Small Bowel Capsule Endoscopy is the first-choice technique for investigating the majority of small bowel diseases. Its most common complications are related to incomplete examinations and capsule retention. There is no consensus on how patients with previous gastrointestinal surgery should receive the capsule.
ObjectiveThe primary endpoint was to compare the rate of complete small-bowel examinations (completion rate) between oral ingestion and endoscopic delivery of the capsule. The secondary endpoint was to compare diagnostic yield and adverse events in the two groups.
MethodsA retrospective observational study was conducted in nine hospitals in Spain. Demographic data, previous surgery, indication for capsule endoscopy, intestinal transit time, diagnosis, completion rate (percentage of capsules reaching the caecum), diagnostic yield (percentage of results compatible with indication for the exam) and adverse events were collected.
ResultsFrom January 2009 to May 2019 fifty-seven patients were included (39 male, mean age 66±15 years). The most common indications for the exam were “overt” (50.9%) and “occult” (35.1%) small bowel bleeding. Previous Billroth II gastrectomy and Roux-en-Y gastric bypass were present in 52.6% and 17.5% of patients respectively. The capsule was swallowed in 34 patients and placed endoscopically in 23 patients. No significant differences were observed between the oral ingestion and endoscopic delivery groups in terms of completion rate (82.4% vs. 78.3%; p=0.742), diagnostic yield (41.2% vs. 52.2%; p=0.432) or small bowel transit time (301 vs. 377min, p=0.118). No capsule retention occurred. Only one severe adverse event (anastomotic perforation) was observed in the endoscopic delivery group.
ConclusionsIn our case series, there were no significant differences between oral ingestion and endoscopic delivery in terms of completion rate, diagnostic yield or safety. Being less invasive, oral ingestion of the capsule should be the first-choice method in patients with previous gastrointestinal surgery.
La cápsula endoscópica representa la técnica de primera elección para investigar la mayoría de las enfermedades del intestino delgado. Sus complicaciones más comunes frecuentes son las exploraciones incompletas y la retención a nivel de intestino delgado. Hasta el momento no hay acuerdo sobre cómo administrar la cápsula a los pacientes que han sido sometidos a una cirugía gastrointestinal previa.
ObjetivoEl objetivo principal fue comparar la tasa de estudios completos entre la ingestión oral y la administración endoscópica de la cápsula. Los objetivos secundarios fueron comparar el rendimiento diagnóstico y los eventos adversos en ambos grupos.
MétodosSe realizó un estudio observacional retrospectivo en 9 hospitales de España. Se recogieron datos demográficos, cirugía previa, indicación de cápsula endoscópica, tiempo de tránsito intestinal, diagnóstico, tasa de estudios completos (porcentaje de cápsulas que llegan al ciego), rendimiento diagnóstico (porcentaje de resultados compatibles con la indicación del examen) y eventos adversos.
ResultadosDesde enero de 2009 hasta mayo de 2019 se incluyeron 57 pacientes (39 hombres, edad media 66 ± 15 años). Las indicaciones más frecuentes para el examen fueron hemorragia de intestino delgado «manifiesta» (50,9%) y «oculta» (35,1%). El 52,6% de los pacientes presentaba gastrectomía Billroth II y el 17,5% bypass gástrico en Y de Roux. La cápsula fue ingerida en 34 pacientes y colocada endoscópicamente en 23 pacientes. No se observaron diferencias significativas entre los grupos de ingesta oral y de colocación endoscópica en cuanto a tasa de estudios completos (82,4% vs. 78,3%; p = 0,742), rendimiento diagnóstico (41,2% vs. 52,2%; p = 0,432) y tiempo de tránsito del intestino delgado (301 vs. 377 min, p = 0,118). No hubo casos de cápsulas retenidas. Solo se observó un evento adverso severo (perforación anastomótica) en el grupo de colocación endoscópica.
ConclusionesEn nuestra cohorte, no hubo diferencias significativas entre la ingesta oral y la colocación endoscópica en cuanto a la tasa de estudios completos, el rendimiento diagnóstico y los eventos adversos. Dado su carácter menos invasivo, la ingesta oral de la cápsula debería ser el método de primera elección en pacientes con cirugía gastrointestinal previa.
Small Bowel capsule endoscopy (SBCE) is currently the first-choice technique to accurately diagnose the majority of small bowel (SB) diseases.1 The two most common indications for the procedure are small bowel bleeding (SBB) and Crohn's disease (CD), whilst less common indications are represented by SB tumours and coeliac disease.2 The use of SBCE has grown significantly over the years thanks to its simplicity of use and low invasiveness3; this has led to the acceptance of SBCE as the main procedure for diagnosis and deep enteroscopy as a complementary procedure for biopsy specimens and treatment.4,5
SBCE is effective, painless and safe.6 However, the frequency of incomplete examinations may be up to 20%, above all in patients with high risk factors such as older age and CD, and may culminate in repeating the examination. Moreover, a 1–2% risk of capsule retention exists, meaning that endoscopic or surgical procedures might be needed to retrieve the capsule or treat intestinal obstructions.7
Usually the SBCE is swallowed by the patient with a glass of water. However, in case of swallowing disorders, anatomical abnormality or patient's fear, an endoscopic delivery can be performed, placing the capsule directly into the stomach or the proximal small bowel, usually the duodenal bulb.8 In general population, it has been shown that there is no difference between oral ingestion (OI) and endoscopic delivery (ED) of the capsule in terms of procedure completion rate and total intestinal transit time.9 ED can be performed with two techniques: one uses a Roth net to grab and drop the capsule in the intestine and has been demonstrated safe and efficient in patients with anatomical abnormalities, gastroparesis and dysphagia10; more recently, a capsule loading device (AdvanCE®, US Endoscopy, Ohio, United States) was described, allowing to mount the capsule in a cup at the tip of the endoscope and drop it in the SB.11
Surgically altered gastro-intestinal (GI) anatomy is considered a risk for OI of the capsule as it may delay the intestinal transit and cause capsule retention. This may be due to three mechanisms: gastric dysmotility following partial denervation of the stomach,12 responsible for delayed gastric emptying13; the presence of surgical non structuring anastomoses, which may be rigid and impede transit of the capsule14,15; finally, the presence of an afferent (excluded) limb where the capsule might be retained.16
Nowadays, the increase rate of obesity and cancer surgery frequently exposes gastroenterologists to patients with surgically altered anatomy. At the moment, there is no agreement on the best delivery method of the capsule in these patients. Most studies are case-reports or small case series so there are no sufficient data in this field.
The primary endpoint of the present study was to compare the rate of complete small-bowel examinations (completion rate) between OI and ED in patients with surgically altered GI anatomy. Secondary endpoints were to compare diagnostic yield and adverse events between the two groups.
Materials and methodsStudy designA retrospective multicenter observational study was conducted in 9 tertiary referral centres in Spain from January 2009 to June 2019. As a clinical, retrospective audit with anonymised data, the study was exempt from the need of written informed consent. The study protocol conforms to the ethical guidelines of the 1964 World Medical Association Declaration of Helsinki and its later amendments and it was approved by the local Ethical Review Board of Hospital Clínic of Barcelona on the 16th March 2020 (protocol number HCB/2020/0036).
Patients’ selectionInclusion criteria were: (1) any age or gender; (2) at least one SBCE examination performed; (3) previous GI surgery including Billroth II gastrectomy, Roux-en-Y gastric bypass, Whipple procedure and Roux-en-Y distal gastrectomy. Exclusion criteria were: (1) use of prokinetic drugs for the procedure; (2) incomplete data in electronical medical records. In case of patients with more than one SBCE examination, only the first one was collected and subsequent procedures in the same patient were not considered for the analysis.
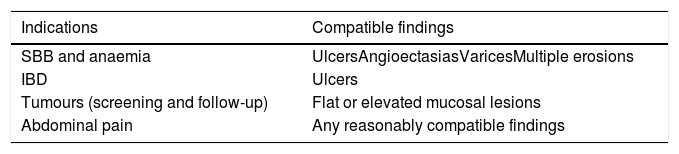
Data collectionPatients’ demographic characteristics, indication for the procedure and type of previous GI surgery were collected. Outcome variables analyzed were: completion rate, defined as percentage of procedures with SBCE reaching the caecum; diagnostic yield, defined as percentage of SBCE examinations with results compatible with the indication for the procedure, according to adapted performance measures for small-bowel endoscopy.17 (Table 1).
Correlation between SBCE indications and endoscopic findings.
| Indications | Compatible findings |
|---|---|
| SBB and anaemia | UlcersAngioectasiasVaricesMultiple erosions |
| IBD | Ulcers |
| Tumours (screening and follow-up) | Flat or elevated mucosal lesions |
| Abdominal pain | Any reasonably compatible findings |
SBB: small bowel bleeding; IBD: inflammatory bowel disease. (Adapted from Spada et al. United Eur. Gastroenterol. J. 2019).
Total intestinal transit time was defined as time from capsule ingestion to the first caecal image. Gastric transit time was calculated from the first gastric image to the first duodenal image. Small bowel transit time was calculated from the first duodenal image to the first caecal image.
Capsule delivery methodsIn the OI group, the endoscopic capsule was ingested with a glass of water; depending on local protocols, an antifoaming agent was added to the water in order to reduce bowel gas bubbles.
In the ED group, either Roth's net or Advance® device were used to correctly deliver the capsule distally to the surgical anastomosis in the jejunal limb. Endoscopy was performed under deep or conscious sedation depending on local protocols.
StatisticsAll variables were tested with Kolmogorov-Smirnov test for normality. Continuous variables were presented as mean and Standard deviation (SD) if normally distributed or as median and interquartile range (IQR); categorical variables were shown as absolute number and percentage. Comparisons between two groups were carried out with independent T-test for continuous variables and Chi-square test or Fisher's exact test for categorical variables. A p-value<0.05 was considered statistically significant. All statistical analyses were done using SPSS 23.0 [IBM Corp, Armonk, NY, USA].
ResultsTen thousand two hundred and five patients submitted to SBCE during the study period were identified; only 57 of them corresponding to unique patients complied with inclusion and exclusion criteria and were finally analyzed (Table 2).
Characteristics of study population and SBCE examinations.
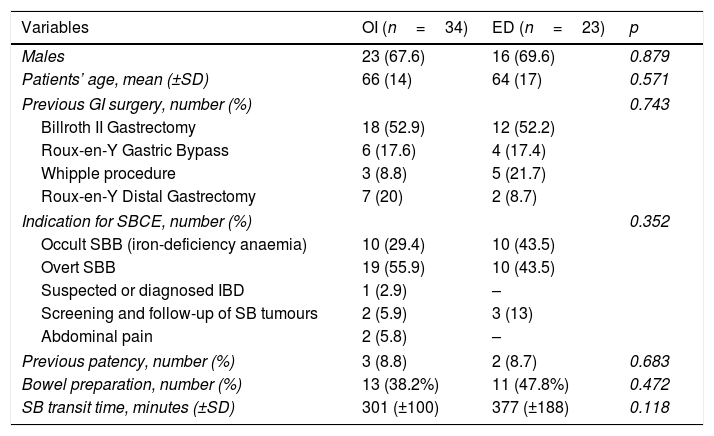
| Variables | OI (n=34) | ED (n=23) | p |
|---|---|---|---|
| Males | 23 (67.6) | 16 (69.6) | 0.879 |
| Patients’ age, mean (±SD) | 66 (14) | 64 (17) | 0.571 |
| Previous GI surgery, number (%) | 0.743 | ||
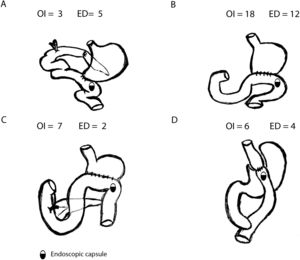
| Billroth II Gastrectomy | 18 (52.9) | 12 (52.2) | |
| Roux-en-Y Gastric Bypass | 6 (17.6) | 4 (17.4) | |
| Whipple procedure | 3 (8.8) | 5 (21.7) | |
| Roux-en-Y Distal Gastrectomy | 7 (20) | 2 (8.7) | |
| Indication for SBCE, number (%) | 0.352 | ||
| Occult SBB (iron-deficiency anaemia) | 10 (29.4) | 10 (43.5) | |
| Overt SBB | 19 (55.9) | 10 (43.5) | |
| Suspected or diagnosed IBD | 1 (2.9) | – | |
| Screening and follow-up of SB tumours | 2 (5.9) | 3 (13) | |
| Abdominal pain | 2 (5.8) | – | |
| Previous patency, number (%) | 3 (8.8) | 2 (8.7) | 0.683 |
| Bowel preparation, number (%) | 13 (38.2%) | 11 (47.8%) | 0.472 |
| SB transit time, minutes (±SD) | 301 (±100) | 377 (±188) | 0.118 |
Data are shown as mean and SD for continuous variables and absolute number and percentage for categorical variables. p-value was considered statistically significant if <0.05. SBCE: small bowel capsule endoscopy; GI: gastro-intestinal; SB: small bowel; SBB: small bowel bleeding; IBD: inflammatory bowel disease.
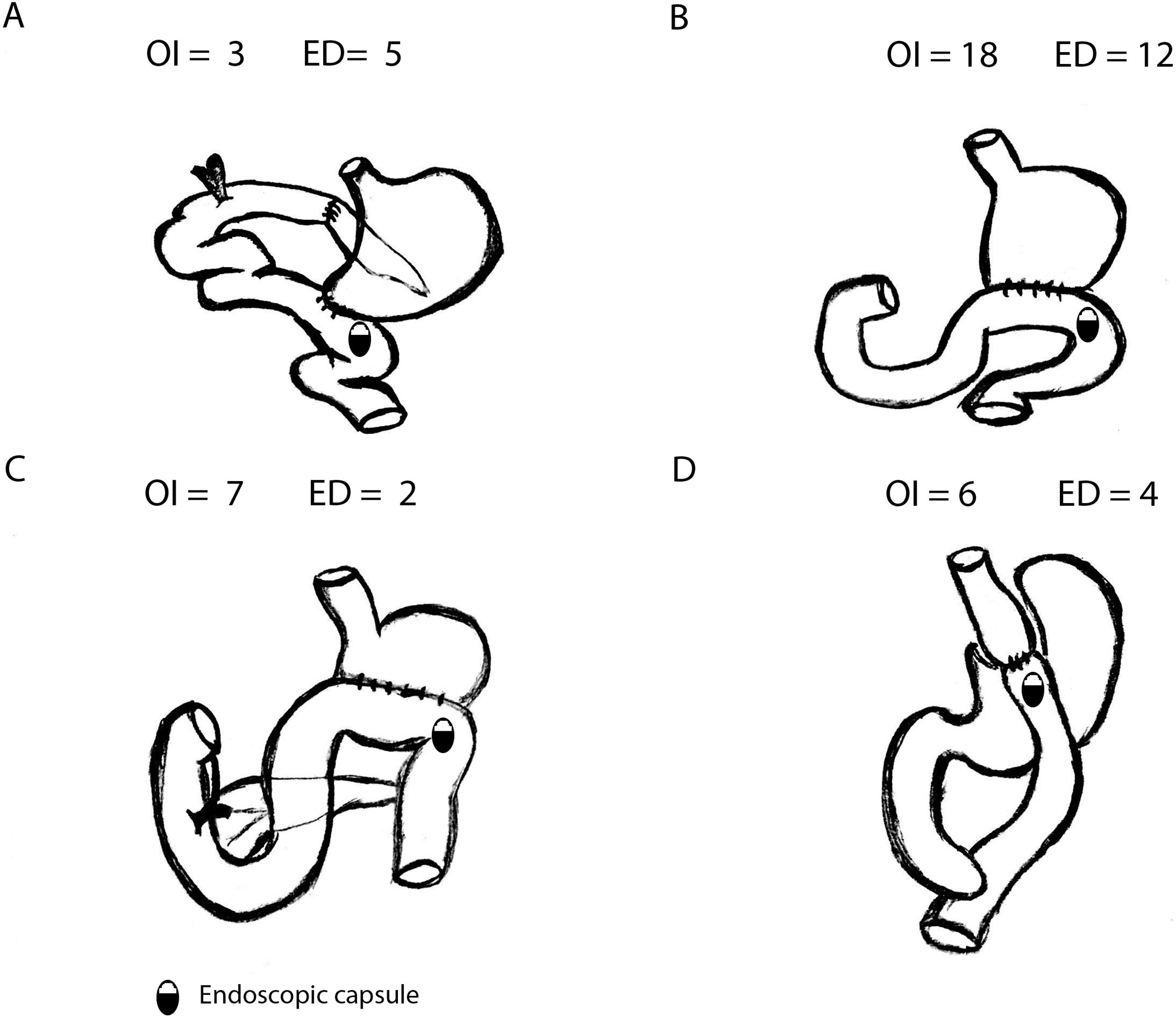
SBCE was ingested in 34 patients (OI) and placed endoscopically (ED) in 23 patients. Most common gastric surgeries were Billroth II gastrectomy (52.6%) or Roux-en-Y Gastric Bypass (17.5%) (Fig. 1).
Comparing OI and ED groups, there were no significant differences as per gender, age and previous GI surgery. No differences were observed in the indications for SBCE (p=0.352) either: most common indications were overt SBB (50.9%) and occult SBB (35.1%). Globally, 5 Agile patency® capsules were performed previously to convencional SBCE with no statistically significant difference between OI and ED group (3 vs. 2, p=0.683): in all cases patency capsules were excreted intact. In OI group, median gastric transit time (GTT) was 2min (IQR 22.75), with 7 patients with GTT>45min (20.6%). Comparing OI and ED, no differences were found in terms of small bowel transit time (301 vs. 377min, p=0.118) or total intestinal transit time (324 vs. 377min, p=0.206).
Technical characteristics of SBCE proceduresDifferent capsule endoscopy platforms were used in the study: 30 Pilllcam SB3 (Medtronic, Minneapolis, USA), 15 Pillcam Crohn's(Medtronic, Minneapolis, USA), 5 Endocapsule-10 (Olympus, Tokyo, Japan), 4 Pillcam SB2 (Medtronic, Minneapolis, USA), 2 Pillcam Colon2 (Medtronic, Minneapolis, USA) and 1 Mirocam (Intromedic, Seul, Korea).
A purgative bowel preparation was prescribed in 24 patients (42.1%) before SBCE (13 OI vs. 11 ED, p=0.472). In OI group, 5 patients also ingested anti-foaming agents in a water solution with the capsule. In ED group, the SBCE was placed with advanCE® device in 17 patients (73.9%) and with Roth net in 6 patients (26.1%). ED was performed under deep sedation in 16 patients (69.6%) and conscious sedation in 7 patients (30.4%).
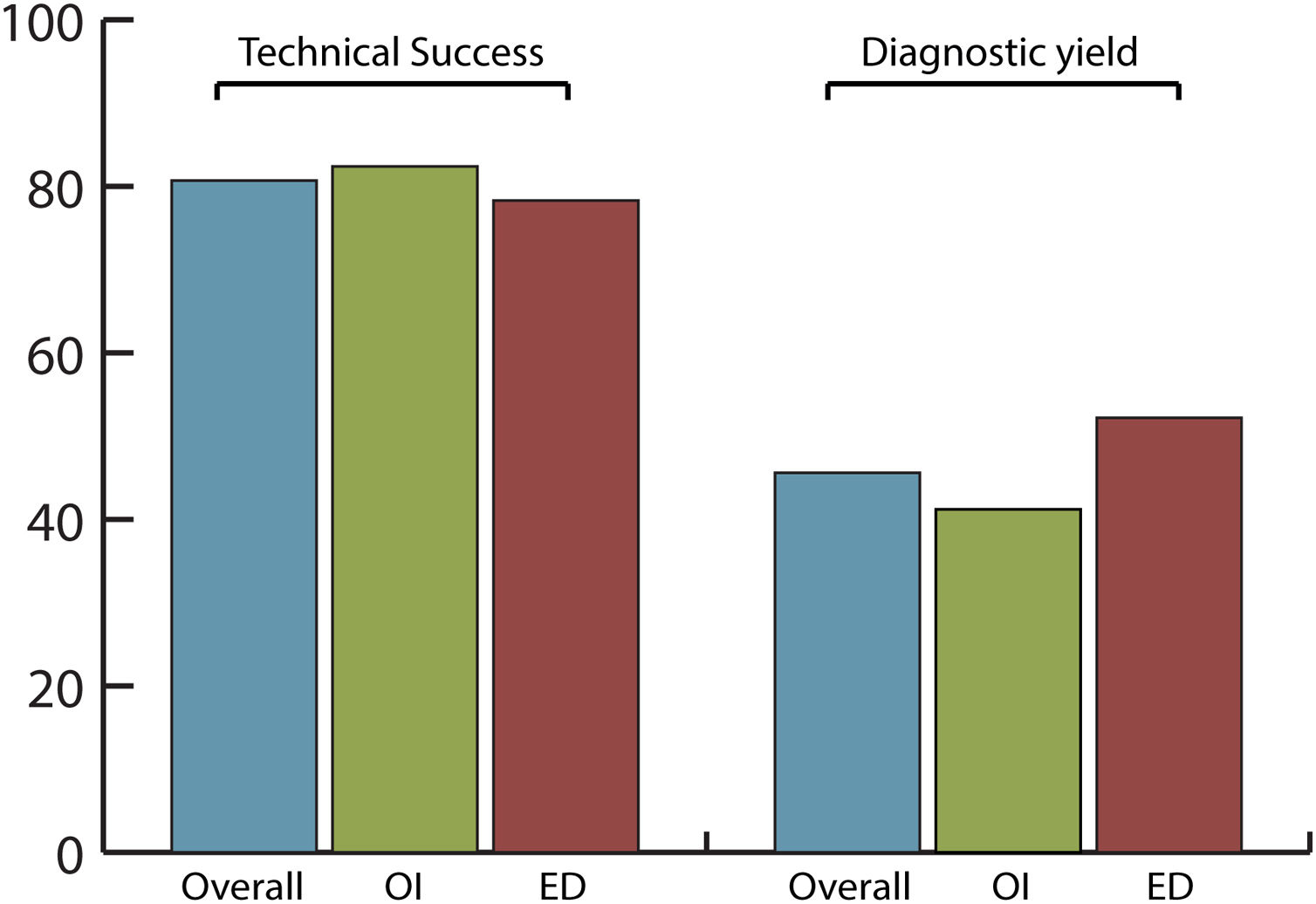
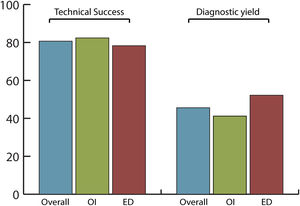
Efficacy and safety of SBCE in previous GI surgeryThe overall completion rate of SBCE was 80.7% with no significant differences between OI and ED (82.4% vs. 78.3%, respectively; p=0.742) being the number of incomplete procedures 5 in OI and 6 in ED group (Fig. 2).
The overall diagnostic yield was 45.6%, with no significant differences (p=0.432) between OI (41.2%) and ED (52.2%) groups. SBCE findings were: no abnormalities (49.1%), angioectasias (21.1%), ulcers (12.3%), active bleeding (7%), erosions (5.3%), varices (3.5%) and gastric abnormalities (1.8%).
There were no cases of capsule retention. Only one severe adverse event related to the procedure was registered. It was a single case of intestinal perforation due to ED with advanCE® at the anastomotic site in a patient with a previous Billroth II surgery. An over-the-scope clip (OTSC®, Ovesco Endoscopy GmbH, Tübingen, Germany) was placed and no other therapeutic actions were needed.
DiscussionSBCE represents the main diagnostic procedure in the study of SB which can be only partially visualized by upper and lower GI endoscopy. With the increasing use of this technique, new issues have emerged in daily practice: evidence is scarce about the management of SBCE in patients with altered anatomy due to previous GI surgery. Usually, these patients represent a minority in daily procedures, although some specific populations, such as operated obese patients, are growing.18 The optimal delivery method for SBCE still remains unclear in this patient population and there is minimal published data to guide clinical decisions. Nowadays, most common clinical practice in our country consists of delivering the capsule to the SB avoiding issues such as capsule retention or incomplete explorations.
The present study compares completion rate, efficacy and safety of two SBCE delivery methods in a Spanish cohort of patients with surgically altered GI anatomy. All hospitals involved were tertiary referral centres with highly experienced operators. To our knowledge this is the largest case-series and the first multicentre study to be published in this population. In contrast, all previous publications were single centre studies with lower sample size. In the study by Sellers et al., 29 patients with upper gastrointestinal surgery evaluated by SBCE were included, showing that completion rate, diagnostic yield and adverse events were not affected by the delivery method.19 In contrast, Stanich et al. demonstrated in 23 patients with previous bariatric surgery and gastric surgery that OI of the capsule could reach a satisfactory completion rate of 81.3%, compared with endoscopic deployment (62.5%), with no capsule retention and with a more favourable cost profile.20
In our study, patient population was divided into two balanced groups, according to OI (34 patients; 59.6%) and ED (23 patients; 40.4%) of the capsule. In overall population, completion rate (80.7%) and diagnostic yield (45.6%) were in line with previously published performance measures for SB endoscopy.17 This confirmed that SBCE is efficient in patients with surgically altered GI anatomy. In the OI group, only a minority of patients (20.6%) had a prolonged gastric transit time>45min, which might contribute to an incomplete examination.21 However, it was demonstrated that OI and ED are equal delivery methods and do not affect the efficacy of the procedure in terms of completion rate (82.4% vs. 78.3%, p=0.742) or diagnostic yield (41.2% vs. 52.2%, p=0.432).
As regards safety, in our patient population there were no cases of capsule retention, which is the most relevant adverse event as for clinical implications. Only 1 adverse event, related to the endoscopic delivery procedure, was observed: this was an anastomosis perforation during ED with advanCE® device, which was solved endoscopically but caused hospital admission of the patient. This shows that endoscopic procedures bear some potential complications that should be avoided when performing a non-invasive examination.
Some factors might affect SBCE examinations in this study. Only a minority of patients (42.1%) received bowel preparation before the procedure and this is one of the reasons that could explain the lower rate of diagnostic yield compared to the European Society of Gastrointestinal Endoscopy (ESGE) Guidelines, together with the high variability of SBCE indications including abdominal pain.17 More than half of patients in ED group (16/23; 69.6%) received deep sedation with propofol, which has been described to slow intestinal transit time while not affecting completion rate.22 However not significant differences were detected in intestinal transit time between OI (301min) and ED (377min).
This study also shows some limitations: first of all, it is a retrospective study in a multicentre cohort of patients which might be biased by loss of information; secondly, this study has a limited sample size, due to the low number of SBCE procedures conducted in this patient population: in our retrospective pool of 10205 procedures, only 0.56% were done in patients with altered GI anatomy. However, the present findings result from a nation-wide sample of patients of high clinical interest.
Data from the present study may be translated into clinical practice by avoiding the use of unnecessary endoscopic delivery in patients with altered GI anatomy, except for other conditions such as swallowing disorders, oesophageal diverticula, oesophago-gastric motility disorders or patient's refusal to oral ingestion. The benefits of this attitude might include the avoidance of unnecessary costs and procedural risks relating to both, endoscopy and sedation.
As a conclusion, our findings suggest that in patients with previous GI surgery the first-choice delivery method should be oral ingestion. Further prospective randomized studies, in a larger cohort, would be helpful to confirm these results.
Specific author contributionBGS, AG designed the study, collected and analyzed data. AEG, FSC, CCR, MAR, JEV, NAL, JLML, CGC, VPB, IFUS collected data. All authors reviewed the final manuscript.
FundingNo funding support was provided for this study.
Conflict of interestThe authors declare that there is no conflict of interest.