Direct-acting antivirals (DAAs) are an opportunity for hepatitis C virus (HCV) elimination. Strategies are needed to diagnose new patients and to attract those diagnosed without evaluation. Patients with other chronic viral diseases who receive satisfactory treatment promote referral of other patients for evaluation. Our aim was to evaluate whether patients who have been treated with DAAs would recommend follow-up and treatment to other patients as well as the characteristics that influence this decision.
Patients and methodsTwo-hundred and 2 HCV-infected patients treated with DAAs were included. Patients were asked about whether they knew other infected people and their willingness to share their experience. A general satisfaction survey and a specific HCV satisfaction survey were carried out. Demographic, socioeconomic and HCV infection variables were recorded.
ResultsDespite the fact that 54.4% of the patients reported knowing others infected, 34.2% would not fully agree to share their experience. The analysis of general and specific satisfaction showed that patients who shared their experience mentioned a perception of greater care from the specialist (4.7 ± 0.4 vs. 4.3 ± 0.6, P = 0.001) and had more information on treatment expectations (4.6 ± 0.5 vs. 4.0 ± 0.7, P = 0.001) and social support (4.5 ± 0.7 vs. 4.0 ± 0.8, P = 0.001).
ConclusionsThe perception by treated patients of general satisfaction with the healthcare process and information about benefits influences the degree of recommendation to other infected people. Knowledge about treatment and perception of improvement in health of treated patients should be enhanced as it can contribute to increasing referrals to specialized consultation.
Los antivirales de acción directa (AAD) representan una oportunidad para la eliminación del virus de la hepatitis C (VHC) por su simplicidad. No obstante, se precisan estrategias dirigidas a diagnosticar nuevos pacientes y a atraer diagnosticados sin evaluación. En este sentido los pacientes con otras enfermedades virales crónicas que reciben un trato satisfactorio promueven la derivación a consulta de otros pacientes. Nuestro objetivo fue evaluar en qué grado los pacientes que han sido tratados con AAD recomendarían seguimiento y tratamiento a otros pacientes, así como las características de los pacientes que influyen en esta decisión.
Pacientes y métodosSe incluyeron 202 pacientes infectados por VHC tratados con AAD. Se les preguntó sobre conocimiento de otros infectados y deseos de compartir su experiencia, y se realizó encuesta de satisfacción general (cuestionario Baker) y específica de VHC (cuestionario HCVTSat). Además, se registraron variables demográficas, socioeconómicas y de la infección por VHC.
ResultadosA pesar de que el 54,4% de los pacientes refería conocer a otros afectados, un 34,2% no estaría totalmente de acuerdo en compartir su experiencia global en consulta. El análisis de satisfacción general y específica mostró que los pacientes que compartirían su experiencia referían una percepción de mayor atención por parte del especialista (4,7 ± 0,4 vs. 4,3 ± 0,6, p = 0,001), tenían más información sobre el tratamiento (4,6 ± 0,5 vs. 4,0 ± 0,7, p = 0,001) y mayor apoyo social (4,5 ± 0,7 vs. 4,0 ± 0,8, p = 0,001).
ConclusionesLa percepción por parte del paciente tratado sobre satisfacción general del proceso de atención sanitaria e información de beneficios influye en el grado de recomendación a otros infectados. Se debe prestar atención y mejorar el conocimiento del tratamiento y la percepción de mejora en salud de los pacientes tratados, ya que puede contribuir a aumentar las derivaciones a consulta especializada.
Chronic hepatitis C virus (HCV) infection has a prevalence of 1.5% in Europe1 and 0.22% in Spain.2 The course of the infection without treatment can have serious consequences,3 and therefore every effort is made nowadays to diagnose, evaluate and treat infected patients in order to attain the World Health Organization (WHO) targets for HCV elimination by 2030.4,5 Furthermore, HCV infection is not just a serious public health problem; it also carries an individual risk of liver and systemic disease, and it has negative psychological, social and familial impacts, becoming a stigmatised disease that can result in the isolation of affected patients.6
Even after the development of direct-acting antivirals (DAAs), despite these treatments’ effectiveness, brief duration and minimal adverse effects, less than 10% of affected individuals are treated and cured.7 There are multiple reasons why patients are not treated8; one of the most notable among them is likely a low rate of referral or of care and follow-up by the specialist, who ultimately prescribes treatment.9 This, furthermore, leads to a lack of treatment availability, making the WHO targets more difficult to reach. Therefore, it is essential to increase rates of diagnosis and referral through specific strategies in this regard.10
It was recently found in patients with human immunodeficiency virus infection that patients’ general satisfaction positively influences adherence to treatment, resulting in achievement of higher viral suppression rates. It also influences the promotion of referral of some patients by other patients.11
However, the role of patients with HCV treated with DAAs and their willingness to share their treatment experience with other patients is unknown. For this, it is important to determine the degree of satisfaction of patients with HCV treated with the new regimens with DAAs without interferon and the factors that influence already treated patients to refer other patients to a specialist. At the same time, it would be interesting to know whether treated patients express the wish to share their experience and are willing to attract cases of HCV infection that they know of in their environment to a specialist, as a new strategy for increasing the referral of patients.
Therefore, we decided to examine which factors dependent on the patients, the treatment and the specialist care received have a direct impact on the degree of satisfaction, to subsequently assess the recommendation that patients would make to other affected persons to receive medical care.
Material and methodsPatientsAll patients consecutively prescribed treatment with DAAs who remained in follow-up by hepatology in the Gastroenterology Department at Hospital Universitario de Canarias [Canary Islands University Hospital] between July 2016 and May 2017 were invited to participate.
The study was conducted in compliance with the ethical principles of the Declaration of Helsinki of October 2013 and approval by the Hospital Universitario de Canarias Institutional Review Board.
After the informed consent form was signed, demographic, socioeconomic, clinical and laboratory data were collected from the patients through the Hospital Universitario de Canarias electronic medical record system. The objective of the study was to evaluate, using specific questions, whether patients knew other patients and intended to share their experience with others. To determine which factors might be influential, such as the degree of satisfaction with care received in different areas, both general12 and specific13 satisfaction surveys were used.
Patients who did not give informed consent to participate in the study and patients who completed the questionnaire incorrectly or illegibly were excluded from the final statistical analysis.
Satisfaction surveysIn the visit after the end of treatment, patients were provided with a survey to complete at home and then deliver to the secretary's office of the Gastroenterology Department. This was a Likert-style survey (1, completely disagree; 2, disagree; 3, neutral; 4, agree; 5, completely agree), comprising an overall satisfaction questionnaire (Appendix B, Supplementary materials 1)12 evaluating different areas related to general aspects of satisfaction, professional care, time dedicated and depth of the physician/patient relationship, and another questionnaire more specific about HCV (HCVTSat questionnaire) (Appendix B, Supplementary materials 2)13 with questions divided into 3 groups evaluating experience with treatment, adverse effects and social factors.
Statistical analysisFor the statistical analysis of the satisfaction surveys, each of them was analysed by grouping the questions based on the area evaluated (Appendix B, Supplementary materials 1 and 2), then calculating the mean sum across all the questions in the same area.
The variables were collected in an SPSS© version 15.0 (SPSS Inc., Chicago, IL, USA) database. Qualitative variables were statistically analysed using the chi-squared test, and the quantitative variables using the Student’s t test or the Mann–Whitney U test if they did not meet normal distribution criteria. Logistic regression analysis was used to identify predictive factors. A p value <0.05 was considered statistically significant.
ResultsPatient characteristicsAfter completing antiviral treatment, a total of 202 patients (median age 55 years, range 27−83; 64.9% men) were invited to participate in this study. A total of 32 patients (15.8%) were excluded for various reasons: 27 did not submit the survey, 3 did not give informed consent to participate in the study, one was incapacitated and one filled in the survey incorrectly.
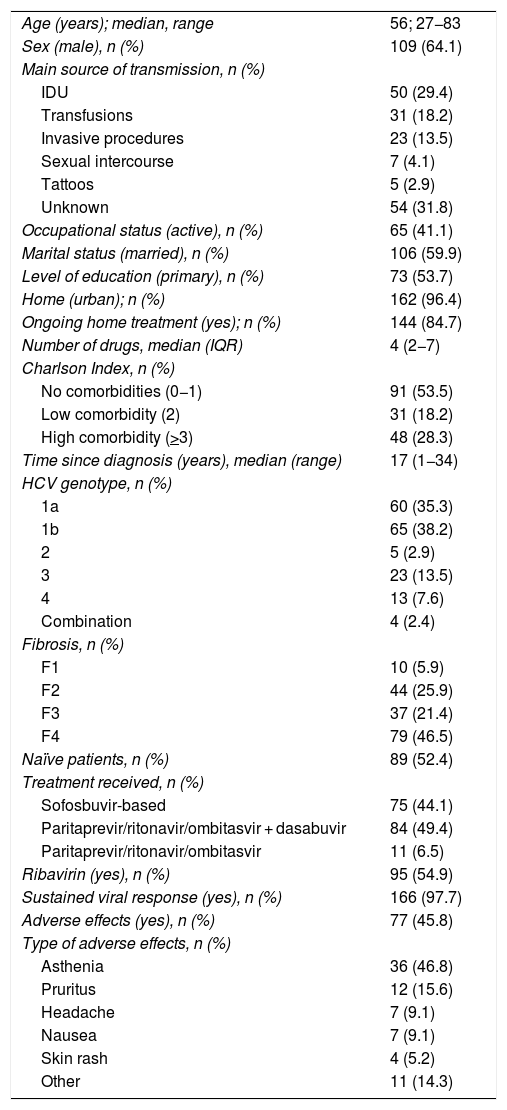
Finally, statistical analysis was performed with a total of 170 patients (median age 56 years, range 27−83; 64.1% men). The characteristics of the patients included are shown in Table 1.
General and HCV-specific characteristics of the patients included.
| Age (years); median, range | 56; 27−83 |
| Sex (male), n (%) | 109 (64.1) |
| Main source of transmission, n (%) | |
| IDU | 50 (29.4) |
| Transfusions | 31 (18.2) |
| Invasive procedures | 23 (13.5) |
| Sexual intercourse | 7 (4.1) |
| Tattoos | 5 (2.9) |
| Unknown | 54 (31.8) |
| Occupational status (active), n (%) | 65 (41.1) |
| Marital status (married), n (%) | 106 (59.9) |
| Level of education (primary), n (%) | 73 (53.7) |
| Home (urban); n (%) | 162 (96.4) |
| Ongoing home treatment (yes); n (%) | 144 (84.7) |
| Number of drugs, median (IQR) | 4 (2−7) |
| Charlson Index, n (%) | |
| No comorbidities (0−1) | 91 (53.5) |
| Low comorbidity (2) | 31 (18.2) |
| High comorbidity (>3) | 48 (28.3) |
| Time since diagnosis (years), median (range) | 17 (1−34) |
| HCV genotype, n (%) | |
| 1a | 60 (35.3) |
| 1b | 65 (38.2) |
| 2 | 5 (2.9) |
| 3 | 23 (13.5) |
| 4 | 13 (7.6) |
| Combination | 4 (2.4) |
| Fibrosis, n (%) | |
| F1 | 10 (5.9) |
| F2 | 44 (25.9) |
| F3 | 37 (21.4) |
| F4 | 79 (46.5) |
| Naïve patients, n (%) | 89 (52.4) |
| Treatment received, n (%) | |
| Sofosbuvir-based | 75 (44.1) |
| Paritaprevir/ritonavir/ombitasvir + dasabuvir | 84 (49.4) |
| Paritaprevir/ritonavir/ombitasvir | 11 (6.5) |
| Ribavirin (yes), n (%) | 95 (54.9) |
| Sustained viral response (yes), n (%) | 166 (97.7) |
| Adverse effects (yes), n (%) | 77 (45.8) |
| Type of adverse effects, n (%) | |
| Asthenia | 36 (46.8) |
| Pruritus | 12 (15.6) |
| Headache | 7 (9.1) |
| Nausea | 7 (9.1) |
| Skin rash | 4 (5.2) |
| Other | 11 (14.3) |
HCV: hepatitis C virus; IDU: intravenous drug use; IQR: interquartile range.
Regarding specific characteristics of HCV, 73.5% of the patients had genotype 1, 67.9% had at least advanced fibrosis or cirrhosis (F > 2) and 52.4% were naïve patients. 44.1% were treated with sofosbuvir-based therapies and 54.9% were also treated with ribavirin. The rate of sustained viral response was 97.7%. Adverse effects in relation to treatment were reported by 46.8% of patients; the most common were asthenia (46.8%) and pruritus (15.6%). The rate of adverse effects was similar regardless of age and prior comorbidity, but higher in patients who received ribavirin in their treatment (65.9% versus 34.1%, p = 0.004).
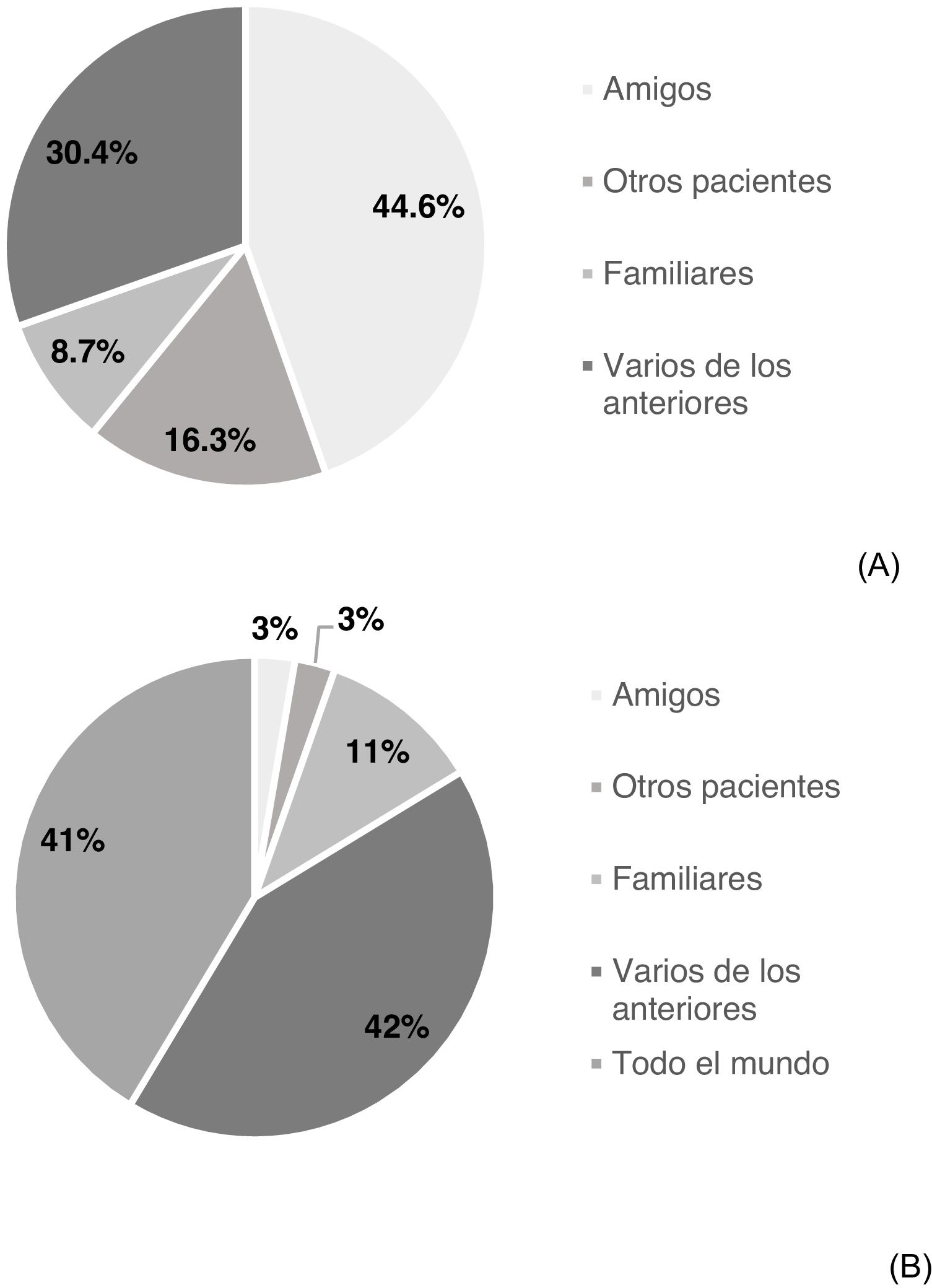
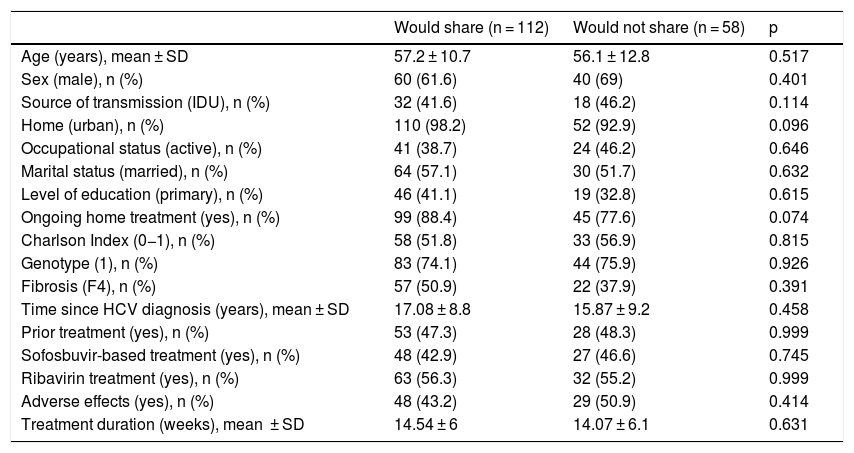
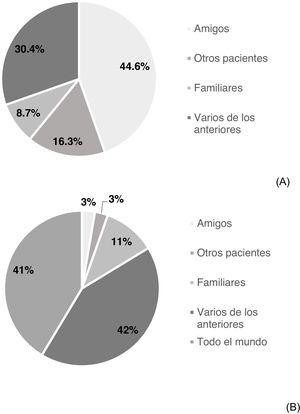
Knowledge of other affected individuals and intention of sharing experienceSome 54.4% of participating patients stated that they knew others affected by HCV (n = 92); 44.6% friends, 8.7% family members, 16.3% other patients and 30.4% several of the above (Fig. 1A). 65.8% (n = 112) of the patients would be completely agreeable to sharing their experience: 2.7% with friends, 10.9% with family members, 2.7% with others affected, 42.3% with several of the above and 41.4% with anybody (Fig. 1B). However, 34.1% would not be completely agreeable to sharing their experience. No significant differences were found in demographic, socioeconomic or HCV-related variables between patients who would share their experience and patients who would not (Table 2).
Comparative analysis of demographic, socioeconomic and HCV-related variables for the group of patients who would share vs. those who would not share their experience.
| Would share (n = 112) | Would not share (n = 58) | p | |
|---|---|---|---|
| Age (years), mean ± SD | 57.2 ± 10.7 | 56.1 ± 12.8 | 0.517 |
| Sex (male), n (%) | 60 (61.6) | 40 (69) | 0.401 |
| Source of transmission (IDU), n (%) | 32 (41.6) | 18 (46.2) | 0.114 |
| Home (urban), n (%) | 110 (98.2) | 52 (92.9) | 0.096 |
| Occupational status (active), n (%) | 41 (38.7) | 24 (46.2) | 0.646 |
| Marital status (married), n (%) | 64 (57.1) | 30 (51.7) | 0.632 |
| Level of education (primary), n (%) | 46 (41.1) | 19 (32.8) | 0.615 |
| Ongoing home treatment (yes), n (%) | 99 (88.4) | 45 (77.6) | 0.074 |
| Charlson Index (0−1), n (%) | 58 (51.8) | 33 (56.9) | 0.815 |
| Genotype (1), n (%) | 83 (74.1) | 44 (75.9) | 0.926 |
| Fibrosis (F4), n (%) | 57 (50.9) | 22 (37.9) | 0.391 |
| Time since HCV diagnosis (years), mean ± SD | 17.08 ± 8.8 | 15.87 ± 9.2 | 0.458 |
| Prior treatment (yes), n (%) | 53 (47.3) | 28 (48.3) | 0.999 |
| Sofosbuvir-based treatment (yes), n (%) | 48 (42.9) | 27 (46.6) | 0.745 |
| Ribavirin treatment (yes), n (%) | 63 (56.3) | 32 (55.2) | 0.999 |
| Adverse effects (yes), n (%) | 48 (43.2) | 29 (50.9) | 0.414 |
| Treatment duration (weeks), mean ± SD | 14.54 ± 6 | 14.07 ± 6.1 | 0.631 |
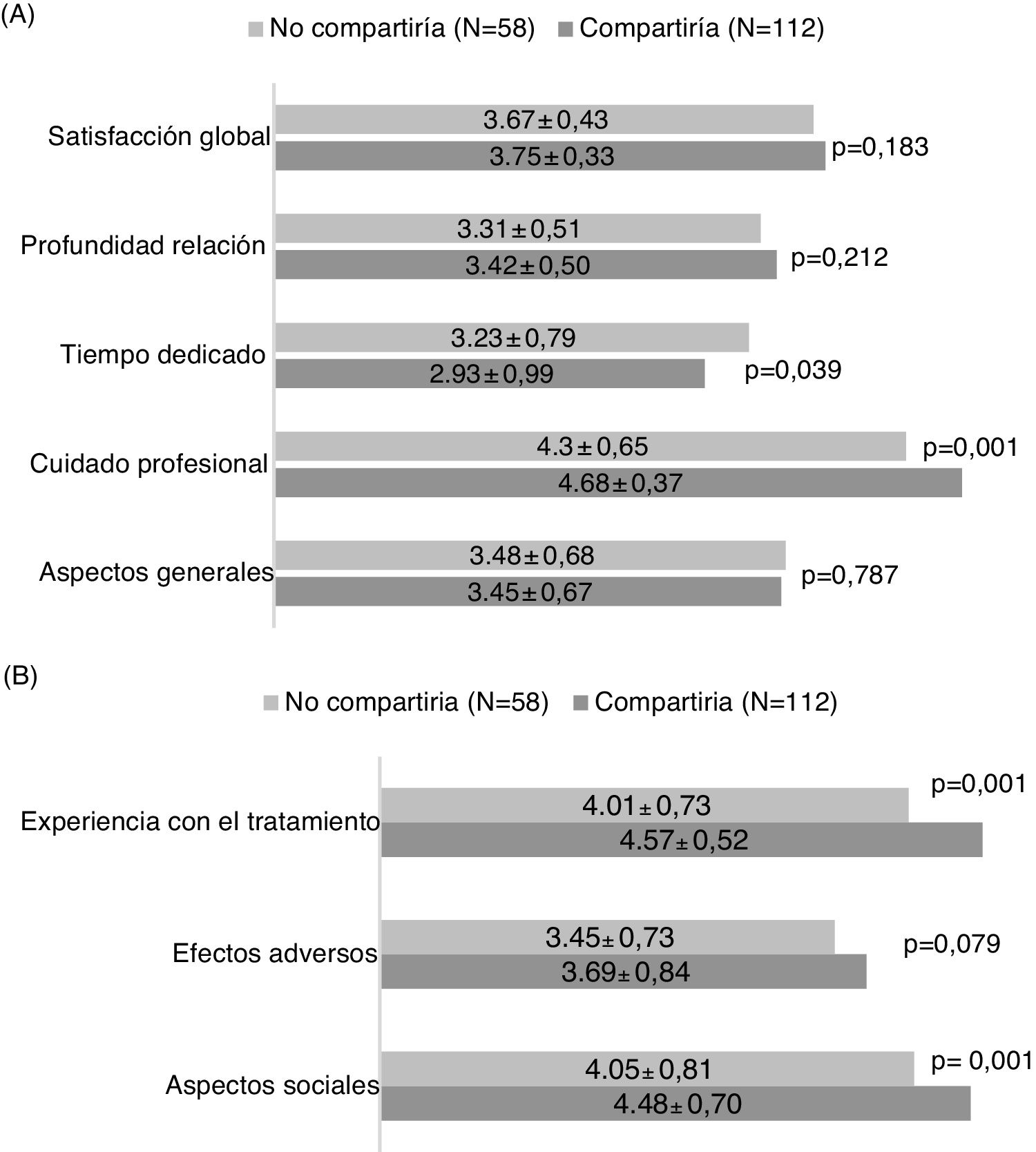
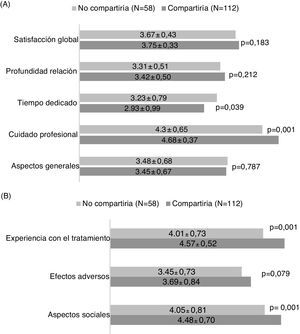
The general satisfaction analysis using the Baker questionnaire found that the patients who would share their experience showed a trend towards greater overall satisfaction with the diagnosis and treatment process (p = 0.183), attaining statistically significant differences in the area of greater care on the part of the medical professional (p = 0.001) and time dedicated (p = 0.039) (Fig. 2A).
Analysis of the HCVTSat specific questionnaire revealed that the patients who were more willing to share their experience were those with the highest score in the area of treatment experience (more information in general and knowledge of the advantages of receiving treatment) and greater social support (Fig. 2B).
The results for each question corresponding to each area and general and specific satisfaction survey appear in Appendix B, Supplementary materials 3 and 4.
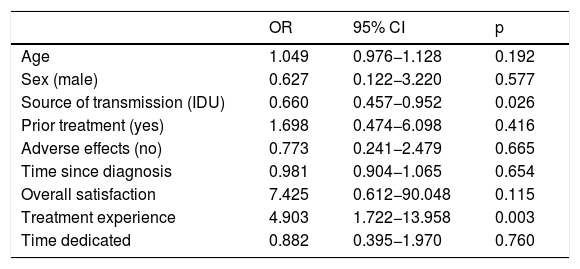
Predictive factorsIn the logistic regression analysis, only having higher expectations and more information with respect to treatment was associated with a higher likelihood of sharing experience with other patients (HR: 4.903, p = 0.003). However, transmission through intravenous drug use was associated with a lower probability of sharing experience (HR: 0.660, p = 0.026) (Table 3).
Factors related to sharing experience after treatment.
| OR | 95% CI | p | |
|---|---|---|---|
| Age | 1.049 | 0.976−1.128 | 0.192 |
| Sex (male) | 0.627 | 0.122−3.220 | 0.577 |
| Source of transmission (IDU) | 0.660 | 0.457−0.952 | 0.026 |
| Prior treatment (yes) | 1.698 | 0.474−6.098 | 0.416 |
| Adverse effects (no) | 0.773 | 0.241−2.479 | 0.665 |
| Time since diagnosis | 0.981 | 0.904−1.065 | 0.654 |
| Overall satisfaction | 7.425 | 0.612−90.048 | 0.115 |
| Treatment experience | 4.903 | 1.722−13.958 | 0.003 |
| Time dedicated | 0.882 | 0.395−1.970 | 0.760 |
IDU: intravenous drug use.
This study is the first to evaluate to what extent patients infected with HCV and treated with DAAs would be willing to share their experience with other infected individuals and which variables related to degree of satisfaction have the greatest impact on the decision to share experience and thus become a potential source of referral of other infected patients to a specialist.
The first finding was that up to 54.4% of treated patients knew other people with the infection. This is important because it gives a sense of the power that each patient has to influence other infected individuals and increase referral rates by talking about their experience. This concept of patients themselves helping to eliminate HCV is novel and, although it might seem to have little impact, it must be borne in mind that population screening programmes would aim to detect 1% of those infected with HCV, a rate lower than that corresponding to each treated patient in influencing the recommendation of evaluation. Therefore, this referral strategy should be investigated within other microelimination programmes for reaching the WHO targets for 2030.
In our study, the majority of the patients knew other infected individuals in the context of friends and family. Moreover, the most common route of transmission corresponded to a history of intravenous drug use, in 29.4% of cases. As this is a vulnerable population, at higher risk of reinfection in cases of active use,14 having a partner or friends or living with family members with active HCV infection may hamper microelimination due to inadvertent reinfection. In addition, closeness among infected persons may be a significant source of information about side effects and advantages of treatment, knowledge which helps to decrease anxiety and fear prior to the start of treatment.15
Our results suggest not only that more than half of treated patients know other infected individuals, but also that more than two thirds would be completely agreeable to sharing their experience. This figure is strikingly high in the context of a stigmatised disease. Therefore, this finding represents an opportunity to extend treatment to people who are aware of their infection status yet for various reasons have not taken steps to get treated.16
With regard to perceived health-related quality of life, this is known to be influenced by different personal variables such as cultural and socioeconomic circumstances; variables related to the disease itself and its symptoms and severity; expectations for the physician/patient relationship; and, undoubtedly, the efficacy and adverse effects expected of the treatment. All of these considerations form a patient perspective that does not always match that of the physician.17 For this reason, it is important to know which variables most influence patients’ perception of quality of life in order to improve them. Our data shows that the degree of general satisfaction of the patients treated was high, and similar whether they were willing to share their experience or not. They stress the care provided by the medical professional and the time dedicated, but not so much the depth of the professional relationship with the physician, suggesting that the latter is not very important to the patients. This is to be expected, since current treatments are brief and patients require just two visits in most cases.18
No differences were observed in demographic, socioeconomic, infection-related or treatment-related characteristics between patients who would share their experience and those who would not. By contrast, the patients who were completely agreeable to sharing their experience, even with people other than their family members and friends, were those who had more information and higher expectations about treatment. Undoubtedly, being aware of the advantages of getting treated and being cured is for many patients motivation enough not only to accept treatment, but also to share their experience.19 In this regard, most of our patients knew about their infection for some time, had received prior treatments and had elevated liver fibrosis when they started treatment. Another important factor that influenced infected individuals' willingness to share their experience was social support. Previously, social stability had already been demonstrated to be associated with higher sustained viral response rates in patients who received treatment with interferon.20 Therefore, our finding is another reason why evaluation and social support measures should be stressed through a more holistic approach to patients with HCV.
By contrast, most of the patients with a history of intravenous drug use would not share their experience, perhaps due to the stigma associated with drug use or fear of legal consequences.21 This is an important argument for requesting more resources for these patients and implementing specific strategies intended for this group of people who are difficult to connect to healthcare.2
This study has limitations. First, the patients included were not the type of patients who are being treated at present since the current ones are less aware of their disease; this undoubtedly may make the dissemination of their experience more difficult.22 However, all patients were treated with DAAs without interferon and current treatments do not differ in terms of efficacy. Second, the number of patients included was limited, but, despite the small size of the sample, the information collected was extensive and all were evaluated using validated surveys. Finally, it was not possible to evaluate the impact of discussing experience and recommending referral of new cases by the patients themselves after being informed of the advantages of being cured.
In conclusion, more than half of the patients treated with DAAs knew other individuals with HCV infection and at least two thirds of them would be fully agreeable to sharing their experience with other infected patients. Considering the degree of satisfaction, and in particular the information that the patients had on the benefits of treatment, which influenced their intention of sharing their positive experience with others, measures must be taken to increase knowledge about treatment and the perception of health improvement among treated patients. In theory, this initiative could help to increase sharing of treatment experience and therefore serve as a powerful source of referral of untreated patients to a specialist.
Conflicts of interestDr M. Hernandez-Guerra has received grant funds and fees for conferences organised by AbbVie, Gilead, Intercept and Bayer.
Please cite this article as: Reygosa C, Morales-Arraez D, Hernández-Bustabad A, Melián Baute L, Hernández-Guerra M. El paciente tratado de hepatitis C como fuente potencial de derivación de nuevos casos. Gastroenterol Hepatol. 2021;44:704–710.