To evaluate the results of isolated liver and combined liver and kidney transplantation in a retrospective series of 32 patients with hepatorenal liver and kidney disease.
Patients and methodsA retrospective observational study that enrolled patients with polycystic liver disease (PLD) and polycystic liver and kidney disease (PLKD) who were evaluated for transplantation between January 1999 and December 2019 at Hospital Clínic de Barcelona [Clinical Hospital of Barcelona].
ResultsOf 53 patients enrolled, 32 (60.3%) had an indication for transplantation, 12 received a single liver transplant and 20 received a double liver and kidney transplant. The mean age was 52 years and 83.9% of the recipients were women. The main indication for liver transplantation was disabling symptomatic hepatomegaly (93.5%). Among the postoperative complications, in the combined liver and kidney transplant group, hepatic artery thrombosis and renal artery thrombosis were detected. In both groups there was an inferior vena cava lesion. Three patients presented acute cellular rejection responding to corticosteroids and one presented humoral rejection which was treated with plasmapheresis. During the follow-up period of 80 (27–121) months, the liver transplant survival rate was 100% and the kidney transplant survival rate was 90%. Two patients in the combined liver and kidney transplant group died (one due to cardiovascular causes and the other due to intestinal adenocarcinoma).
ConclusionsIsolated liver transplantation or combined liver and kidney transplantation in selected patients with polycystic disease yields excellent results, with few complications, very good transplant survival and excellent patient survival (93.8%).
Evaluar los resultados del trasplante hepático aislado y del trasplante combinado hepatorrenal en una serie retrospectiva de 32 pacientes con enfermedad poliquística hepatorrenal.
Pacientes y métodosEstudio observacional retrospectivo en el que se incluyeron los pacientes con enfermedad poliquística hepática (EPH) y enfermedad poliquística hepatorrenal (EPHR) que fueron evaluados para trasplante desde enero de 1999 a diciembre de 2019 en el Hospital Clínic de Barcelona.
ResultadosSe incluyeron 53 pacientes, 32 (60,3%) tenían indicación de trasplante, 12 recibieron trasplante hepático único y 20 doble trasplante hepático y renal. La edad media fue de 52 años y el 83.9% de los receptores fueron mujeres. La principal indicación de trasplante hepático fue la hepatomegalia sintomática incapacitante (93,5%). Dentro de las complicaciones postoperatorias, en el grupo de trasplante hepatorrenal se detectaron una trombosis de arterial hepática y una trombosis de arterial renal. En ambos grupos se produjo una lesión de vena cava superior. Tres pacientes presentaron rechazo celular agudo respondiendo a corticoesteroides y un rechazo humoral que se trató con plasmaféresis. Durante el periodo de seguimiento 80 (27–121) meses, la sobrevida del injerto fue de 100% para el hígado y del 90% para el injerto renal. Fallecieron dos pacientes con trasplante hepatorrenal (uno por causas cardiovasculares y el otro por un adenocarcinoma intestinal).
ConclusionesEl trasplante hepático aislado o combinado hepático y renal en pacientes seleccionados con enfermedad poliquística tiene unos resultados excelentes, con pocas complicaciones, muy buena sobrevida del injerto y excelente supervivencia del paciente (93,8%).
Polycystic liver disease is caused by structural anomalies that occur during embryonic development of the biliary tree. These alterations manifest in adults in the form of three conditions: Von Meyenburg complexes, isolated polycystic liver disease (PLD) and polycystic liver and kidney disease (PLKD).1 PLD’s prevalence is 1/100,000–1/1000,000, while PLKD presents at a rate in keeping with its inheritance pattern: 1/400 to 1/1000 for the autosomal dominant variant (AD-PLKD), which is considered more common, and 1/40,000 for the autosomal recessive variant (AR-PLKD).2,3 The incidence of Von Meyenburg complexes has been estimated at 7–60/1000.4
The treatment options are aspiration of the cyst and injection of sclerosing agents, laparoscopic fenestration, medical treatment with somatostatin analogues and liver transplant, which is considered the only curative treatment.1 Data from the European Liver Transplant Registry show a graft survival rate of 88% and a patient survival rate of 92% at five years.5 Transplant is reserved for those patients with significant morbidity who do not respond to other treatments. In the case of PLKD, a reduction in glomerular filtration to below 40 ml/min is required in order for combined liver and kidney transplant to be indicated.6
Patients who ultimately receive a liver or combined transplant can have a complex post-operative clinical course as a result of the resection and extraction of a large liver with distortion of the vascular structures. The objective of this study is to present our centre’s experience of liver transplant over 20 years in patients with polycystic disease, highlighting survival and associated complications.
Material and methodsPatientsThe characteristics of all PLD and PLKD patients assessed as candidates for liver or combined transplant between 1999 and 2019 in the Liver Transplant Unit of the Hospital Clínic [Clinical Hospital], Barcelona were analysed retrospectively. The presence of complications of PLD such as disabling symptomatic hepatomegaly, portal hypertension (ascites, haemorrhaging oesophageal varices), complications of liver cysts that lack alternative treatment (repeated sepsis in spite of prophylactic medical treatment, structural compression) and malnutrition were considered indications for transplant. The presence of chronic kidney disease with a glomerular filtration rate (GFR) <40 ml/min plus PLD with the criteria listed above were indications for combined transplant.
The usual surgical technique was modified to allow for the use of a venovenous bypass of the inferior vena cava (IVC) as a result of its compression and the difficulty of mobilising the liver. Where there was no hypertension in the portal vein and hepatofugal vessels, a venovenous shunt was created from the splanchnic territory by cannulation of the portal vein after its section. This avoided the venous congestion that would be caused by prolonged clamping of the inferior vena cava and portal vein to complete the hepatectomy and during the anhepatic phase.
In accordance with our immunosuppression protocol, the treatment used initially was based on an induction agent (basiliximab), a calcineurin inhibitor (tacrolimus or cyclosporine) and a proliferation inhibitor (mycophenolate), seeking higher levels of immunosuppression in combined transplant patients due to their greater immunological risk.
The following data were collected: age, sex, complications of polycystic disease and treatments prior to transplant, operative time, transfusion requirements, length of stay in relation to the transplant, explant weight, pre-and post-transplant glomerular filtration rate, clinical and surgical complications, including liver and kidney rejection during follow-up, graft survival and patient survival. Extrahepatic manifestations, including the presence of hypertension, family history of PLKD and the presence of brain aneurysms in those who had an imaging study were intentionally collected.
Statistical analysisWe carried out a descriptive statistical analysis. The categorical variables were represented as percentages and the quantitative variables as median and interquartile range (IQR). The statistical analysis was carried out using the SPSS 23 statistical software package (Statistical Package for the Social Sciences; SPSS, Chicago, IL).
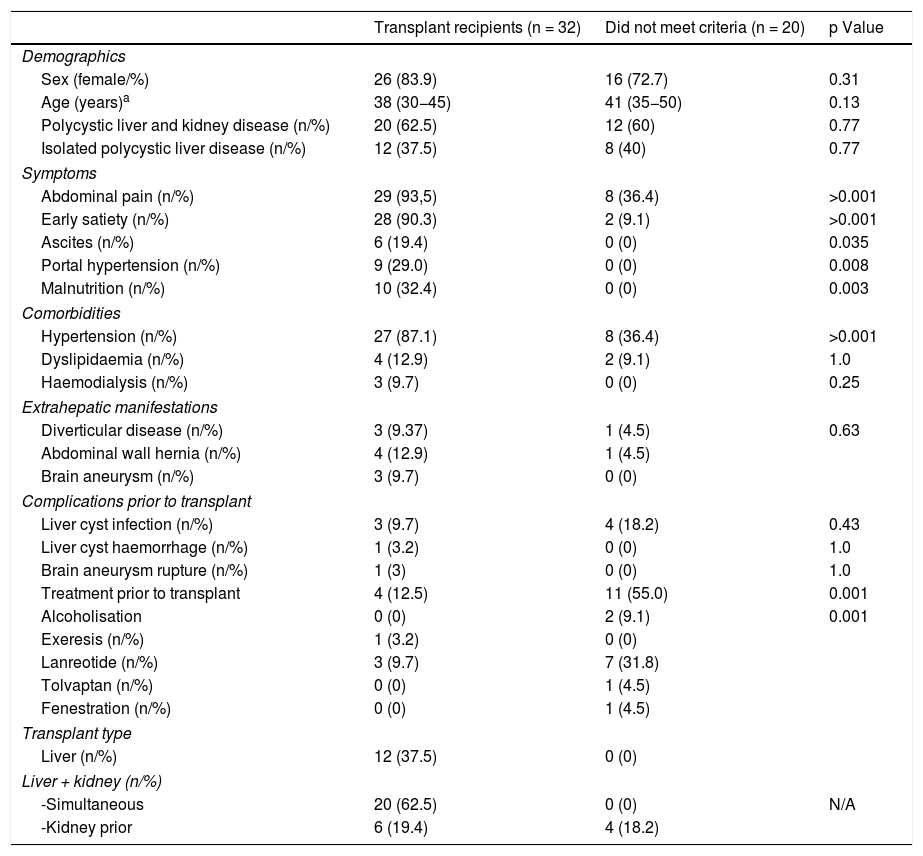
ResultsDuring the period from 1999 to 2019, 53 patients were assessed for PLD and PLKD in our centre (Fig. 1A–B). Of these, transplant was indicated in 32 patients (60.3%); 12 received only a liver transplant and 20 received a combined liver and kidney transplant. Twenty-nine patients (54.7%) had a family history of PLKD. The median age at the time of transplant was 52 years and 83.9% of the recipients were women. The leading indication for transplant was disabling symptomatic hepatomegaly (93.5%), followed by malnutrition (32.4%) and portal hypertension (28.1%). The average weight of the liver explants was 5700 g.
The extrahepatic manifestations in our cohort of assessed patients were hypertension (67.3%), diverticular disease (7.7%) and abdominal wall hernias in 9.6% of patients. Table 1 shows the incidence of these manifestations, comparing patients with and without an indication for transplant. There were no significant differences between the two groups of patients.
General characteristics of the patients assessed.
| Transplant recipients (n = 32) | Did not meet criteria (n = 20) | p Value | |
|---|---|---|---|
| Demographics | |||
| Sex (female/%) | 26 (83.9) | 16 (72.7) | 0.31 |
| Age (years)a | 38 (30−45) | 41 (35−50) | 0.13 |
| Polycystic liver and kidney disease (n/%) | 20 (62.5) | 12 (60) | 0.77 |
| Isolated polycystic liver disease (n/%) | 12 (37.5) | 8 (40) | 0.77 |
| Symptoms | |||
| Abdominal pain (n/%) | 29 (93,5) | 8 (36.4) | >0.001 |
| Early satiety (n/%) | 28 (90.3) | 2 (9.1) | >0.001 |
| Ascites (n/%) | 6 (19.4) | 0 (0) | 0.035 |
| Portal hypertension (n/%) | 9 (29.0) | 0 (0) | 0.008 |
| Malnutrition (n/%) | 10 (32.4) | 0 (0) | 0.003 |
| Comorbidities | |||
| Hypertension (n/%) | 27 (87.1) | 8 (36.4) | >0.001 |
| Dyslipidaemia (n/%) | 4 (12.9) | 2 (9.1) | 1.0 |
| Haemodialysis (n/%) | 3 (9.7) | 0 (0) | 0.25 |
| Extrahepatic manifestations | |||
| Diverticular disease (n/%) | 3 (9.37) | 1 (4.5) | 0.63 |
| Abdominal wall hernia (n/%) | 4 (12.9) | 1 (4.5) | |
| Brain aneurysm (n/%) | 3 (9.7) | 0 (0) | |
| Complications prior to transplant | |||
| Liver cyst infection (n/%) | 3 (9.7) | 4 (18.2) | 0.43 |
| Liver cyst haemorrhage (n/%) | 1 (3.2) | 0 (0) | 1.0 |
| Brain aneurysm rupture (n/%) | 1 (3) | 0 (0) | 1.0 |
| Treatment prior to transplant | 4 (12.5) | 11 (55.0) | 0.001 |
| Alcoholisation | 0 (0) | 2 (9.1) | 0.001 |
| Exeresis (n/%) | 1 (3.2) | 0 (0) | |
| Lanreotide (n/%) | 3 (9.7) | 7 (31.8) | |
| Tolvaptan (n/%) | 0 (0) | 1 (4.5) | |
| Fenestration (n/%) | 0 (0) | 1 (4.5) | |
| Transplant type | |||
| Liver (n/%) | 12 (37.5) | 0 (0) | |
| Liver + kidney (n/%) | |||
| -Simultaneous | 20 (62.5) | 0 (0) | N/A |
| -Kidney prior | 6 (19.4) | 4 (18.2) | |
A brain imaging study was conducted in 32 patients (60%) as an early detection test of brain aneurysms. A brain aneurysm was found in three, and one patient had presented a brain haemorrhage due to rupture of an aneurysm located in the middle cerebral artery. This haemorrhage had occurred prior to the transplant and had resolved without sequelae.
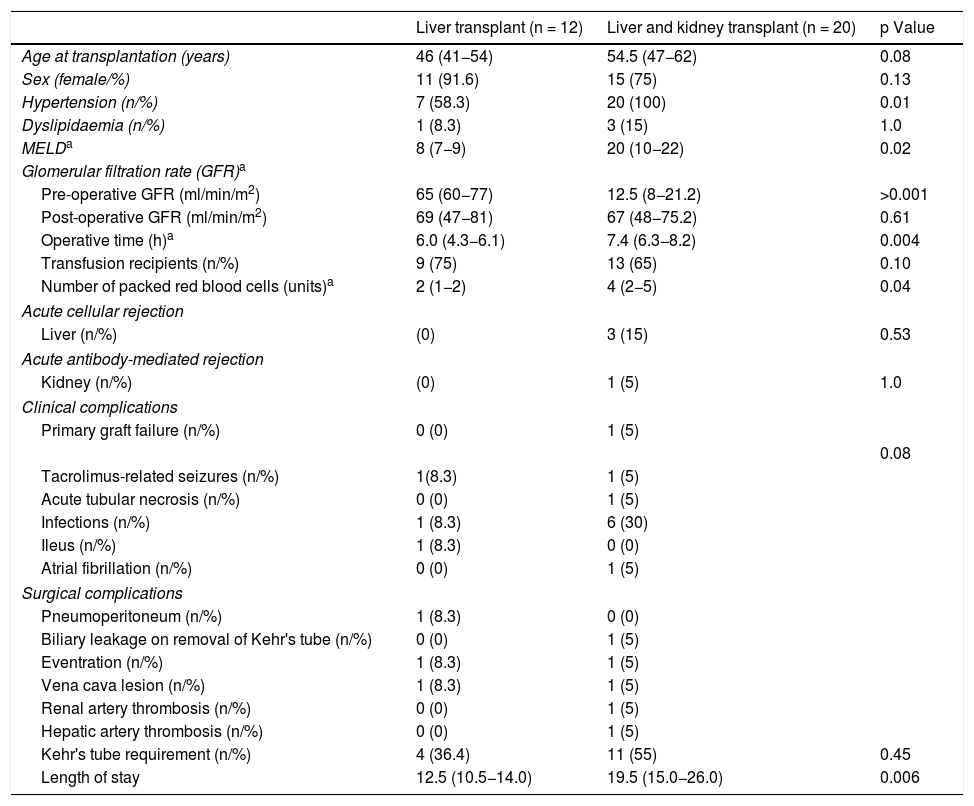
Perioperative complications and survivalTable 2 describes the postoperative complications in the transplant patients, comparing liver transplant only with combined transplant. The operative time for combined transplant was 7.4 h, exceeding that for liver transplant only, which was 6.2 h. Transfusion (packed red blood cells) requirements were greater in the combined transplant group (4 vs. 2 units) (p < 0.04).
Characteristics of transplant recipients.
| Liver transplant (n = 12) | Liver and kidney transplant (n = 20) | p Value | |
|---|---|---|---|
| Age at transplantation (years) | 46 (41−54) | 54.5 (47−62) | 0.08 |
| Sex (female/%) | 11 (91.6) | 15 (75) | 0.13 |
| Hypertension (n/%) | 7 (58.3) | 20 (100) | 0.01 |
| Dyslipidaemia (n/%) | 1 (8.3) | 3 (15) | 1.0 |
| MELDa | 8 (7−9) | 20 (10−22) | 0.02 |
| Glomerular filtration rate (GFR)a | |||
| Pre-operative GFR (ml/min/m2) | 65 (60−77) | 12.5 (8−21.2) | >0.001 |
| Post-operative GFR (ml/min/m2) | 69 (47−81) | 67 (48−75.2) | 0.61 |
| Operative time (h)a | 6.0 (4.3−6.1) | 7.4 (6.3−8.2) | 0.004 |
| Transfusion recipients (n/%) | 9 (75) | 13 (65) | 0.10 |
| Number of packed red blood cells (units)a | 2 (1−2) | 4 (2−5) | 0.04 |
| Acute cellular rejection | |||
| Liver (n/%) | (0) | 3 (15) | 0.53 |
| Acute antibody-mediated rejection | |||
| Kidney (n/%) | (0) | 1 (5) | 1.0 |
| Clinical complications | |||
| Primary graft failure (n/%) | 0 (0) | 1 (5) | |
| 0.08 | |||
| Tacrolimus-related seizures (n/%) | 1(8.3) | 1 (5) | |
| Acute tubular necrosis (n/%) | 0 (0) | 1 (5) | |
| Infections (n/%) | 1 (8.3) | 6 (30) | |
| Ileus (n/%) | 1 (8.3) | 0 (0) | |
| Atrial fibrillation (n/%) | 0 (0) | 1 (5) | |
| Surgical complications | |||
| Pneumoperitoneum (n/%) | 1 (8.3) | 0 (0) | |
| Biliary leakage on removal of Kehr's tube (n/%) | 0 (0) | 1 (5) | |
| Eventration (n/%) | 1 (8.3) | 1 (5) | |
| Vena cava lesion (n/%) | 1 (8.3) | 1 (5) | |
| Renal artery thrombosis (n/%) | 0 (0) | 1 (5) | |
| Hepatic artery thrombosis (n/%) | 0 (0) | 1 (5) | |
| Kehr's tube requirement (n/%) | 4 (36.4) | 11 (55) | 0.45 |
| Length of stay | 12.5 (10.5−14.0) | 19.5 (15.0−26.0) | 0.006 |
MELD: Model For End-Stage Liver Disease.
In the combined transplant group there were two cases of arterial thrombosis: one patient presented a renal infarction due to thrombosis of the renal artery and another patient developed primary liver graft failure due to thrombosis of the hepatic artery, requiring a kidney and a liver transplant, respectively. In addition, one patient with combined transplant and one with liver transplant only presented a lesion of the superior vena cava that was repaired without consequences. One patient in each group presented surgical wound eventration, one at three months and the other at one year after transplant. A Kehr's "T" tube was used in 11/20 (55%) of the patients with combined transplant and 4/12 (33.3%) of the patients with liver transplant only. In the combined transplant group, one patient presented biliary leakage on removal of said tube.
In the immediate postoperative period, seven patients presented an infectious process (21.8%). In the combined transplant group, urinary tract infection was diagnosed in two patients, as well as one surgical wound infection, one case of bacteraemia caused by Pseudomonas aeruginosa, one herpes zoster virus infection and four cases of nosocomial pneumonia. In the liver transplant only group, there was one case of nosocomial pneumonia.
In the combined transplant group, three patients presented acute cellular rejection of the liver and one presented antibody-mediated rejection of the kidney, all diagnosed by biopsy. The three cases of acute cellular rejection of the liver and the case of antibody-mediated rejection of the kidney responded to high doses of corticosteroids and plasmapheresis, respectively. The length of stay in hospital was significantly longer in the combined transplant group at 19.5 (15–26) days compared with the liver transplant only group at 12.5 (10.5–14) days (p < 0.006).
The mean follow-up of the transplant patients was 80 (27–121) months. Four patients presented complications: de novo autoimmune hepatitis at 24 months post-transplant, mild acute diverticulitis resolved with antibiotics, basal cell carcinoma of the skin at eight months post-transplant and disseminated adenocarcinoma of intestinal origin at 16 months post-transplant.
Two patients presented post-transplant chronic renal failure at 10 months and eight years respectively, and underwent retransplantation. No liver graft required retransplantation.
Two of the 32 patients died (6.2%): one patients died during the transplant procedure of cardiovascular causes and the other died at 16 months post-transplant of disseminated intestinal adenocarcinoma. Thirty patients remain alive with adequate graft function, improvement of the symptoms that led to the transplant and a median graft survival of 94 months for liver transplant cases and 70 months for combined transplant cases.
DiscussionPLD has a prevalence in the general population of 1/1000,000, while the prevalence of PLKD varies between 1/400 and 1/1000, representing approximately 80%–90% of cases of polycystic disease.1 Although considered a benign disease, in a minority of patients (3%) liver cyst expansion causes severe abdominal symptoms and in extreme cases can cause death.7 Liver transplant is indicated in those cases with significant morbidity that cannot be controlled by other means (disabling abdominal pain, recurrent infection, portal hypertension, significant impact on nutrition).8 Conservative and surgical treatment options lead to improvement of symptoms in patients with small cysts, but in patients with multiple large cysts such treatments only lead to transient improvement.9 Two matters have been debated with regard to transplant in PLD and PLKD: on the one hand, the low mortality without transplant and the maintenance of normal liver function in many cases, and on the other hand, the surgical difficulties associated with extracting large organs (the mean liver weight in this series was 5000 g) and consequent vascular distortion. However, the results from our series indicate that liver or combined transplant is associated with a high survival rate in these patients (100% for PLD and 90% for PLKD), there are few surgical complications, in keeping with those observed in transplants in other pathologies,10 and that no increase in infectious complications, episodes of rejection or transfusion requirements was detected. Although it was not specifically assessed using questionnaires, all patients presented an important improvement in their quality of life. All of this indicates that liver or combined transplant in PLKD is a good treatment in those select cases that meet the established criteria.
Progression to end-stage liver disease is generally exceptional in nature and is seen in cases where the liver reaches extremely high volumes. In general, liver function remains intact, meaning that symptomatic patients with very significant hepatomegaly can have a very low Model For End-Stage Liver Disease (MELD) score.11,12 The factor that most influences the MELD score in these patients is kidney functions in PLKD cases, which is conserved in patients with only liver involvement. For these reasons, PLKD, and particularly PLD, are considered an exception and extra points should be awarded for equitable inclusion on waiting lists. The following are assessed for list inclusion and prioritisation: presence of clinical signs of portal hypertension, intractable complications of the cysts, or malnutrition. Extra points are awarded as time on the waiting list increases.13,14 Of all of the patients we assessed over the 20 years, the leading indications for transplant were symptomatic hepatomegaly, malnutrition and portal hypertension.
It has been demonstrated that kidney function is one of the most important factors in predicting the prognosis of patients with polycystic disease. The choice of whether to perform only liver or combined liver and kidney transplant depends on kidney function prior to transplant.15 In the patients in our study with combined transplant, 10/20 patients (50%) were on dialysis prior to transplant. Following the transplant, kidney function improved significantly from a GFR of 12.5 (8−21.2) ml/min/1.73 m2 pre-transplant to 67 (48−75.2) ml/min/1.73 m2 (p > 0.001).
Surgical problems have been described during the hepatectomy due to the significant hepatomegaly in these patients. Vascular complications are the most common and occur more often in combined transplant patients.16 In our study, there were very few vascular complications, with a slightly higher incidence (3/20) in the combined transplant group versus the liver transplant only groups (1/12) (not significant). Moreover, we think the use of a venovenous bypass contributed to maintaining haemodynamic stability in our patients during the transplant procedure, minimising blood loss and maintaining better splanchnic and renal perfusion, by avoiding venous congestion. However, other authors consider venovenous bypass to be unnecessary in these patients, so it is difficult to establish an incontrovertible recommendation in this respect.17 IVC substitution may be necessary on occasions to avoid venous drainage problems in the graft.
With regard to extrahepatic complications of PLKD, the presence of intracranial aneurysms is noteworthy. PLKD is the most common hereditary disease associated with intracranial aneurysms, which have a prevalence within PLKD between 4% and 40%.18,19 In this complaint, detection is highest when there are other family members with brain aneurysms. Magnetic resonance imaging and computed tomography are the screening methods of choice due to their non-invasive nature.19 A cohort of 113 patients in a Korean hospital was found to have a prevalence of 20% (23 patients), of whom 8% (nine patients) had a history of aneurysm rupture at an average age of 34.9 years, with the most common location being the middle cerebral artery (35%).18 In another systematic review of nine studies, the prevalence was 11.5% for asymptomatic intracranial aneurysms and 1.9% for aneurysms with rupture, with a average age at rupture of 42 years.20 In our cohort of 53 assessed patients, 33 (60%) had an early detection imaging study, with an asymptomatic intracranial aneurysm found in three of these (5.6%), and another case had a history of intracranial haemorrhage due to aneurysm rupture. At present, there is no clear definition of the action to take when faced with these asymptomatic aneurysm in patients who are to undergo combined liver and kidney transplant. Some factors need to be taken into account when deciding on management, such as: location, size, morphology, existence of a thrombus within the aneurysm, age, history of subarachnoid haemorrhage and family history of subarachnoid haemorrhage, with aneurysm size being the most important predictor of rupture. The treatment options are clipping, and now, endovascular coiling.19
Following the transplant, the quality of life of these patients improves dramatically with the disappearance of abdominal fullness and pain, malnutrition and kidney failure in the case of combined transplants. The questionnaire most widely used to assess quality of life in these patients is the 36-Item Short Form Survey (SF-36), which covers physical, emotional and social aspects.21 Kirchner et al. assessed quality of life following transplant based on two questionnaires covering topics relating to physical health, mental health and lifestyle changes. This study found an improvement in quality of life in 100% of liver transplant patients and 91% of combined liver and kidney transplant patients.22 Being a retrospective study, quality of life was not adequately assessed. In general, patients experienced an improvement in symptoms and improved quality of life, although it is not possible to quantify this nor to specify the specific areas in which this improvement occurred.
Our study shows the experience of selection, inclusion on waiting lists, transplantation and associated complications in a cohort of 53 patients with PLD and PLKD. Liver transplant or combined liver and kidney transplant is an excellent option in the treatment of these patients, with a survival rate above 90% at eight years from transplant. Potential surgical complications do not pose a significant problem in practice.
ConclusionIn patients with polycystic liver disease, where this is severe, liver transplant or combined liver and kidney transplant should be considered as a treatment option. Although the surgical technique is more complex in these cases and requires adaptation to the specific conditions of these patients when appropriately selected, it has excellent long-term results.
FundingNo funding was received for this study.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Rodríguez-Aguilar EF, Sastre L, Colmenero J, García-Valdecasas JC, Fondevila C, García Juárez I, et al., Trasplante hepático y renal en la enfermedad poliquística hepatorrenal, Gastroenterol Hepatol. 2021;44:552–558.