Ischemic type biliary lesions (ITBLs), a particular subset of non-anastomotic biliary strictures (NAS), are characterized by intra and extrahepatic strictures that occur in the absence of either hepatic artery thrombosis or stenosis. When they occur within the first year after liver transplantation their development is mostly related to ischemia–reperfusion injury (IRI). The indocyanine green plasma disappearance rate (ICG-PDR) might be able to predict the probability of IRI-induced graft damage after liver transplantation.
ObjectiveOur aim was to evaluate the association between ICG-PDR and the occurrence of ITBLs. Secondly, we searched for evidence of IRI in patients presenting ITBLs.
MethodsThis retrospective single-center observational study assessed a cohort of 60 liver transplant patients. Each patient underwent ICG-PDR on the 1st postoperative day. ITBLs were identified by means of either cholangiography or magnetic resonance imaging evidence of a deformity and narrowing of the biliary tree in the absence of hepatic artery thrombosis/stenosis.
ResultsITBLs were discovered in 10 patients out of 60 liver recipients (16.67%) within one year after transplantation. A low ICG-PDR value was found to be a significant predictive factor for ITBL development, with an OR of 0.87 and a 95% CI of 0.77–0.97. Liver biopsies were performed in 56 patients presenting unexplained abnormal liver function test results. A statistically significant association was found between the development of ITBLs and anatomopathological evidence of IRI.
LimitationsRetrospective, single-center study.
ConclusionsThe findings from this study show a relationship between low ICG-PDR values on first post-operative-day and the occurrence of ITBLs within 1 year after transplantation.
Las lesiones biliares de tipo isquémico (ITBL) representan un subconjunto de estenosis biliares no anastomóticas, caracterizadas por estenosis intra y extrahepáticas, que ocurren en ausencia de trombosis o estenosis de la arteria hepática. Cuando ocurren dentro del primer año después del trasplante de hígado, están relacionadas principalmente con la lesión por isquemia-reperfusión (IRI). La tasa de desaparición del plasma con verde de indocianina (ICG-PDR) podría estimar el daño del injerto inducido por IRI después de un trasplante.
ObjetivoNuestro objetivo es evaluar la asociación entre ICG-PDR y la aparición de ITBL. También investigamos la evidencia de IRI entre los pacientes que presentaron ITBL.
MétodosEstudio observacional, retrospectivo, unicéntrico, realizado en una cohorte de 60 receptores trasplantados con determinacion del ICG-PDR el primer día posoperatorio. Las ITBL se definieron mediante colangiografía o evidencia por resonancia magnética de deformidad del árbol biliar en ausencia de trombosis/estenosis de la arteria hepática.
ResultadosDe 60 receptores, se descubrieron ITBL en 10 pacientes (16,67%) en el primer año. El valor bajo de ICG-PDR es un factor predictivo significativo para ITBL, con OR=0,87 y un IC (95%)=0,77-0,97. Se analizaron 56 biopsias hepáticas para la presencia de IRI, si los receptores presentaban una prueba de función hepática anormal inexplicable, encontrando asociación significativa entre ITBL y evidencia anatomopatológica de IRI.
LimitacionesEstudio retrospectivo, unicéntrico.
ConclusionesEste estudio encontró una relación entre los valores bajos de ICG-PDR en el primer día posoperatorio y la aparición de ITBL dentro de un año posterior al trasplante.
Biliary complications are the most frequent complication after liver transplantation, with an incidence of 10–35%.1 Despite the improvements in surgical and medical care, they continue to be a relevant cause of morbidity, moderate mortality rates (2–7%),2 graft loss and death after orthotropic liver transplantation (OLTx).2,3 The majority of biliary complications include leaks, strictures and ischemic cholangiopathy (IC). Anastomotic strictures are the most frequent biliary complications while non-anastomotic strictures (NAS) tend to be less frequent and generally occur later in the liver-transplanted patient history.4
Ischemic-type biliary lesions (ITBLs), a particular subset of non-anastomotic biliary strictures (NAS), tipically occur in the absence of hepatic arterial thrombosis (HAT) or stenosis (HAS) and are characterized by intra-and extrahepatic strictures.5,6 Due to their multifactorial pathogenesis ITBLs may arise via different mechanisms. Among the main factors, prolonged cold (CIT) and warm (WIT) ischemia times, acute and chronic graft rejection as well as bile salts acidity seem to play a significant role.2,5–7
Immune-mediated bile duct injuries are typically responsible for the pathogenesis of ITBLs that occur over one year after OLTx.2,4,5 In addition to the above-mentioned causes, it is supposed that ITBLs are related to the phenomenon of ischemia–reperfusion injury (IRI), mainly when they present within 1 year after tranplantation.8 As a result of the blood supply interruption that occurs during all transplantation procedures, liver sinusoidal endothelial cells, cholangiocytes and hepatocytes often fall victim to cell injury and apoptosis-induced cell death.9,10 The absence of oxygen and nutrients creates a condition that leads to inflammation and oxidative stress following the restoration of circulation, with consequent damage to the tissues involved, instead of the resumption of normal function.11–13 In particular, the peribiliary arteriolar dysfunction that occurs secondarily to the cold ischemic storage and IRI leads to microvascular thrombosis and subsequent ischemia of the biliary epithelium. This phenomenon is strongly associated with the development of ITBLs, frequently resulting in a picture of diffuse strictures over the intra and extrahepatic biliary tree.9,11,14,15 The rate of indocyanine green clearance measured using the pulse-densitometric method (ICG-PDR) might represent a method to indirectly assess IRI.11,16 Plevris et al. evaluated indocyanine green (ICG) clearance in 41 livers within 24h of transplantation and the maximal rise in reactive oxygen intermediates was measured in peri-operative phases. An ICG clearance cut-off value of 200ml/min was identified as a predictor of reperfusion injury grade and graft outcome.11 The spectrum of duct injuries resulting from IRI, as shown by liver biopsy, ranges from full-thickness necrosis to superficial epithelium cell damage. It is the fibrotic reaction accompanying these lesions that leads to the development of the narrow stenoses characterizing ITBLs.13,15,17 Considering that ICG-PDR provides a means to estimate IRI following liver transplantation, this study assessed the association between ICG-PDR and the development of ITBLs. In addition, we investigated whether a significant association existed between ITBLs and the presence/grade of IRI at the time of graft biopsy.
Materials and methodsStudy populationThis study constitutes a post hoc analysis of the data collated in our previous observational study in which ICG-PDR was measured as a diagnostic and prognostic tool16 after orthotopic liver transplantation (OLTx) from Dead Brain Donors (DBD). The study was approved on 3 February 2020 by the Internal Review Board (IRB) of the University of Udine, Italy, with a protocol identifier number 001/2020_IRB. Due to the retrospective design of the study, patient consent was waived, but the European Privacy law 2016/679 for General Data Protection Regulation (GDPR) was respected. The patient inclusion criterion was: all adult transplanted patients from donors after brain death (DBDs) having ICG-PDR testing on post-operative day one. The exclusion criteria were: patients undergoing OLTx due to autoimmune liver disease (autoimmune hepatitis, primary sclerosing cholangitis), the onset of primary graft non-function (PNF) with need for re-transplantation within 72h from OLTx, biliodigestive anastomosis, open abdomen, hepatic artery thrombosis (HAT), hepatic artery stenosis (HAS), known iodine allergy and acute vascular complications in the immediate postoperative phase.18 Sixty patients fulfilling the above-mentioned criteria were enrolled in our study. We only included elective patients with end-stage liver disease as assessed using the Model for End-stage Liver Disease (MELD) score.
Surgical noteAll grafts harvested from DBDs were perfused through the portal vein and the hepatic artery during procurement and on the back table with Servator C Salf (CS) 1L (sterile pyrogen-free solution for the perfusion and hypothermic preservation of thoracic and abdominal organs, SALF spa Laboratorio Farmacologico, 24069, Cenate di Sotto (BG), Italy). The biliary tract was washed with Celsior solution (CS) during procurement and on the back table to avoid stagnation of cytotoxic bile salts. We used the piggyback technique as surgical approach. In accordance with our institute's transplantation protocols, all grafts were re-perfused simultaneously via the portal vein and hepatic artery.19 The majority of the recipients underwent biliary duct-to-duct anastomosis, whereas a Roux-en-Y hepaticojejunostomy anastomosis was necessary in a minority of cases. Placement of the Kehr's Tube (Rüsch, Teleflex®, Willy Rüsch GmbH, Willy-Rüsch-Strasse 4-10, 71394 Kernen, Germany) was determined by the surgical team in case of size discrepancy or steatotic (≥30%) grafts and was not protocol-driven.
Immunosuppressive protocolConsidering the role of immune-mediated mechanisms in the genesis of ITBLs, we analyzed the immunosuppressive drug concentrations used in the transplant patient's immunosuppressive therapy. After surgery, all patients received the same immunosuppressive therapy, involving extended-release tacrolimus or everolimus, mycophenolate mofetil (MMF) and steroids, in according to the protocol suggested by the Italian Society of Organ Transplantation.20 In particular, steroids were administered during surgery at the time of graft reperfusion at a dose of 1000mg. A de-escalation scheme was carried out during the first month after OLTx. Tacrolimus was started on the second day post-OLTx; the initial dose was calculated on the basis of creatinine clearance and liver function in accordance with the literature.21 In our center, daily administration is preferred. Tacrolimus was switched to cyclosporine in dysmetabolic patients, in patients with severe diabetes, or in the case of adverse effects to tacrolimus. In the case of insufficient blood serum concentration (less of 5ng/ml), tacrolimus was switched to everolimus. Everolimus was also evaluated as an alternative in the case of CNI intolerance (nephrotoxicity or neurotoxicity). If possible, Everolimus was started at least one month after OLTx due to the risk of artery thrombosis, as suggested in the literature.22 Immunosuppression therapy was managed with the therapeutic drug monitoring (TDM) in all patients. We recorded all available TDM values available on our medical records management system from the beginning of the immunosuppressive therapy to the end of the first post-OLTx year. In our center, mycophenolate mofetil (MMF) treatment is started in all patients three months after OLTx in order to be able to reduce the administration of other immunosuppressors, or when corticosteroid withdrawal is desirable or to reduce the risk of renal damage by calcineurin inhibitor (CNI). MMF was introduced earlier in patients with renal failure.23,24 As per our Institute's policy, we do not use basiliximab, ursodeoxycholic acid (UDCA) or N-Acetylcysteine. Blood counts, liver function profiling and graft ultrasound scans were performed daily in all patients for the first postoperative week. Liver function and blood counts were subsequently monitored on a weekly basis for the next three months after OLTx.
ICG-PDR by pulse spectrophotometryICG-PDR assessed by pulse spectrophotometry has been demonstrated to provide a picture of a transplanted graft's metabolic function at the time of surgery.16,25 The ICG-PDR test can be performed noninvasively after a central line injection of the dye. Pulse spectrophotometry is performed using a finger-clip sensor (LiMON®, Pulsion Medical Systems AG, Munich, Germany) that detects the plasma disappearance rate of indocyanine green, giving us a value of its hepatic clearance.25 In all patients, ICG-PDR determinations were tested in the intensive care unit 24h after surgery to detect the evidence of microcirculation dysfunction.26–29 Common side effects attributable to green dye are cough, difficulty swallowing, dizziness, tachycardia, hives or welts, itching, skin rash, puffiness or swelling of the eyelids or around the eyes, face, lips, or tongue, redness of the skin, tightness in the chest, tiredness or weakness.
ITBLs definition and diagnosisMost of the ITBLs that occur within one year after transplantation are secondary to IRI.2,5,6 We only considered ITBLs occurring within one year after OLTx. In our protocol, all transplanted patients systematically undergo trans-T-tube cholangiography, endoscopic retrograde cholangiography (ERC), or magnetic resonance imaging (MRI) at 3, 6 and 12 months after surgery. However, if patients presented any clinical signs suggestive of biliary complications, they underwent instrumental examinations before the timepoints foreseen by our protocol. A diagnosis of ITBL is recorded when hepatic artery stenosis (HAS), hepatic artery thrombosis (HAT), portal thrombosis, autoimmune hepatitis (AIH), primary sclerosing cholangitis (PSC) and group AB0 type incompatibility have all been ruled out.
ITBLs were identified after a careful review of the cholangiography images and/or MRI scans and defined as evidence of (i) focal or extensive mucosal irregularities (tortuosity and deformity of the biliary tree, cholangiectasis of the intrahepatic ducts, accumulation of biliary sludge) or (ii) narrowing of the biliary lumen, ductal dilatation.2,4–7
Liver biopsyWe performed a liver parenchyma biopsy in transplanted recipients when they presented unexplained abnormal liver function tests30 and when acute rejection was suspected; as we know from the literature, liver biopsy is the gold standard for the diagnosis of acute graft rejection.31,32 These biopsies were analyzed by a senior pathologist with more than 10 years of experience who identified the presence of any IRI-induced damage. We used a Tru-Cut needle liver biopsy (Bard Max-Core, Bard Peripheral Vascular Inc., Tempe, Arizona, USA). The available histological graft needle biopsies were revised to identify any signs of graft rejection or IRI-induced damage; if IRI signs were present, we scored the IRI severity.32
Outcome measuresThe primary outcome was to assess the predictive value of ICG-PDR for ITBLs. The data recorded for each patient were also compared to the corresponding anatomopathological findings obtained from the liver graft biopsy.
Statistical evaluationConsidering the results of the Shapiro–Wilk test for normality, quantitative variables were investigated using ANOVA and the non-parametric Kruskal–Wallis and Mann–Whitney U tests for independent samples to compare and analyze the associations between variables (age, MELD score, DRI score, BMI, SOFA score, ICG-PDR value, cold ischemia times, warm ischemia times, red blood cell count, volume of plasma transfusions, lactates and other biochemical parameters, immunosuppression therapy levels, complications and length of hospital stay. Univariate logistic regression analyses were used to study how patient ICG-PDR influenced the occurrence of ITBLs. The significance of associations between dichotomic variables as the presence/absence of ITBLs vs IRI or ITBLs vs acute rejection was tested using the (χ2) chi-square test and Fisher's exact test. Statistical significance was considered for p-values≤0.05. MedCalc Statistical Software version 16.4.3 (MedCalc Software bv, Ostend, Belgium; 2016) was used to perform the statistical analyses.
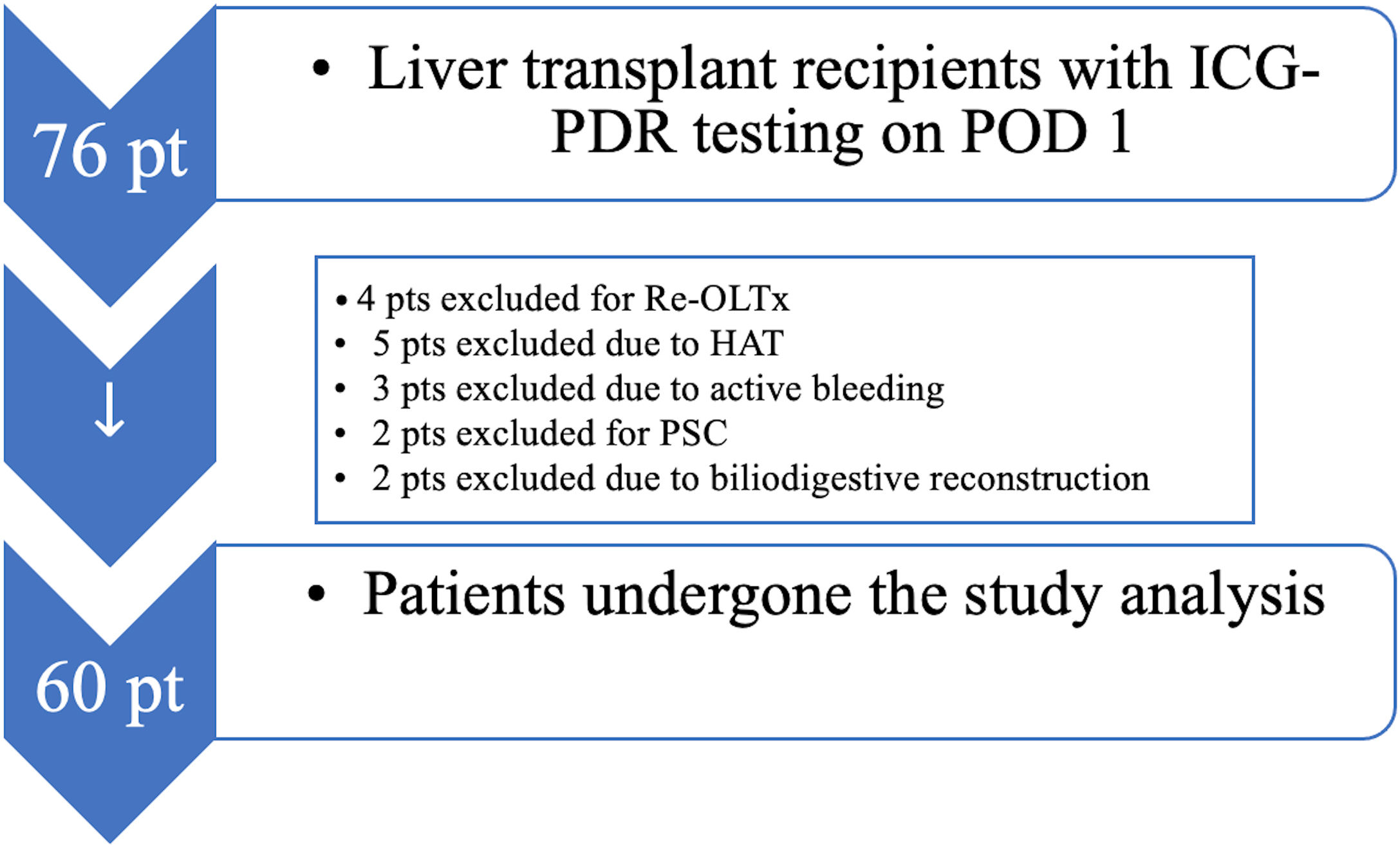
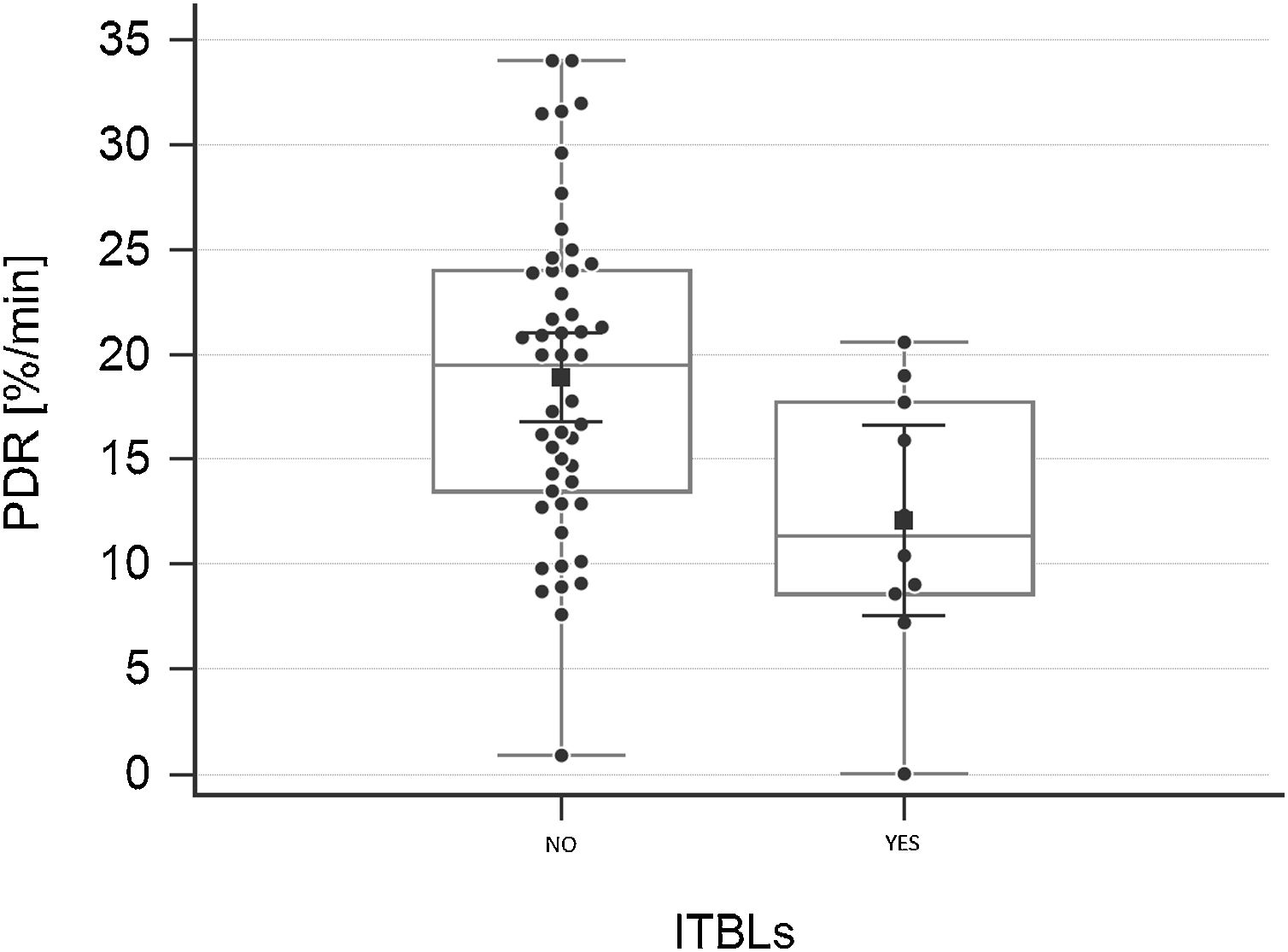
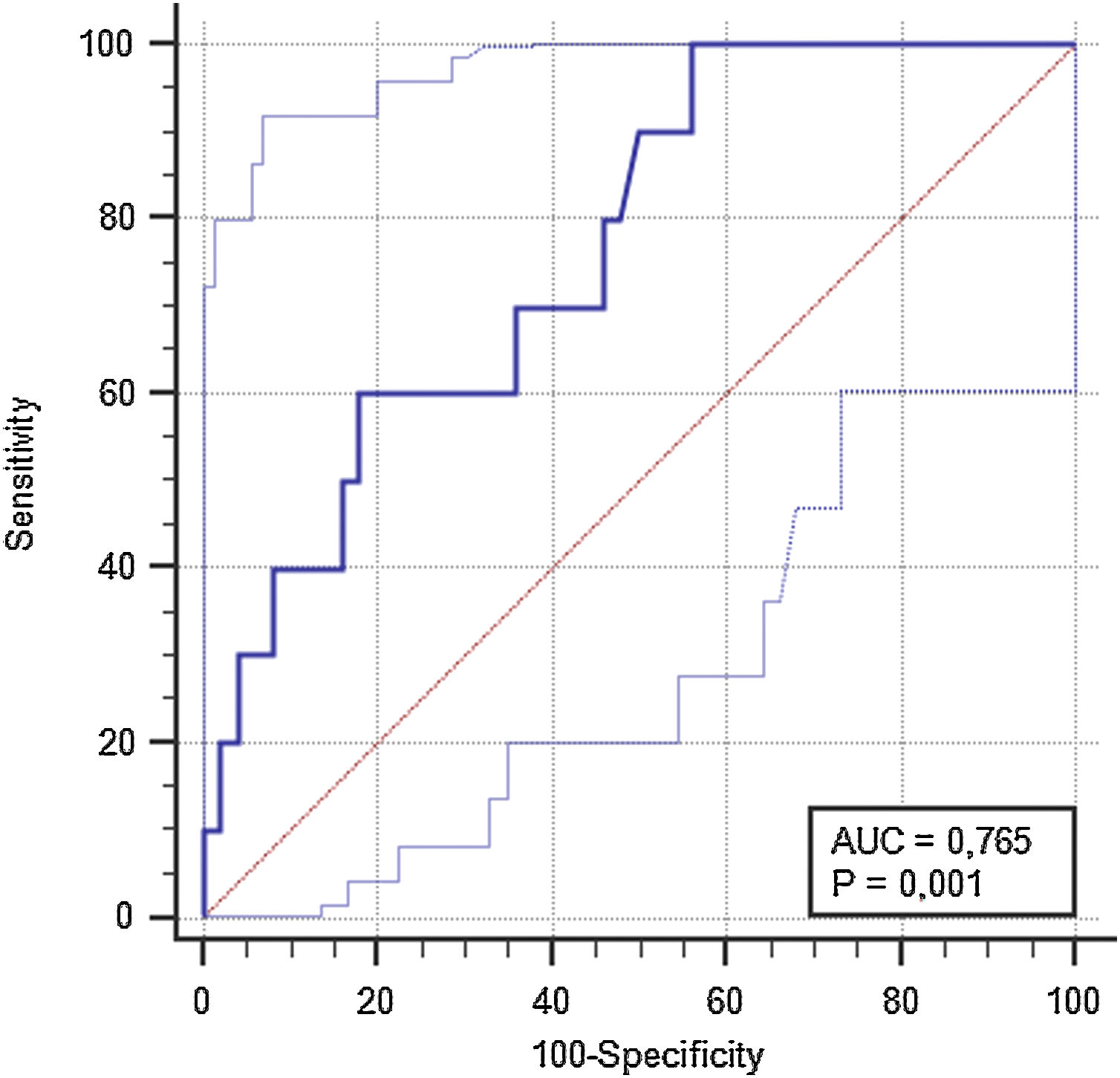
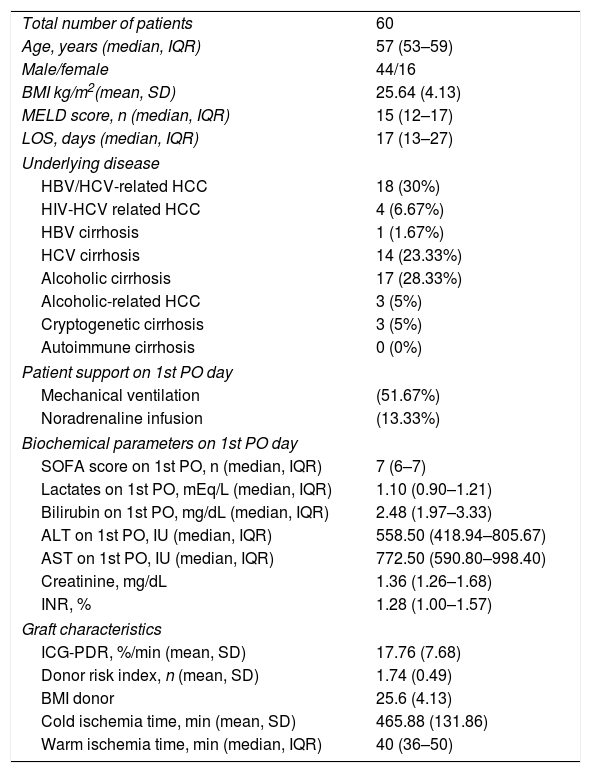
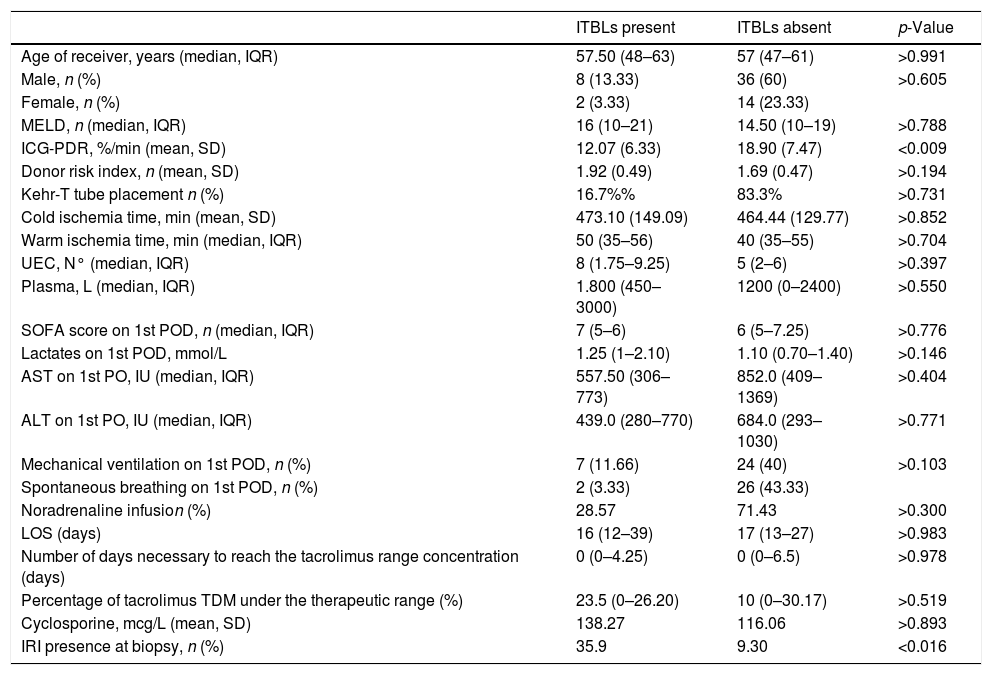
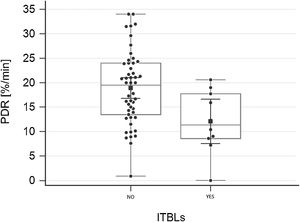
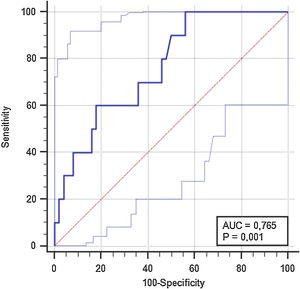
ResultsTo investigate the prognostic value of the relationship between ICG-PDR data and ITBLs, 60 OLTx patients meeting the study criteria were included in this post hoc analysis. The flow chart of the study is shown in Fig. 1. All patients underwent ICG-PDR determination on the 1st PO. Table 1 reports the demographic characteristics, MELD scores, graft characteristics and all other relevant data for this cohort. The median length of stay (LOS) in hospital was 17 days. One year after OLTx, ITBLs were discovered in 10 patients (16.67%). ANOVA and Kurskal–Wallis tests were performed to study the association between the ICG-PDR as assessed on the 1st PO day and the analyzed variables. An ICG-PDR value of less than 12.07%/min (SD±6.33) was significantly associated with the development of ITBLs (p=0.009). Patients without ITBLs showed a mean ICG-PDR value of 18.90%/min (SD±7.47). Fig. 2 shows box plots for the ICG-PDR data from patients with vs without ITBLs. None of the following variables were significantly associated with the occurrence of ITBLs: age, sex, MELD score, DRI, LOS, Kehr T-tube placement, cold and warm ischemia times, or transfusion volume (Table 2). A ROC curve was generated for ICG-PDR values on the 1st PO day and ITBLs; the AUC was 0.77 with a 95% confidence interval (CI) of 0.64–0.87 and a p-value of 0.001 (Fig. 3). Logistic regression analysis was then performed and revealed that a 1st PO day ICG-PDR value≥16%/min was prognostic of a low risk of ITBLs (p<0.017) with an odds ratio (OR) of 0.87 and a 95% CI of 0.77–0.97. Of the 60 patients included in the analysis, 56 biopsies were available, of which 17 were positive for the presence of IRI. The principal characteristics were: the presence of neutrophilic infiltrate, apoptosis and cell count dropout. The χ2 analysis demonstrated a statistically significant association between the development of ITBLs and anatomopathological evidence of ischemic reperfusion injury, with a p-value of 0.015. These data showed an association between ITBLs and cholestatic damage (p-value<0.01). Neither the percentage of out-of-range TDM values nor the number of days to reach the target concentrations correlated with the occurrence of ITBLs (p=0.616 and p=0.978, respectively; Kurskal–Wallis tests). No association was found between ITBLs and anatomopathological evidence of acute graft rejection (p=0.391) and any patient from the study had anatomopathological evidence of chronic graft rejection.
Patient demographic, clinical and laboratory data.
| Total number of patients | 60 |
| Age, years (median, IQR) | 57 (53–59) |
| Male/female | 44/16 |
| BMI kg/m2(mean, SD) | 25.64 (4.13) |
| MELD score, n (median, IQR) | 15 (12–17) |
| LOS, days (median, IQR) | 17 (13–27) |
| Underlying disease | |
| HBV/HCV-related HCC | 18 (30%) |
| HIV-HCV related HCC | 4 (6.67%) |
| HBV cirrhosis | 1 (1.67%) |
| HCV cirrhosis | 14 (23.33%) |
| Alcoholic cirrhosis | 17 (28.33%) |
| Alcoholic-related HCC | 3 (5%) |
| Cryptogenetic cirrhosis | 3 (5%) |
| Autoimmune cirrhosis | 0 (0%) |
| Patient support on 1st PO day | |
| Mechanical ventilation | (51.67%) |
| Noradrenaline infusion | (13.33%) |
| Biochemical parameters on 1st PO day | |
| SOFA score on 1st PO, n (median, IQR) | 7 (6–7) |
| Lactates on 1st PO, mEq/L (median, IQR) | 1.10 (0.90–1.21) |
| Bilirubin on 1st PO, mg/dL (median, IQR) | 2.48 (1.97–3.33) |
| ALT on 1st PO, IU (median, IQR) | 558.50 (418.94–805.67) |
| AST on 1st PO, IU (median, IQR) | 772.50 (590.80–998.40) |
| Creatinine, mg/dL | 1.36 (1.26–1.68) |
| INR, % | 1.28 (1.00–1.57) |
| Graft characteristics | |
| ICG-PDR, %/min (mean, SD) | 17.76 (7.68) |
| Donor risk index, n (mean, SD) | 1.74 (0.49) |
| BMI donor | 25.6 (4.13) |
| Cold ischemia time, min (mean, SD) | 465.88 (131.86) |
| Warm ischemia time, min (median, IQR) | 40 (36–50) |
Abbreviations: BMI, Body Mass Index; MELD, Model for End-stage Liver Disease; LOS, Length of Stay; HBV, Hepatitis B Virus; HCV, Hepatitis C Virus; HCC, HepatoCellular Carcinoma; IU, International Units; SOFA score, Sequential Organ Failure Assessment score; ALT, Alanine AminoTransferase; AST, Aspartate Amino-Transferase; INR, International Normalized Ratio; ICG-PDR, IndoCyanine Green dye Plasma Disappearance Rate; IQR, Interquartile Range; SD, Standard Deviation; n, number; min, minutes; 1st PO, first Post-Operative Day.
Association with presence/absence of ITBLs.
| ITBLs present | ITBLs absent | p-Value | |
|---|---|---|---|
| Age of receiver, years (median, IQR) | 57.50 (48–63) | 57 (47–61) | >0.991 |
| Male, n (%) | 8 (13.33) | 36 (60) | >0.605 |
| Female, n (%) | 2 (3.33) | 14 (23.33) | |
| MELD, n (median, IQR) | 16 (10–21) | 14.50 (10–19) | >0.788 |
| ICG-PDR, %/min (mean, SD) | 12.07 (6.33) | 18.90 (7.47) | <0.009 |
| Donor risk index, n (mean, SD) | 1.92 (0.49) | 1.69 (0.47) | >0.194 |
| Kehr-T tube placement n (%) | 16.7%% | 83.3% | >0.731 |
| Cold ischemia time, min (mean, SD) | 473.10 (149.09) | 464.44 (129.77) | >0.852 |
| Warm ischemia time, min (median, IQR) | 50 (35–56) | 40 (35–55) | >0.704 |
| UEC, N° (median, IQR) | 8 (1.75–9.25) | 5 (2–6) | >0.397 |
| Plasma, L (median, IQR) | 1.800 (450–3000) | 1200 (0–2400) | >0.550 |
| SOFA score on 1st POD, n (median, IQR) | 7 (5–6) | 6 (5–7.25) | >0.776 |
| Lactates on 1st POD, mmol/L | 1.25 (1–2.10) | 1.10 (0.70–1.40) | >0.146 |
| AST on 1st PO, IU (median, IQR) | 557.50 (306–773) | 852.0 (409–1369) | >0.404 |
| ALT on 1st PO, IU (median, IQR) | 439.0 (280–770) | 684.0 (293–1030) | >0.771 |
| Mechanical ventilation on 1st POD, n (%) | 7 (11.66) | 24 (40) | >0.103 |
| Spontaneous breathing on 1st POD, n (%) | 2 (3.33) | 26 (43.33) | |
| Noradrenaline infusion (%) | 28.57 | 71.43 | >0.300 |
| LOS (days) | 16 (12–39) | 17 (13–27) | >0.983 |
| Number of days necessary to reach the tacrolimus range concentration (days) | 0 (0–4.25) | 0 (0–6.5) | >0.978 |
| Percentage of tacrolimus TDM under the therapeutic range (%) | 23.5 (0–26.20) | 10 (0–30.17) | >0.519 |
| Cyclosporine, mcg/L (mean, SD) | 138.27 | 116.06 | >0.893 |
| IRI presence at biopsy, n (%) | 35.9 | 9.30 | <0.016 |
Abbreviations: MELD, Model for End-stage Liver Disease; ICG-PDR, IndoCyanine Green dye Plasma Disappearance Rate; UEC N°, Number of Units of packed red blood cells; n, number; SOFA score, Sequential Organ Failure Assessment score; ALT, Alanine AminoTransferase; AST, Aspartate Amino-Transferase; IQR, Interquartile Range; LOS, Length of Stay; SD, Standard Deviation; TDM, Therapeutic Drug Monitoring; n, number; min, minutes; L, liters.
In our cohort, only one case of Cytomegalovirus (CMV) infection was detected, but this patient was not affected by ITBLs.
DiscussionThe main findings of our study are twofold. Firstly, the ICG-PDR value on the 1st PO day was related to the occurrence of ITBLs. Secondly, a significant association was present for evidence of IRI damage assessed by needle biopsy and ITBLs. Biliary complications are presently the most frequent lesions following OLTx and have an enormous impact on patients’ quality of life, with high morbidity and mortality rates.2,3 In particular, NAS may occur as a result of hepatic artery thrombosis or due to the absence of hepatic artery patency. The genesis of ITBLs (a subset of NAS) is intricate and multifactorial; nevertheless, experimental and clinical studies have identified some of the significant events/factors associated with their development such as ischemia–reperfusion damage, bile salt toxicity and immune processes.2,5,33–35 Bile salt toxicity and an altered salt/phospholipid ratio are known to bring about direct cell damage via destruction of the cellular lipid membrane, causing secondary fibrosis and scarring.33,35 Indeed, it is due to the known detrimental effects of intraductal bile, which may persist during organ preservation, that retrograde flushing of the biliary tree is generally practiced during liver procurement.36 Instead, immune-mediated bile duct injuries, are likely to be responsible for the pathogenesis of ITBLs occuring later than one year after liver transplantation.2,4,5 For this reason, the present study did not analyze patients presenting ITBLs occurring later than one year, and we focused only on ITBLs presenting within one year, which are mainly related to ischemia and reperfusion injury.35 Ischemia and reperfusion injury leads to a broad spectrum of lesions, ranging from biliary epithelium damage to full-thickness necrosis and even perforation.9,17 The fibrosing response of the duct wall that follows these lesions leads to the formation of the narrowing and stenoses that characterize ITBLs.2,4,9 The dysfunction of the peri-biliary vascular plexus contributes significantly to the death of cholangiocytes.9,17 In 1999, Plevris et al. showed IRI could be accurately measured by indocyanine clearance11 and considerable evidence supports the use of ICG-PDR assessment on liver transplanted patients to predict the occurrence of early allograft dysfunction (EAD) or primary nonfunction (PNF).16,29 Noack et al. demonstrated a greater tolerance of hepatocytes to IRI compared with biliary cells, which might explain why complications more frequently affect the biliary structure rather than the parenchymal component.9 For these reasons, we considered ICG-PDR as a measurement of IRI and assessed the association between ICG-PDR and the development of ITBLs. In our study, and in accordance with literature (which reports incidences ranging from 1.4 to 26%), the rate of ITBLs occurrence was 16.67%. A 1st PO day ICG-PDR value lower than 16%/min, confirmed its relationship with ITBL occurrence. We also reviewed all the biopsy data pertaining to our OLTx patients to assess the presence or absence of signs of liver rejection and/or IRI. As mentioned above, liver biopsies were performed in transplant recipients when they presented unexplained abnormal liver function tests30 suggestive of acute graft rejection; as we know from literature, liver biopsy is the gold standard for the diagnosis of acute rejection.31,32 Histopathological assessment of ITBL-affected patients has always confirmed the presence of IRI signs. No cases of chronic graft rejection were discovered in our biopsies. To the best of know knowledge, this study is the first to show that a single ICG-PDR measurement performed on the 1st PO day has the potential to reliably predict ITBLs. When we stratified the population according to an ICG-PDR cut-off of 16.0%/min, the two resulting subgroups showed significantly different odd ratios (OR) regarding the development of ITBLs within one year. In our study, ICG PDR was the only variable to correlate with ITBL development. None of listed factors reported in Table 2 showed any significant association with the incidence of ITBLs. Note, however, that other studies with much larger and better characterized populations, have established many of the factors listed in Table 2 as risk factors for ITBLs.2,4,9,12,17,34,37 This discrepancy may be because we only analyzed only ITBLs that occurred within one year after OLTx and may also reflect the small number of patients involved in our study. These reasons may also explain why we did not reveal any statistically significant association between immunosuppressive therapy and ITBLs. As discussed above, a part of the NAS, occurring after one year following liver transplantation are likely to be caused by immune-mediated bile duct injury.2,5,35 Moreover, very few of our grafts exceeded 9h of cold ischemia time, cited in the literature as a risk factor for later development of ITBLs.15 None of the transplanted patients were affected by primary sclerosing cholangitis (PSC), autoimmune hepatitis (AIH) and AB0 incompatibility which were defined by literature as risk factors as well.35,37 Our results are in agreement with some other studies from in literature that reveal the IRI-induced degree of peribiliary vascular injury as a significant independent risk factor for the development of ITBLs. 12,13 This stands to explain how ICG-PDR testing may be able to predict IRI-related ITBLs. Future work will be directed toward understanding if and how ICG-PDR data can be implemented to plan strategies to prevent biliary complications. The mechanisms underlying IRI in transplant surgery need to be elucidated to develop new targeted therapies that can reduce the damage caused by ischemia and reperfusion, with the ultimate aim of improving the mortality and morbidity rates in liver transplant patients. Some limitations of our study also need to be acknowledged. First, this study constitutes a post hoc analysis of our previous study; it is also a single-center and retrospective study. Even the fact that the decision to obtain an ICG-PDR measurement on the 1st PO day in some patients was based on the clinician's assessment and not necessarily protocol-driven may have introduced a source of bias. The small sample size also limits the potency of our results, which need to be confirmed by further prospective studies; indeed, we did not perform any power analysis in relationship to the study's primary aim, but simply analyzed all available data. Biopsies and ICG-PDR were not performed at the same time, therefore a reliable relationship between low ICG-PDR values and evidence of IRI was difficult to establish. Moreover, it is possible that sicker patients were more likely to undergo ICG-PDR assessment to assist clinical judgment. Finally, only a single ICG-PDR measurement was obtained on the 1st PO day, but an average value would have been preferable, particularly in cases of high donor risk index (DRI), an unstable intraoperative period or post-transplant sepsis.
ConclusionsThis study was able to define a relationship between low ICG-PDR values on the first post-operative-day and the occurrence of ITBLs within one year after transplantation. In addition, biopsies from patients presenting with ITBLs were significantly related to the anatomopathological evidence of IRI. As a consequence of the study-related limitations, related to the small number of patients, further studies are needed for clarify the potential of the indocyanine green test as simple tool in the daily postoperative assessment for liver transplanted patients. Early identification of ITBLs in high-risk patients may help plan for corrective therapy and prevent disease progression.
The authors whose names are listed above certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed within in this manuscript.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestThe authors declare that they have no conflict of interest.