,46–51 have consistently shown the efficacy of low FODMAP diet for IBS. Solid evidence supporting the efficacy of low FODMAP diet relies on five controlled trials, four of these being randomized controlled trials (RCT) (Table 2).20,52–55 The non-RCT compared low FODMAP diet to standard dietary advice for IBS, according to recommendations from The National Institute for Health and Care Excellence (NICE).52 Substantially more patients on a low FODMAP diet reported improvement in overall symptoms as well as satisfaction with response at a 2–6 months follow-up visit compared with those receiving standard advice.52 However, the lack of randomization and follow-up only in patients who returned to the clinic are major limitations to this study.
Key randomized trials supporting the efficacy of low FODMAP diet for IBS.
| Author, year (country) | Study design (duration) | Subjects | Results |
|---|---|---|---|
| Ong, 2010 (Australia)20 | Single-blind crossover RCT | IBS (n=15) | ↓H2 excretion, score symptoms and lethargy on low-FODMAP diet in IBS patients |
| High vs. low-FODMAP diet, (2-day periods) | Healthy (n=15) | ||
| Staudacher, 2011 (UK)52 | Non-RCT, Low-FODMAP vs. standard IBS diet (NICE) (2–6 months) | IBS (n=82): | ↓Symptom score on low-FODMAP diet. Improvement in symptom score: 86% vs. 49% (p=0.002) |
| Low-FODMAP diet (n=43) | |||
| Standard IBS diet (n=39) | |||
| Staudacher, 2012 (UK)53 | RCT (Non blinded), Low-FODMAP vs. usual diet (4 weeks) | IBS (n=41): | ↓Symptom score with the low-FODMAP diet. Adequate relief after diets: 68% vs. 23% (p=0.005). |
| Low-FODMAP diet (n=22) | |||
| Usual diet (n=19) | |||
| Halmos, 2014 (Australia)54 | Single-blind crossover RCT | IBS (n=28) | ↓Symptom score on low-FODMAP diet in IBS (p<0.001) |
| Low-FODMAP vs. Australian diet (3 weeks/period) | Healthy (n=8) | ||
| Böhn, 2015 (Sweden)55 | Single-blind RCT, Low-FODMAP vs. standard IBS diet (NICE) (4 weeks) | IBS (n=75): | ↓Symptom score with both diets (p<0.0001), without differences between them (p=0.62). |
| Low-FODMAP diet (n=38) | |||
| Traditional IBS diet (n=37) | |||
Abbreviations: NICE: The National Institute for Health and Care Excellence; RCT, randomized clinical trial.
Thus far, four RCTs have investigated the effect of FODMAP restriction on IBS symptoms, two of which are controlled studies in which food was provided and carefully controlled, and the other two based upon dietary advice in the clinical setting. The first study compared the effect of two 2-day diets comparing low- and high-FODMAP diets (9g vs. 50g per day) and showed that symptom scores were substantially reduced during low FODMAP diet.20 In the second RCT, this approach was compared with the usual diet.53 Adequate relief of symptoms was reported in 68% of patients receiving dietary intervention compared with 23% of control patients on their usual diet. However, the treatment group was not blinded to the intervention, a common problem in dietary intervention trials. The third study was a randomized, controlled, crossover trial that demonstrated a statistically significant reduction in overall symptoms, pain, bloating and flatulence in patients with IBS consuming a low-FODMAP diet as compared with a typical Australian diet.54 Improvement in overall gastrointestinal symptoms was observed in 70% of participants. The Australian typical diet in which food was provided and carefully controlled, however, does not mimic the real-life challenges associated with sustaining a restricted diet in free-living individuals. In real life, the low FODMAP diet is dietitian-taught, and dietary restriction may have varying degrees of compliance since depends on the patients’ degree of understanding and motivation.
Finally, a recent single-blinded RCT compared in a clinical practice setting a low FODMAP diet with traditional IBS diet based on NICE recommendations during 4 weeks.55 IBS symptom severity improved in both groups, with any differences between the groups. At the end of the study 56% of patients in the low-FODMAP diet and 46% in the traditional IBS diet were responders to the treatment (IBS severity scoring system reduction ≥50 at the end of the treatment relative to baseline). Food diaries demonstrated good adherence to the dietary advice.
The results of a recent meta-analysis of RCT evaluating the effectiveness of low FODMAP diet in patients with IBS (not including the recent trial of Böhn et al.55) consistently support the utility of this dietary intervention with an estimated number needed to treat (NNT) of 2.2 (95% confidence interval: 1.89–2.51).56
In general, these uncontrolled and controlled trials indicate that, in patients with IBS, the symptoms most responsive to FODMAP restriction are bloating, flatulence, abdominal pain, urgency and altered stool output, with up to 70% of patients reporting symptomatic benefit.
The efficacy of low FODMAP diet for non-celiac gluten sensitivityNon-celiac gluten sensivity (NCGS) is an emerging disorder characterized by intestinal and extraintestinal symptoms related to the ingestion of gluten-containing food, in patients who are not affected by either celiac disease or wheat allergy. Due to the absence of reliable biomarkers, NCGS remains a diagnosis of exclusion of celiac disease and most patients are self-diagnosed and voluntarily start a gluten-free diet (GFD).57 A recent systematic review on NCGS has highlighted the lack of evidence supporting a GFD for NCGS.58 Two trials first showed recently contrasting results for the culprit agent in NCGS. In the first study, gluten was believed to trigger symptoms after comparing small supplements of gluten or placebo while on a GFD.59 In contrast, in a second placebo-controlled rechallenge study from the same group, FODMAPs but not gluten were found to induce symptoms in NCGS.60
A more recent cross-over, placebo-controlled trial from Italy has shown again a significant increase in gastrointestinal symptoms during 1 week of intake of small amounts of gluten in NCGS patients.61 In contrast, several upcoming trials, from Italy as well, will show a higher benefit of low FODMAP diet over GFD for NCGS, which is sustained during follow-up and not improved by additional restriction of gluten.62–64 Whether gluten, FODMAPs or other components in wheat trigger symptoms in IBS is a matter of ongoing research.65
Criticisms to low FODMAP dietIt is clear that IBS treatments are unsatisfactory in many cases. This is related to different aspects, ranging from the complex and incompletely understood pathophysiology of IBS to its enormous clinical heterogeneity. Moreover, some IBS medications (received with great expectations) had to be withdrawn from the market because of side effects.66 New promising drugs have been launched, and some others will be in the next future, but its final place in the treatment algorithm of IBS in the clinical setting will not be known before accumulated experience of their use is available, and in some cases price can be a concern. In the meanwhile, a huge amount of patients and clinicians are waiting for the perfect treatment to arrive: (1) specifically driven to a given pathophysiological mechanism; (2) useful for all IBS subtypes and symptoms; (3) devoided of side effects and (4) cheap.
Is low FODMAP diet this marvelous treatment? According to Google (more than 600,000 citations), the media, and many low FODMAP fans this is the case. Such enthusiasm is supported by conclusions, such as the one published in a journal as prestigious as Gastroenterology: “A diet low in FODMAPs effectively reduced functional gastrointestinal symptoms. This high-quality evidence supports its use as a first-line therapy”. Nevertheless, this conclusion, obtained from a well design clinical trial, came from a study only including 30 patients with IBS and 8 healthy individuals.54 It is true that some other studies have successfully evaluated low FODMAP in IBS patients, but today (May 1, 2015) there is still much controversy about it and it remains unclear whether all of these expectations will be confirmed as of real clinical value in a long lasting chronic disorder as IBS. Putative limitations of a low FODMAP diet are displayed in Table 3.
Putative limitations of a low FODMAP diet for IBS.
| Limitations related to the low FODMAP concept |
| High level of restriction due to carbohydrate additive effects |
| Avoidance of most wheat sources may lead to reduced gluten intake in the low FODMAP diet |
| The mechanisms by which FODMAPs induce lethargy and other extradigestive manifestations remain to be elucidated |
| The reintroduction process is yet to be clarified and may be difficult due to carbohydrate additive effects |
| Limitations related to reported literature |
| Lack of medium- and long-term results of efficacy, the majority not beyond 6 weeks |
| Concerns with different placebo and comparator group (placebo, standard diet) |
| Blinding is complex and may not reflect a “real life” scenario |
| Other therapeutic interventions (standard dietary advice for IBS, gut-directed hypnotherapy, probiotics) have been recently shown not inferior to a low FODMAP diet for IBS |
| Limitations related to the safety of the diet |
| Impact on richness and diversity of gut microbiota (fructans and GOS are prebiotics) |
| Nutritional inadequacy, mostly related to dairy restriction |
| Potential increased cardiovascular diseases or colorectal cancer due to reduced fiber intake |
| Limitations for health providers |
| Counseling by an expert dietitian is mandatory |
| Limitations for the IBS patient |
| Following a low FODMAP diet requires highly motivated patients |
| It is more expensive than standard diet |
| Long-term adherence is not easy |
| Social life is hampered by restrictive diets |
| Reduced intake of fiber may worsen constipation-related symptoms |
| Lack of predictors of response to a low FODMAP diet (which IBS patient will benefit the most: constipation, diarrhea, abdominal pain, alternate, mixed?) |
Furthermore, when it comes to any dietary intervention for IBS, collecting high quality evidence might be hard to accomplish. Good clinical trials in dietary research, fulfilling all research conditions, including adequately powered, well-controlled, double-blind, placebo-compared, are difficult to perform. In fact, most of the published studies evaluating the efficacy of low FODMAP diet have some limitations, such as the choice of placebo (in many cases country specific customary diets), short-term evaluation (from 3 days to 6 weeks) and lack of reliable blinding. As pointed out by Staudacher et al.,22 controlled feeding studies enable diets with a precise composition to be provided in a laboratory setting, but blinding is still problematic because participants might become aware of their group allocation when consuming specific foods. In addition, controlled feeding studies do not reflect “real life” eating behavior and it is not known whether the same symptom response would occur when a participant attempts to incorporate the intervention into their habitual diet. In contrast, studies in which participants are given dietary advice better reflects what happens in clinical practice and provides an understanding of the degree of dietary change, and symptom response, that a patient is likely to achieve. However, it is difficult to provide ‘control dietary advice’ unless a comparator dietary intervention is chosen. An excellent review about challenges associated with the design and reporting in dietary intervention trials has been recently published.67
Limitations related to the low FODMAP conceptOne limitation when performing dietary research is that restriction of one constituent often influences the intake of another. For example, restriction of fructans from wheat may lead to reduced gluten intake [only oats (limited intake), bread crumbs and bread made with spelt (limited intake) and barley-containing drinks (limited intake) are allowed as gluten sources]. This situation might explain the considerable clinical and therapeutic overlap between low FODMAP diet and a GFD.
Long-term liberalized low FODMAP diet is recommended. After response to a low FODMAP diet, a re-challenge process should be commenced to identify individual's specific food triggers and tolerance limit.68 Every patient should be challenged with small quantities of each individual carbohydrate group to check symptom relapse and tolerance by increasing up gradually the dose. Then, a combination of various FODMAP should also be trialed and finally the patient should ingest any food well tolerated. However, this combination after individual challenge, apart from not reported yet in clinical studies, is questionable as the rationale basis for the FODMAP concept is that global carbohydrate restriction is deemed to be necessary in order to avoid additive effects.21
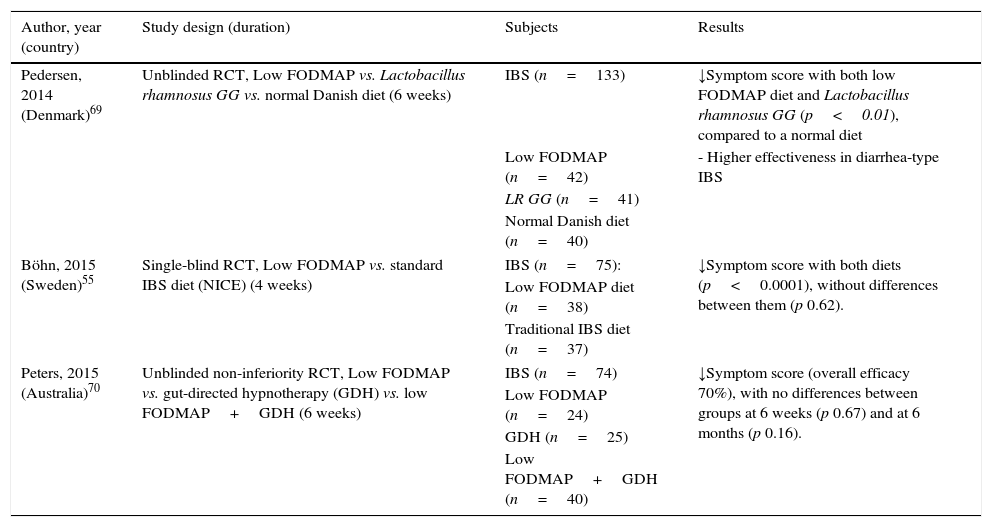
Limitations related to recent comparative studiesBesides the aforementioned limitations regarding dietary interventions study, one of the most controversial issues recently raised with the low FODMAP diet is the lack of advantage over alternative and simpler therapeutic interventions. Recent studies comparing low FODMAP diet and traditional IBS dietary advice,55 probiotic administration (Lactobacillus rhamnosus GG)69 and gut-directed hypnotherapy70 have shown similar clinical benefits for IBS. The results of these studies are summarized in Table 4.
Recent comparative studies showing no advantage of a low FODMAP diet compared to other dietary, pharmacological and psychological interventions for IBS patients.
| Author, year (country) | Study design (duration) | Subjects | Results |
|---|---|---|---|
| Pedersen, 2014 (Denmark)69 | Unblinded RCT, Low FODMAP vs. Lactobacillus rhamnosus GG vs. normal Danish diet (6 weeks) | IBS (n=133) | ↓Symptom score with both low FODMAP diet and Lactobacillus rhamnosus GG (p<0.01), compared to a normal diet |
| Low FODMAP (n=42) | - Higher effectiveness in diarrhea-type IBS | ||
| LR GG (n=41) | |||
| Normal Danish diet (n=40) | |||
| Böhn, 2015 (Sweden)55 | Single-blind RCT, Low FODMAP vs. standard IBS diet (NICE) (4 weeks) | IBS (n=75): | ↓Symptom score with both diets (p<0.0001), without differences between them (p 0.62). |
| Low FODMAP diet (n=38) | |||
| Traditional IBS diet (n=37) | |||
| Peters, 2015 (Australia)70 | Unblinded non-inferiority RCT, Low FODMAP vs. gut-directed hypnotherapy (GDH) vs. low FODMAP+GDH (6 weeks) | IBS (n=74) | ↓Symptom score (overall efficacy 70%), with no differences between groups at 6 weeks (p 0.67) and at 6 months (p 0.16). |
| Low FODMAP (n=24) | |||
| GDH (n=25) | |||
| Low FODMAP+GDH (n=40) | |||
Abbreviations: GDH: gut -directed hypnotherapy; LR GG: Lactobacillus rhamnosus GG; NICE: The National Institute for Health and Care Excellence; RCT, randomized clinical trial.
It is now well established that food, along with infectious exposures and antibiotics, is major factor controlling gut microbial diversity and activity.71 Among FODMAPs, fructans and GOS exert prebiotic effects and, accordingly, a marked reduction in the diet will likely reduce overall bacterial abundance. In a first study after a 4-week low FODMAP in IBS patients, a marked reduction in luminal Bifidobacteria concentration was observed, with no effects on other bacterial group or total numbers of bacteria.53 Another recent study investigated fecal bacteria from IBS patients comparing a controlled low FODMAP and a standard Australian diet for 3 weeks.72 Specific and marked reduction (by 47%) in the relative abundance of Clostridium coccoides (a strong butyrate-producing species) and Akkerkmansia muciniphila (key to healthy mucus-associated microbiota) were observed, with a relative increase in Ruminococcus torques, an unfavorable mucus-consuming bacterium. Interestingly, no specific reduction of Bifidobacteria was observed in this study.53 The functional significance and health implications of such changes might lead to caution in recommendation of strict FODMAP intake reduction in the longer term.
Other main concern is that low FODMAP diet could lead to nutritional inadequacy in the long-term. In one study evaluating the effect of fermentable carbohydrate restriction compared with control diet no difference was found in micronutrient intake, except for a lower calcium intake,53 presumably as a result of lower dairy products intake. This might be a problem mainly in children and post-menopausal women, and high-calcium alternative foods should be sought.
As fiber intake is reduced in the low FODMAP diet, constipation might worsen in constipation-type IBS. This fact has not been confirmed in a first systematic review, showing a trend to a short-term efficacy in this subset of patients.73 More rigorous studies are needed to ascertain which specific IBS patient among their diverse phenotypes will benefit most from the low FODMAP diet. Finally, whether some other long-term risks, such as cardiovascular diseases74 or colorectal cancer75 related to reduced dietary fiber are increased following a low FODMAP diet, remains to be elucidated.
Limitations for health providersPrescribing a low FODMAP diet is not easy. Much knowledge about dietary and nutritional aspects is needed as the diet is complex and most gastroenterologists do not have it. Inadequate prescription and education of the patient poses the risk of nutritional inadequacy. Therefore, low FODMAP diet should be taught by motivated dietitians with experience in the area.68 A recent study first comparing different methods of teaching the low FODMAP diet (one-to-one vs. group) did show group education was cost effective and might be a first step to implement it in the clinical practice setting.76 Similarly, adherence to therapy should be monitored by expert dietitians.
Limitations for IBS patientsThe low FODMAP diet is a complex diet, which may not be easy to follow. The diet is more expensive than a standard diet, and it is difficult to keep such a restrictive diet during normal social life and at work. Hence, following a low FODMAP diet requires considerable effort from a highly motivated patient to achieve an adequate adherence. This is important because a prospective, long term follow-up study from New Zealand49 in 90 IBS patients, showed that 75% of patients were adherent to the diet and the degree of adherence correlated with symptom improvement. However, the impact of the diet on the quality of life of patients and their relatives (e.g., by deciding not to eat out-of-home and hampering social life) should also be taken into consideration.
Conflict of interestThe authors declare no conflict of interest.
We kindly appreciate the comments provided by Dr. Peter Gibson and his team from the Monash University in Australia.