Hepatitis E virus (HEV) is a virus of the Herpesviridae family, of which there are four genotypes in humans. In developed countries, genotype 3 predominates, producing sporadic infections which are usually asymptomatic and self-limiting, with age, immunosuppression and chronic liver disease being the main factors for poor prognosis.1
We present the case of a 76-year-old male with a history of chronic coronary heart disease, on treatment with enalapril and atorvastatin and no known prior liver disease. The patient was admitted with jaundice and elevated liver enzymes (GOT: 1694U/l [range: 4–50]; GPT: 1927U/l [range: 5–40]; total bilirubin: 5.02mg/dl; platelets: 66,000/μl; INR: 1.25); GFR: 43.81ml/min (baseline GFR: 60ml/min) without fever. An acute hepatitis study was carried out with RNA (PCR), IgG and IgM (ELISA-Dia.Pro®) positive for HEV, with other results proving normal (hepatotropic virus, autoimmunity, iron/copper metabolism). In the urinalysis, no proteinuria or haematuria was observed. The patient said he had not consumed game meat, had contact with animals or traveled.
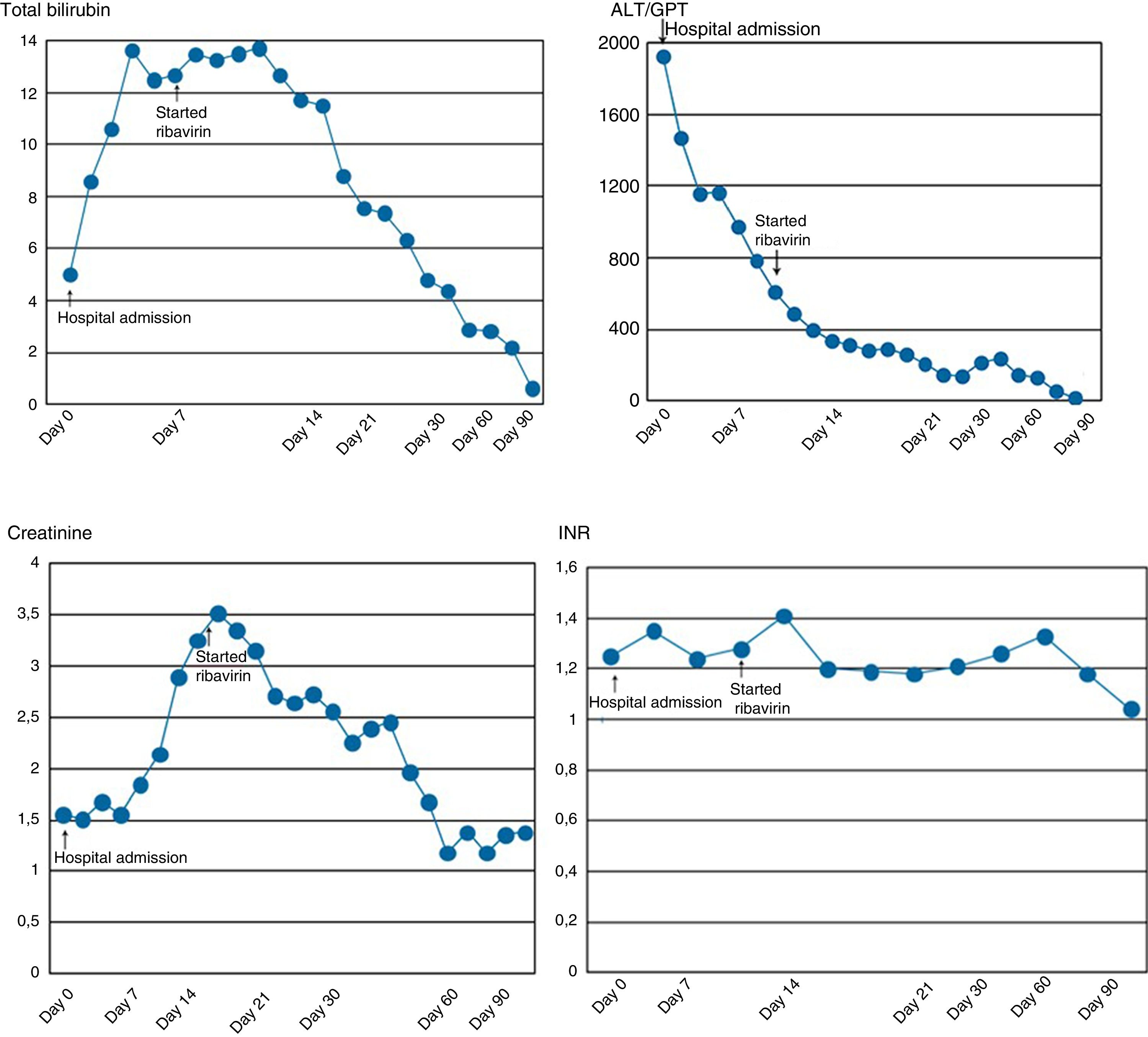
Abdominal CT showed no signs of chronic liver disease, but there was mild ascites. While in hospital, there was a slow but progressive decrease in the patient's transaminase levels, but elevation of bilirubin, prolongation of prothrombin time and deterioration of kidney function requiring haemodialysis (Fig. 1). He also developed moderate ascites and grade II hepatic encephalopathy.
The patient was initially treated with support measures. Once we discovered he was HEV positive, in view of his progressive deterioration, treatment was started with ribavirin adjusted according to kidney function: 200mg/day in the first month which, following an improvement in kidney function, was adjusted to 400mg/day. The patient tolerated ribavirin well, besides developing anemia (Hb: 9.2mg/dl), but no transfusion, dose reduction or use of erythropoietin was required. Progress was very slow but in the right direction. Ribavirin was withdrawn after 90 days of treatment when the liver biochemistry returned to normal. We did not determine the HEV viral load on completion of the treatment. Now, two years later, the patient is asymptomatic.
In Spain, the seroprevalence of HEV is 0.6–7.3%,2 with the majority in rural areas and most often in men over 50 years of age. The main mechanism of transmission in our setting is the ingestion of contaminated meat from pigs, wild boar and deer (genotypes 3 and 4), but cases of transmission through transfusions and solid organ transplants have been described.3
In terms of clinical manifestations, these can range from patients being asymptomatic to fulminant hepatitis.3
There is no well-established consensus on the treatment of acute and chronic infection. In solid organ transplant recipients HEV can cause chronic infection and cirrhosis, and a three-month course of ribavirin is therefore recommended as the treatment of choice.3 Blood testing should be frequent because of the risk of severe anemia as a side effect.4
In immunocompetent patients the infection is usually self-limiting with clearance of the virus within a few weeks, so the most common treatment is supportive. Reports in the literature of isolated cases of immunocompetent patients without previous liver disease suggest that ribavirin improves prognosis in severe acute hepatitis.4 The optimal duration of treatment has not been established, but three-month regimens are recommended or until the viral load is undetectable, with doses ranging from 600 to 1200mg a day.4
In HEV infection, there can be multiple extra-gastrointestinal manifestations, such as neurological and hematological signs, and glomerulonephritis.3 Although the physiological/pathogenic mechanism of renal involvement is not fully understood, one hypothesis is that a deposit of IgG antigen–antibody complexes and rheumatoid factor forms in the glomerulus. Both the HEV antigen and RNA have been detected in the urine of patients with chronic HEV infection, but there is no evidence that HEV is directly nephrotoxic or that it can replicate in renal cells.5 Moreover, clearance of the virus can improve kidney function.5 In our patient, no possible triggers of prerenal or toxic kidney injury were found. No kidney biopsy was performed due to the patient's coagulopathy, but urinalysis was not compatible with glomerulonephritis.
In this case of severe acute hepatitis E complicated by kidney failure, ribavirin was effective and well tolerated, with recovery of liver and kidney function.
Please cite this article as: Ríos León R, Rodríguez Gandía MA, Rodríguez de Santiago E, Sánchez Rodríguez E, Guerrero García A, Flores de Miguel A, et al. Tratamiento con ribavirina de la hepatitis E aguda con afectación renal grave en un paciente inmunocompetente. Gastroenterol Hepatol. 2019;42:102–103.