Despite unique immunoregulatory properties pointing toward tolerance, the liver allograft can be negatively impacted by humoral alloreactivity, with immediate as well as long-term harmful consequences. With regard to the unmet need of long-term outcomes improvement after liver transplantation, donor-specific antibodies have recently been the matter of intense study in this context. We review here recent advances regarding the understanding of the impact of preformed as well as de novo anti-human leukocyte antigen donor-specific antibodies in liver transplantation and discuss potential strategies to overcome this problem.
A pesar de sus propiedades inmunorreguladoras únicas que apuntan a la tolerancia, el aloinjerto hepático puede verse afectado negativamente por la alorreactividad humoral, con consecuencias nocivas inmediatas y también a largo plazo. Recientemente, y en relación con la necesidad no cubierta de mejorar los resultados a largo plazo tras el trasplante de hígado, se han realizado estudios exhaustivos sobre los anticuerpos específicos del donante. En el presente artículo, revisamos los últimos avances a la hora de comprender el impacto de los anticuerpos contra el antígeno leucocitario humano específicos del donante, preformados o de novo, en el trasplante de hígado y estudiamos posibles estrategias para resolver este problema.
Liver transplantation (LT) remains currently the most successful treatment for end-stage liver disease. Remarkable improvements in short-term liver allograft and transplant recipient survival have been achieved in the last three decades, due in particular to the improvement in immunosuppressive protocols and the refinement in surgical techniques and intensive care. However, long-term outcomes remain unsatisfactory, with a morbidity and mortality superior to the ones of the general population.1 The 5- and 10-year patient survival, as reported by registries across Europe and the USA, is in fact ranging between 70–80% and <70%, respectively. These suboptimal figures can be related to cardiovascular and metabolic diseases, de novo malignancies, chronic kidney disease and recurrent liver disease. In addition to these conditions, a precise understanding of the consequences of anti-human leukocyte antigen (HLA) donor-specific antibody-mediated injury is required to improve patient and graft survival after LT.
The development of new sensitive assays in antibody detection has uncovered the pathogenic role of preexisting or de novo anti-HLA donor-specific antibodies (DSA) after kidney transplantation,2 and more recently also after lung,3 heart4 and pancreas transplantation.5 DSA are indeed a risk factor for the development of acute and chronic rejection, graft loss and patient death after solid organ transplantation.6,7 Of note, even though the detrimental effects of DSA on outcomes following renal transplantation have been recognized since 50 years,8 the understanding of their pathologic impact continues to be refined. Several studies highlight the role of antibody class, amount and specificity, functional properties, epitope binding, as well as antigen density and location, as determinants of the ultimate outcome of organ transplantation.9–15
In the context of LT, DSA were until recently considered to be clinically irrelevant, despite early reports of increased graft loss rates in recipients who had a positive crossmatch.16–18 Moreover, most of the liver transplant centers still do not take HLA matching into account in their allocation algorithms. Our knowledge on DSA in LT has however evolved over the last decade, thanks to numerous studies which showed their detrimental effects on the liver graft, yet their role in clinical practice still needs to be defined. Here, we review recent advances regarding the impact of preformed as well as de novo DSA in adult ABO-compatible LT, the complications associated with DSA, and discuss potential strategies to overcome this problem.
Pathophysiology of antibody-mediated rejection and methods to detect anti-HLA antibodiesAntibodies that can mediate rejection include those against HLA molecules, endothelial-cell antigens and ABO blood-group antigens on endothelial cells and red cells.19,20 Non-HLA antigens, whose mismatch between donor and recipient has been described to be potentially harmful, include anti-endothelial cell antibodies,21 angiotensin-II type-1 receptor,22 anti-glutathione-S-transferase T123 and MHC class-I related chain A.24 Donor-specific antibodies (DSA) are by definition antibodies specifically directed to antigens expressed in the transplanted organ. In this review, we will focus on anti-HLA antibodies; thus, DSA will refer here to anti-HLA DSA only. DSA can be classified into 2 categories: (1) preformed DSA and (2) de novo DSA, depending on whether they are present at the time of transplantation or develop at any time after the procedure, respectively. Most recipients do not have antibodies against HLA molecules before transplantation but some are sensitized by previous exposure to alloantigens, mainly through pregnancy, blood transfusions or previous transplantation.
Hyperacute rejection can occur after organ transplantation in the presence of preformed DSA. This phenomenon, which remains very rare in LT,25 involves the deposition of preformed antibodies against HLA antigens expressed on the endothelium of the allograft and underlies the activation of the classical pathway of the complement cascade, followed by endothelial activation, platelet deposition and local coagulation.26
Complement is the prime immune effector system in hyperacute and acute antibody-mediated rejection (AMR) to mismatched organs, as preformed DSA activate complement through the classical pathway, which in turn targets endothelium and causes inflammation and injury.27,28 Both innate and adaptive immune responses are induced. The damaged endothelial cells release various molecules, such as von Willebrand factor and P-selectin that promote platelet aggregation, cytokines and chemokines (interleukin-1α, interleukin-8, chemokine C–C motif ligand 2) that lead leukocytes to adhere to capillaries, and chemoattractants (C3a, C5a).19 C4d, a marker of classical complement activation, is frequently found in peritubular capillaries of kidney26 and also in the portal tract capillaries of liver allografts undergoing AMR.29 C3b binds to the surface and acts as an opsonin. C5b-9 (or membrane-attack complex, MAC) is inserted in the membranes and causes localized endothelial necrosis and apoptosis.19,28 Microthrombi with hemorrhage and arterial wall necrosis and infarction can occur in severe cases. T cell alloreactivity is increased and B cell CR2 binding of C3d may amplify their response to target antigens.28 Antibody-dependent cellular cytotoxicity (ADCC) seems also to be a mechanism involved in antibody-mediated rejection.30 Following the response of the adaptive immune system, NK cells can typically further destroy allograft endothelium by ADCC, triggered through cross-linking of the CD16 Fc receptor by DSA bound to allograft.31
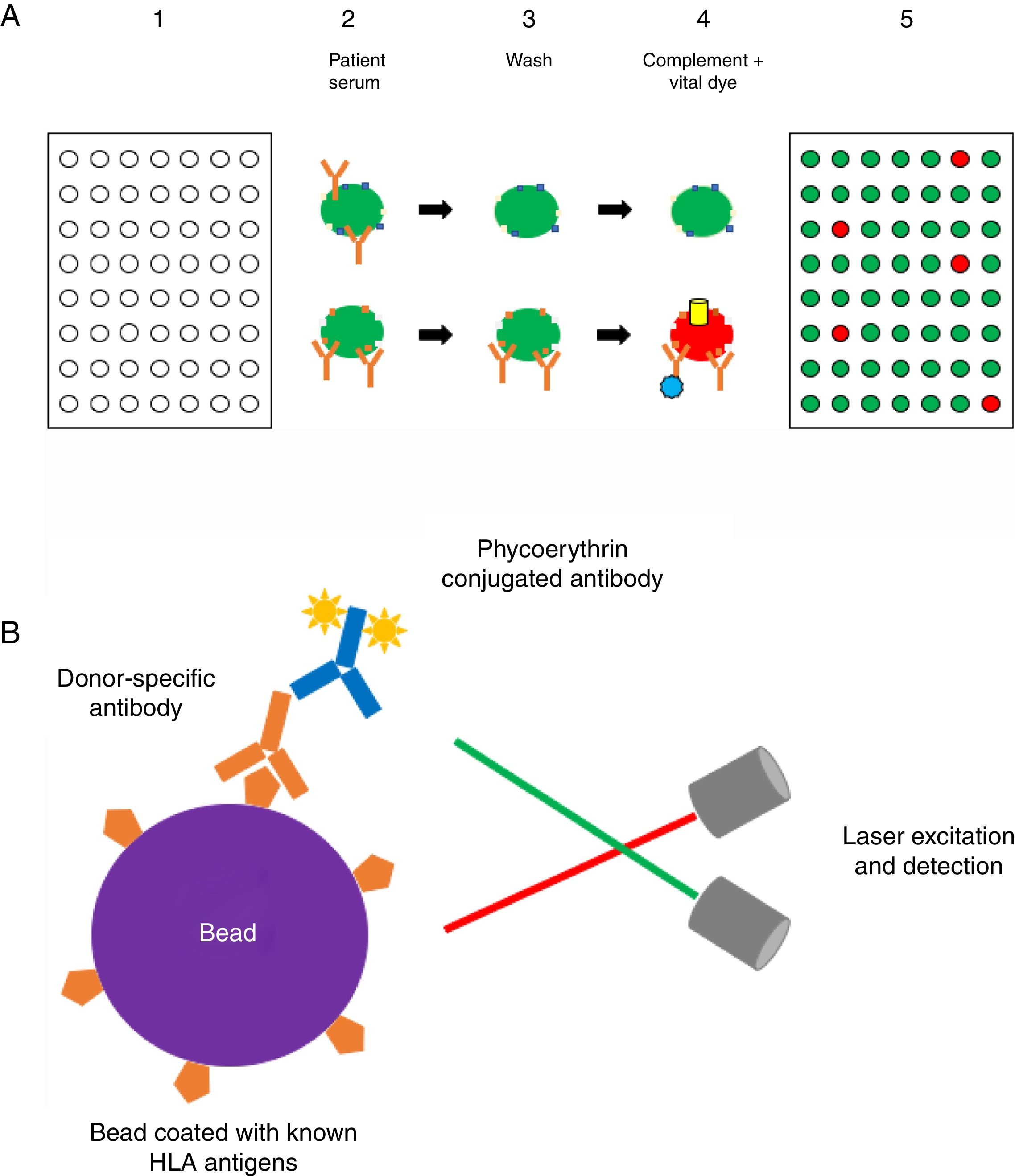
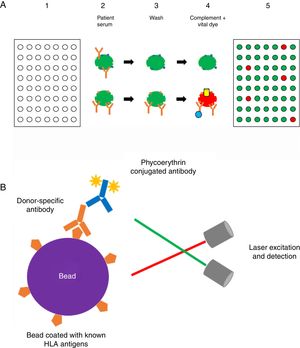
Methods to detect anti-HLA antibodies: The complement-dependent cytotoxicity (CDC) assay (Fig. 1a) has been for many years the gold standard method for anti-HLA antibody detection. This cell-based assay involves the binding of antibodies, which are present in the recipient serum, to HLA antigens expressed on the lymphocyte surface and allows the identification of high concentrations of anti-HLA antibodies. The serum is incubated with T and/or B cells from a HLA-typed panel (meant to be representative of the HLA antigen distribution in the same population from whom deceased donors may be selected) to allow formation of an immune complex which, after complement addition, results in cell lysis.8,32,33 Lysed cells are stained by a fluorescent vital dye to discriminate positive and negative reactions. The result is then expressed as a panel reactive antibody (PRA) value, defined as the percentage of cells in the panel that give a positive reaction with serum.32
(A) Complement-dependent cytotoxicity (CDC) assay. (1) A 56- (class I HLA) or 26-well (class II HLA) plate contains frozen living cells expressing HLA antigens representative of the HLA antigen distribution in the population of interest. (2) Patient serum is added to each well and donor-specific antibodies (DSA), if present, bind to the corresponding HLA antigens (upper panel: serum without DSA; lower panel: serum with DSA). (3) Non donor-specific antibodies are washed away, whereas DSA remain attached to their complementary HLA antigens. (4) Complement (blue star) and vital fluorescent dye are added to the wells, resulting in cell lysis through a membrane attack complex (yellow cylinder)-dependent mechanism only in patients with DSA; lysed cells are stained by the dye (viable cell in green, lysed cell in red). (5) The plate is finally read with a fluorescence microscope: in each well, the cell population is described as viable or dead; and the result is expressed as a panel reactive antibody (PRA) value, defined as the percentage of cells/wells in the panel that give a positive reaction (in our example, 5/56=9%). (B) Solid-phase assay (SPA). Fluorescent beads (each bead has a specific known color) coated with known HLA antigens are incubated with patient serum. If present, HLA-specific antibodies bind to the HLA antigens on the beads. The red laser excites the fluorochrome within the beads and classifies the bead, while the green laser detects the fluorescence signal of phycoerythrin conjugated to the secondary antibody. The combination of the two signals defines antibody specificity. Data are acquired, processed and analyzed by the Luminex platform and fluorescence intensity is expressed as mean fluorescence intensity (MFI).
Solid-phase assays (SPA) include ELISA and flow technology. As the former technology is now abandoned in most of transplant immunology centers, we will focus here on the latter. The Luminex® technology (Fig. 1b) is a high-throughput platform for DSA detection consisting in a flow-based bead assay. Antibody screening is performed with a set of polystyrene microparticles or beads containing different fluorochromes, coated with specific or different purified HLA antigens. The recipient serum is added to the bead mix and anti-HLA antibodies, if present, bind to specific antigen coated on the beads. A secondary phycoerythrin-conjugated anti-human IgG antibody (PE) is then added, which binds to the primary anti-HLA antibody. The Luminex® analyzer is a flow cytometer with an excitation system comprising two solid-state lasers: the red classification laser excites the fluorochromes in beads, while the green reporter laser excites the fluorescence of the PE molecules bound to anti-HLA antibodies on each bead. The combination of the two signals defines antibody specificity and is expressed as a median fluorescence intensity (MFI) bead value.32–34 Class I and class II HLA antibody can be easily distinguished by using class-specific beads, and by manufacturer design, isotype detection can be limited to IgG. Precise specificities may be determined by using beads that each binds only one unique HLA antigen.35
Liver transplantation: a unique immunological scenario of solid organ transplantationThe liver exhibits intrinsic immunoregulatory properties and responds differently than other organs to rejection and immune-mediated injuries.36–38 Major differences between liver and other solid organ allografts may contribute to create a microenvironment with a unique potential to develop spontaneous and induced tolerance in the context of transplantation. First of all, the liver has a dual, portal and arterial, afferent blood supply and has a greater mass.30,37,20 Portal venous blood is rich in food- and potentially drug-derived antigens and in bacterial intestinal degradation products such as endotoxin. Constant exposure to these microbial products leads to a phenomenon referred to as endotoxin tolerance39 and fosters a tolerogenic microenvironment with relatively low co-stimulatory and MHC class II expression on antigen-presenting cells (APC). Sinusoids are the majority microvasculature, lined by liver sinusoidal endothelial cells (LSEC) and Küpffer cells (KC), which scavenge particles and antigens, and regulates immune responses, liver regeneration and fibrogenesis.37 Moreover, the liver has tremendous parenchymal regenerative abilities which can reverse fibrosis after elimination of immune injury. Finally, there are a variety of immune leukocytes as normal hepatic inhabitants, as well as hematopoietic stem cells.
Human liver allografts have been considered to be more resilient to antibody-mediated damage compared to heart or kidney allografts.40 To specifically explain this relative resistance to humoral responses, in addition to the unique liver characteristics raised earlier, it was suggested that liver allografts were able to release soluble class I MHC antigens into the recipient circulation and that these antigens could form immune complexes with anti-class I DSA which would eventually be absorbed and cleared by KC.41 These cells were also suspected to exert other effects that could enhance the hepatic resistance to AMR, such as phagocytosis of activated platelets and complement components.42 Furthermore, as mentioned above, the liver exhibits a limited distribution of HLA class II expression under stable conditions. Other mechanisms (e.g. increase in Foxp3+ CD25+ CD4+ regulatory T cells) have been proposed more recently to explain the switch from immunogenicity to immune tolerance in the context of liver transplantation, but going into further details is beyond the scope of this review. However, significant sensitization can exceed the liver defenses against antibodies, as demonstrated in experimental rat models.43 Hence, the “two-hit hypothesis” has been proposed by certain authors.44 According to this theory, in the presence of DSA (first hit), a liver injury such as a viral illness or a lower donor allograft quality (second hit) could upregulate HLA class II antigens on the microvascular endothelium, facilitate anti-class II DSA binding and downstream complement cascade activation.
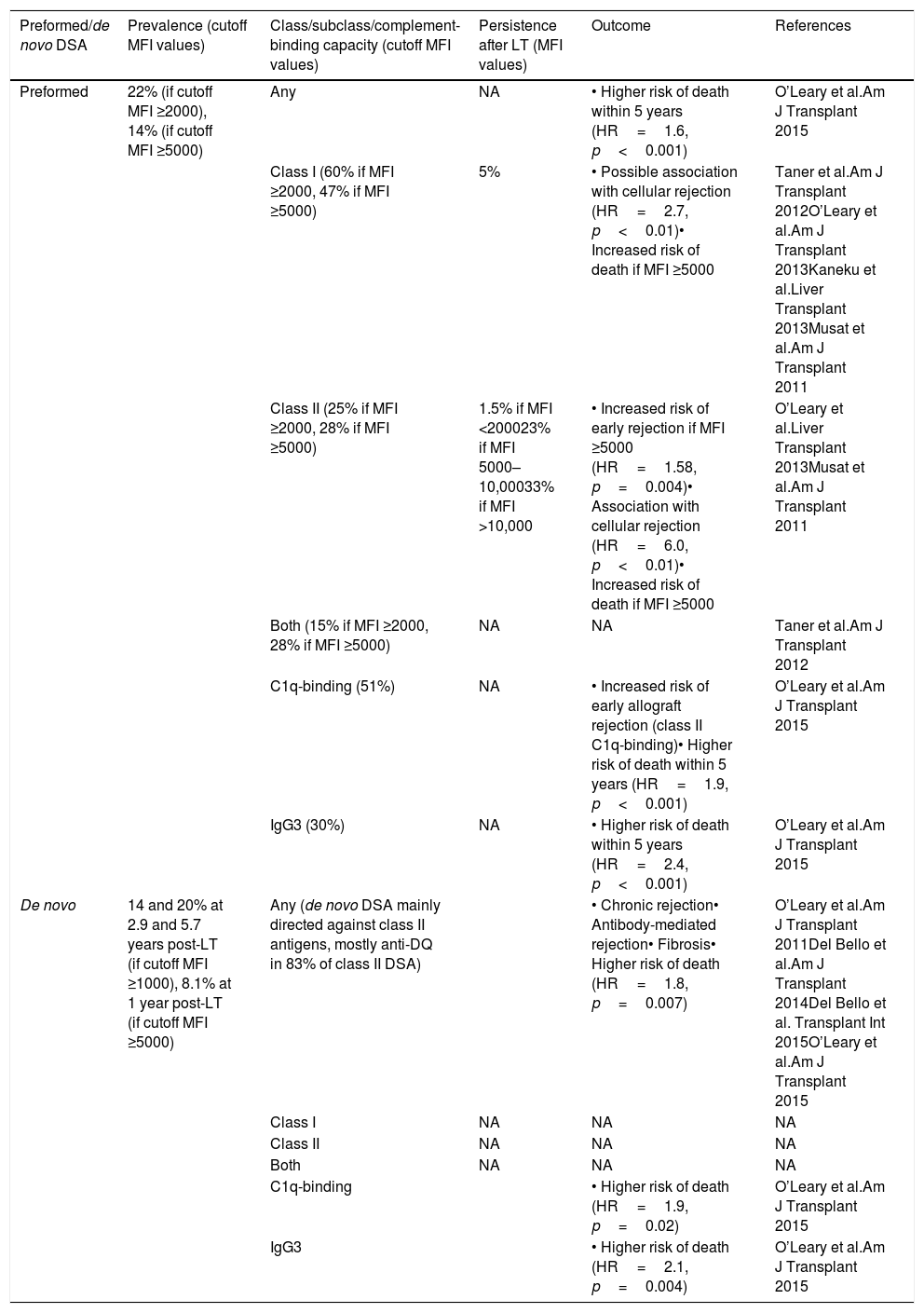
Preformed donor-specific antibodies in liver transplantation (Table 1)Regarding the prevalence of preformed DSA, Taner et al. reported that, out of 90 consecutive LT recipients, 22% of patients had DSA with a MFI ≥2000 (60% against class I HLA antigens, 25% against class II, 15% against both class I and II).45 Patients with DSA were more likely to be female (female 80% vs. male 40%, p=0.002), to have undergone re-transplantation (re-transplantation 25% vs. first LT 5%, p=0.04) and with a history of previous blood transfusions (positive history 70% vs. negative history 41%, p=0.04). Among these immunized patients, 80% had a positive crossmatch at the time of transplantation.
Prevalence and outcomes of preformed and de novo donor-specific antibodies.
| Preformed/de novo DSA | Prevalence (cutoff MFI values) | Class/subclass/complement-binding capacity (cutoff MFI values) | Persistence after LT (MFI values) | Outcome | References |
|---|---|---|---|---|---|
| Preformed | 22% (if cutoff MFI ≥2000), 14% (if cutoff MFI ≥5000) | Any | NA | • Higher risk of death within 5 years (HR=1.6, p<0.001) | O’Leary et al.Am J Transplant 2015 |
| Class I (60% if MFI ≥2000, 47% if MFI ≥5000) | 5% | • Possible association with cellular rejection (HR=2.7, p<0.01)• Increased risk of death if MFI ≥5000 | Taner et al.Am J Transplant 2012O’Leary et al.Am J Transplant 2013Kaneku et al.Liver Transplant 2013Musat et al.Am J Transplant 2011 | ||
| Class II (25% if MFI ≥2000, 28% if MFI ≥5000) | 1.5% if MFI <200023% if MFI 5000–10,00033% if MFI >10,000 | • Increased risk of early rejection if MFI ≥5000 (HR=1.58, p=0.004)• Association with cellular rejection (HR=6.0, p<0.01)• Increased risk of death if MFI ≥5000 | O’Leary et al.Liver Transplant 2013Musat et al.Am J Transplant 2011 | ||
| Both (15% if MFI ≥2000, 28% if MFI ≥5000) | NA | NA | Taner et al.Am J Transplant 2012 | ||
| C1q-binding (51%) | NA | • Increased risk of early allograft rejection (class II C1q-binding)• Higher risk of death within 5 years (HR=1.9, p<0.001) | O’Leary et al.Am J Transplant 2015 | ||
| IgG3 (30%) | NA | • Higher risk of death within 5 years (HR=2.4, p<0.001) | O’Leary et al.Am J Transplant 2015 | ||
| De novo | 14 and 20% at 2.9 and 5.7 years post-LT (if cutoff MFI ≥1000), 8.1% at 1 year post-LT (if cutoff MFI ≥5000) | Any (de novo DSA mainly directed against class II antigens, mostly anti-DQ in 83% of class II DSA) | • Chronic rejection• Antibody-mediated rejection• Fibrosis• Higher risk of death (HR=1.8, p=0.007) | O’Leary et al.Am J Transplant 2011Del Bello et al.Am J Transplant 2014Del Bello et al. Transplant Int 2015O’Leary et al.Am J Transplant 2015 | |
| Class I | NA | NA | NA | ||
| Class II | NA | NA | NA | ||
| Both | NA | NA | NA | ||
| C1q-binding | • Higher risk of death (HR=1.9, p=0.02) | O’Leary et al.Am J Transplant 2015 | |||
| IgG3 | • Higher risk of death (HR=2.1, p=0.004) | O’Leary et al.Am J Transplant 2015 |
DSA, donor-specific antibodies; HR, hazard ratio; LT, liver transplantation; MFI, mean fluorescence intensity; NA, data not available.
“Any” refers to donor-specific antibodies as a whole, i.e. directed indistinctly against either class I or II HLA antigens. “Both” refers to donor-specific antibodies directed against both class I and II HLA antigens in the same patient.
In a retrospective study of 1270 LT patients with prospectively stored serum samples, O’Leary et al. found that 14% of the patients had class I and/or class II DSA with MFI ≥5000 (6.6% against class I HLA antigens, 3.9% against class II, 3.9% against both class I and II).46 Preformed class I DSA were found to persist only in 5% of the cases and there was no association with rejection, whereas preformed class II DSA persisted in 23% and 33% of the cases, whether the MFI was comprised between 5000 and 10,000 or superior to 10,000 respectively. Of note, when preformed class II DSA were lower than 2000, there was a 1.5% persistence after LT. Patients with DSA were more likely to be female with an auto-immune disease, to have a high pre-transplant MELD score and to have received no immunosuppressive induction treatment.46
With regard to the outcomes, many recent studies have concluded that DSA are associated with an increased risk of graft loss after LT.46–51 O’Leary et al. described that AMR was often combined with acute cellular rejection and that it could be the cause of early graft loss in approximatively 10% of the cases.49 Moreover, preformed class II DSA with a MFI ≥5000 were associated with increased risk of early rejection (i.e. during the 6 first months post-LT; HR=1.58, p=0.004) while both class I and II preformed DSA with a MFI ≥5000 were independently correlated with a greater risk of death (HR=1.51, p=0.02).46 In contrast, preformed DSA with low MFI (1000–5000) were not related to an increased risk of rejection. Other studies reported a significant association between preformed class I DSA and acute cellular rejection, with an HR of 2.7 and 6, respectively.48 Of note, the authors also described in a previous study that both DSA and C4d positive staining were present in 54% of acute cellular rejection episodes.52 Finally, the association between preformed DSA and patient survival is linked to the immunoglobulin subclass and their ability to bind complement. Compared to DSA negative patients, the highest risk of death was related to IgG3 DSA (HR=2.4, p<0.001) whereas C1q-binding DSA and standard DSA also had a significant increased risk of death (HR 1.9 and 1.6, respectively). Preformed C1q-binding class II DSA had a strongest correlation with early allograft rejection (HR 1.7, p<0.03). Importantly, up to 30% of preformed DSA were IgG3 and that 51% of them were C1q-binding.50 In contrast with these results, Taner et al. reported no difference in patient or graft survival between LT recipients with or without DSA.45 This finding was also described in another study by Kim et al. in the setting of living donor LT.53 Of note, as a phenomenon already described above, DSA at 1 week post-LT showed an important drop of MFI in 90% of the patients and 75% of positive crossmatches became negative by 2 weeks after LT.45,47
De novo donor-specific antibodies in liver transplantation: incidence, risk factors and consequences (Table 1)The observed incidence of de novo DSA after LT depends on the MFI threshold and the study design. Using a MFI ≥1000, in two large studies, the incidence of de novo DSA ranged from 14% to 20% at 2.9 and 5.7 years after LT respectively.54,55 In contrast, using a cutoff MFI ≥5000, the incidence of novo DSA at 1 year after LT was only 8.1%. Interestingly, the probability of presenting de novo DSA after LT was not linear, being lower within 6 months after LT56 and rising up to 40% at 17 years after LT.54 Some of these de novo DSA disappeared shortly after their detection, especially those with low MFI.56 This time-dependent and non-persisting pattern partly explains the discordant prevalence of de novo DSA in cross-sectional studies. While some studies reported a 6.1% prevalence at 3–12 months,51 others showed a 17.8% prevalence at 77 months after LT57 or up to 21.5% during the entire post-transplant follow-up.58 More than 25% of patients developed at least one DSA after LT and these DSAs were predominantly directed against class II HLA and targeted the DQ locus (85%).59
Reported risk factors for the development of de novo DSA included a low MELD (<15) at transplantation, previous transplant, younger age (<60 years) and a lesser degree of immunosuppression.54,56 A cyclosporine-based immunosuppression (instead of tacrolimus), the non-compliance to immunosuppression, a low-dose of calcineurin inhibitor (CNI) treatment (tacrolimus trough level <5ng/ml) increased by more than twofold the risk of developing DSA.55,56 Recently, the number of class II HLA epitope/eplet mismatch between donor and recipients and high tacrolimus intra-patient variability have been described as additional risk factors for de novo DSA development.56,60 The role of mammalian target of rapamycin inhibitors on the risk of de novo DSA is controversial.55,61
The development of de novo DSA is likely to increase the risk of rejection after LT but the magnitude of this risk is yet to be ascertained. Del Bello et al. reported a high risk of rejection in a small cohort of patients (52%; 11/21) who developed de novo DSA within 3 years after LT as compared to those without de novo DSA (23%; 30/131). In those with positive de novo DSA, 78% (7/9) of the episodes of rejection consisted were, i.e. combining T-cell and antibody-mediated features, while only two patients developed a pure acute AMR.55 Some authors have reported that LT recipients presenting with acute allograft dysfunction due to biopsy-proven T cell-mediated rejection (TCMR) often (up to 50%) have positive DSA and that strong class II DSA (mean fluorescence intensity ≥10,000) was associated with steroid-resistant rejection, as well as increased rejection severity and risk of chronic rejection with ductopenia.62,48,63
Importantly, in a large single center study conducted by O’Leary et al., de novo DSA led to a higher risk of death (1.8-fold) and the development of IgG3 subclass DSA was associated with a twofold increased risk of death.50
Acute AMR, clinical manifestations, histological findings, potential treatment optionsAcute AMR after LT was first described in ABO-incompatible LT. These recipients developed microvasculitis, focal fibrinoid necrosis, and platelet–fibrin thrombi 2–6h post-transplantation, leading to early allograft failure.16 Acute AMR after ABO-compatible LT is however an uncommon phenomenon with an estimated incidence between 0.5% and 6% in LT.64 It usually presents within the first 3 months after LT in highly sensitized recipients with graft dysfunction with hyperbilirubinemia, elevation of transaminases, occasionally fever, thrombocytopenia, decreased serum complement levels and persistence of DSA (especially against class II HLA).20,55,48,65–67 O’Leary et al. reported a 10% incidence of acute AMR in patients with preformed DSAs, especially in those with preformed C1q-fixing class-II DSA.46 These LT recipients exhibited a 70% increased risk of early rejection compared to the 40% increased risk in recipients with non-complement-binding DSA.46 Importantly, acute AMR was considered to be the primary cause of allograft failure in 5% of cases and was suspected to have contributed to it in another 5%. The development of IgG3 subclass DSA presented a threefold increased risk of graft loss.68
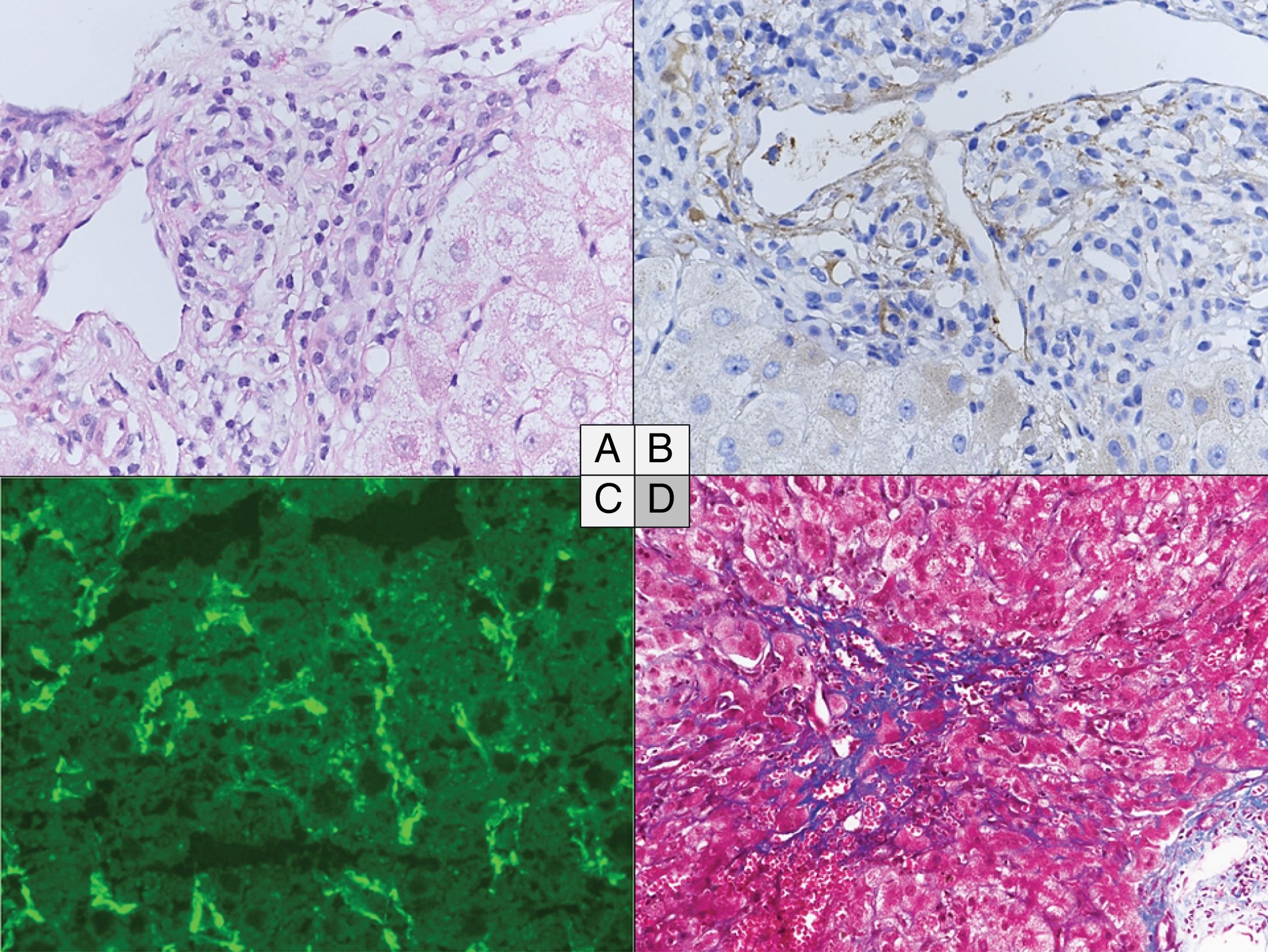
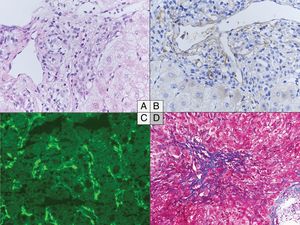
The main histological characteristics of acute AMR after LT have been described by many authors, summarized in the 2016 Banff consensus and illustrated in Fig. 216,37,20,48,65–67:
- a)
Portal changes: A majority of portal tracts with endothelial hypertrophy/enlargement if portal veins, capillaries, and inlet venules, portal vein with cytoplasmic eosinophilia, occasional platelet aggregates and leukocyte (macrophages, neutrophils, eosinophils) margination involving the portal vein branches. Portal and peribiliary plexus capillaries extending into sinusoids, periportal edema, and ductular reaction are other features. These changes are associated with diffuse portal microvasculature C4d staining, including more than 50% of portal tracts. Lymphocytic intimal inflammation and necrotizing arteritis are rare but also diagnostic of acute AMR when combined with diffuse C4d deposits. Importantly, detection of microvascular complement deposition with C4d staining alone is not specific of AMR.
- b)
Central zone: Central vein with occasional platelet aggregates, endothelial cell enlargement/hypertrophy, and “microvasculitis” with centrilobular hepatocellular swelling can be observed.
- c)
Lobular changes: Sinusoidal C4d deposition with hepatocanalicular cholestasis and hepatocyte apoptosis.
Histological changes in acute (A to C) and chronic (D) antibody-mediated rejection of the liver allograft. (A–C) Acute antibody-mediated rejection with mild to moderate hypertrophy of the endothelials cells of the portal microvessels and monocyte margination (A) and diffuse C4d positivity both on immunoperoxydase (B) and immunofluorescence (C). (D) Perivenular fibrosis in the centrolobular vein area in the chronic antibody-mediated rejection. (A) Hematoxylin and eosin, original magnification 40×. (B) C4d immunochemistry, original magnification 40×. (C) C4d immunofluorescence, original magnification 40×. (D) Masson's Trichrome, original magnification 20×.
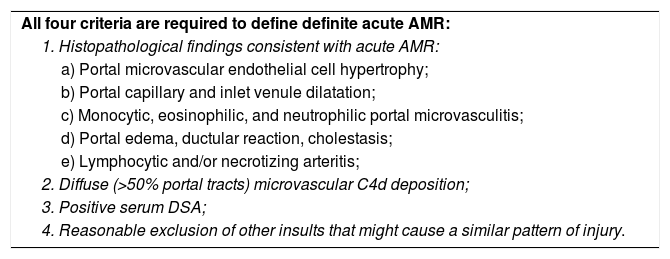
According to the 2016 Comprehensive Banff Update,20 the diagnostics of acute AMR (Table 2) are based on: (i) presence of detectable DSA in the serum, (ii) compatible findings from hematoxylin/eosin stained liver biopsy, (iii) positive C4d stain, and (iv) the exclusion of other causes of similar types of injury. These findings may coexist with TCMR.69
Criteria for acute AMR in ABO-compatible liver allografts.
| All four criteria are required to define definite acute AMR: |
| 1. Histopathological findings consistent with acute AMR: |
| a) Portal microvascular endothelial cell hypertrophy; |
| b) Portal capillary and inlet venule dilatation; |
| c) Monocytic, eosinophilic, and neutrophilic portal microvasculitis; |
| d) Portal edema, ductular reaction, cholestasis; |
| e) Lymphocytic and/or necrotizing arteritis; |
| 2. Diffuse (>50% portal tracts) microvascular C4d deposition; |
| 3. Positive serum DSA; |
| 4. Reasonable exclusion of other insults that might cause a similar pattern of injury. |
Since the pathophysiology of AMR is partially known, the optimal treatment for acute AMR in adult ABO-compatible LT remains unclear. The utilization of T and B cell targeting agents has been proposed despite the limited experience on its use after LT. Different treatment regimens combining steroid pulses, plasmapheresis with polyclonal immunoglobulin infusion, rituximab, bortezomib and thymoglobulin have been attempted to treat acute AMR.47,55,70–72 Recently, treatment with eculizumab, a humanised monoclonal antibody binding complement C5 protein and blocking consecutively terminal complement pathway, has also been reported to be efficient in acute AMR.73,74 Nevertheless, we want to underline the fact that none of these strategies have proven any benefit over the others in the LT setting and that their clinical impact is still to be determined. Moreover, the risk of severe adverse events, mainly opportunistic infections, should always be balanced with the potential benefits of the treatment. More work is needed to determine the efficacy of these new therapeutic options in acute AMR in LT.
Chronic AMR and other manifestations associated with donor-specific antibodiesChronic liver allograft AMR lacks a typical clinical or biochemical presentation. In fact, many histopathological features potentially associated with chronic AMR have been described in protocol liver biopsies of clinically stable recipients with normal liver tests. The identification of specific lesions of chronic AMR is slowly emerging from studies including liver biopsies of long-term follow-up of pediatric recipients, and recipients suboptimally immunosuppressed or off immunosuppression.75 The common histological findings of chronic AMR after LT correlate with those seen in chronic AMR after kidney transplantation: vascular inflammation (portal and perivenular in the liver), tissue damage (interface activity in the liver) and fibrosis with C4d deposits, being less prominent after LT compared to kidney transplantation. According to the 2016 Comprehensive Banff Update,20 the diagnostics of chronic AMR are based on histological findings with less emphasis on C4d deposition, circulating DSA, and exclusion of other causes of liver injury (Table 3). Low-grade portal, periportal, and perivenular lymphoplasmacytic inflammation, with low-grade interface and perivenular necro-inflammatory activity and non-inflammatory fibrosis, are the main features of chronic AMR according to this consensus document (Fig. 2). However, the diagnosis may be difficult because the deposition of complement is weak and the histological criteria may overlap with those described in cases of late TCMR. Indeed, in a recent study employing both histopathology and gene expression which included surveillance liver biopsies from stable pediatric liver transplant recipients exhibiting portal inflammaton, interface activity and fibrosis, the transcriptional profiles observed were indistinguishable from those typically detected at the time of conventional TCMR.76 This is in contrast to what has been observed in the setting of chronic AMR in kidney transplantation, were the presence of circulating DSA is clearly associated with a distinct transcriptional pattern.77 Finally, it should be emphasized that, while the 2016 Comprehensive Banff Update criteria for acute AMR allow to obtain three levels of diagnosis certitude (definite, suspicious and indeterminate), the criteria for chronic active liver allograft AMR allow to obtain only two levels of diagnosis certitude (probable or possible). In this context, especially in chronic AMR, there remains an unmet need for more robust and unequivocal diagnostic criteria for AMR in LT.
Criteria for chronic AMR in ABO-compatible liver allografts.
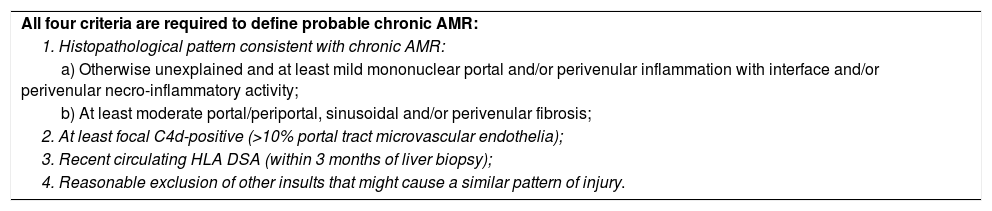
| All four criteria are required to define probable chronic AMR: |
| 1. Histopathological pattern consistent with chronic AMR: |
| a) Otherwise unexplained and at least mild mononuclear portal and/or perivenular inflammation with interface and/or perivenular necro-inflammatory activity; |
| b) At least moderate portal/periportal, sinusoidal and/or perivenular fibrosis; |
| 2. At least focal C4d-positive (>10% portal tract microvascular endothelia); |
| 3. Recent circulating HLA DSA (within 3 months of liver biopsy); |
| 4. Reasonable exclusion of other insults that might cause a similar pattern of injury. |
Possible chronic active AMR: as above, but C4d staining is minimal or absent.
Adapted from the 2016 Comprehensive Update of the Banff Working Group on Liver Allograft Pathology.20
Importantly, the presence of DSAs has also been associated with liver allograft fibrosis of unknown origin on the long-term follow-up, especially in pediatric LT recipients78 but also in adults.55,79–81 To address the fact that existing scores (such as METAVIR and Ishak scores) were not originally developed to assess transplanted livers and are focused exclusively on portal tract fibrosis, Venturi et al. proposed a novel liver allograft fibrosis score, called liver allograft fibrosis semi-quantitative scoring system (LAFSc) and which evaluates separately portal, sinusoidal and centrolobular areas.82 This score was found to reflect more accurately liver allograft fibrosis than METAVIR and Ishak and its use waw recommended in the 2016 Comprehensive Banff Update.20 Finally, a recent study by Dao et al. confirmed in particular that perivenular fibrosis (but also portal inflammation) in late pediatric liver allograft biopsies was a feature of chronic AMR.83
Other potential manifestations of DSA-mediated damage are anastomotic biliary strictures84 or other biliary complications,85 nodular regenerative hyperplasia with/without obliterative arteriopathy,30 and chronic idiopathic hepatitis.86 The management of chronic AMR is yet to be elucidated, but optimization of CNI-based immunosuppression has been proposed.
DSA in immunosuppression withdrawal trialsThe potential relevance of DSA monitoring during or after immunosuppression withdrawal was first proposed by Girnita et al. in 2010.87 Employing an ELISA, the authors observed no DSA in 19 successfully weaned LT recipients, as compared to recipients under minimal or normal immunosuppression. These results were not confirmed by Feng et al. in a prospective multicentre drug withdrawal trial in living-donor LT pediatric recipients (WISP-R).88 In this study, neither HLA mismatch nor presence of DSA (assessed by single antigen bead assays) were associated with the outcome of immunosuppression withdrawal. In the 5-year histological and serological follow-up of the 12 operationally tolerant patients, no cases of chronic rejection, graft loss or death were reported, with no increase in inflammation nor fibrosis as well.89 Nevertheless, 3 subjects showed intermittent de novo class I DSA, 4 subjects showed persistent de novo class II DSA and 5 subjects showed persistent preformed class II DSA. Class II DSA was predominantly against donor DQ antigens, often of high MFI and capable of C1q-binding, but rarely of the IgG3 subclass. The absence of association between DSA (detected by ELISA, complement-dependent cytotoxicity or flow cytometry) and immunosuppression withdrawal outcome was also pointed out in the study by Benítez et al.90 On the other hand, in a Japanese cross-sectional study of 81 pediatric living-donor LT recipients, anti-HLA-DRB1 DSA (detected by single antigen bead assay), as well as anti-angiotensin II type 1 receptor antibodies, were found to be associated with the presence of long-term progressive graft fibrosis.80
In summary, DSA are a reflection of donor sentitization and could therefore represent a potential barrier for the establishment of tolerance. However, in the setting of LT, as it has been shown that not all DSA seem to be deleterious, this finding is difficult to interpret in the context of the prediction of spontaneous operational tolerance. Currently, the presence of preformed or de novo DSA is not an exclusion criteria in recent or ongoing immunosuppression withdrawal trials in LT (LIFT NCT02498977, iWITH NCT01638559 and OPTIMAL NCT02533180). These prospective immunosuppression withdrawal trials in pediatric (iWITH) and adult (LIFT, OPTIMAL) LT recipients are expected to give information on the precise role of DSA in LT of paramount importance in this field.
Future developmentsThanks to the recent development of tissue HLA-typing techniques, innovative concepts, such as HLA-matching at the epitope level, have emerged.91,92 Each HLA allele represents a series of epitopes that can be classified by eplets, i.e. small consecutive or non-consecutive arrangements of polymorphic amino acid configurations on the HLA molecular surface, to which anti-HLA antibodies could be directed.93 Eplet structure, position and conformation will eventually determine antibody accessibility, recognition and reactivity.92 HLAMatchmaker, a computer algorithm able to determine HLA compatibility between donors and recipients by assessing the 3-dimensional molecular design of the epitope–paratope interface, can calculate the number of eplet mismatches for each donor–recipient pair, using typically a high-resolution 4-digit HLA typing.91 A growing body of evidence demonstrates that HLA locus-specific eplet mismatches are associated with the development of DSA and adverse outcomes in kidney,94 but also in lung and heart transplantation.11,95 Recently, the same associations have been described in LT, in particular de novo DSA formation and rejection, as well as portal fibrosis.96,97 Further studies are awaited in order to define the utility of eplet mismatch analysis in stratifying the immunological risk of LT patients.
ConclusionsDSA play a relevant role in the alloimmune responses after LT, yet the underlying mechanisms are to be elucidated. Current data support that preformed DSA increase the risk of early rejection and that de novo DSA are associated with a higher risk of rejection, acute or chronic AMR, and probably mixed rejection. Moreover, DSA are associated with long-term allograft fibrosis and higher risk of short- and long-term allograft loss and death after LT. The optimal periodicity for DSA monitoring, the interpretation of MFI levels and the optimal strategy for the patients with positive DSA are currently unsolved questions for the management of our patients. Because the evidence for the possible benefits of measuring DSA in liver transplantation remains small, further studies are needed before an anti-HLA antibody systematic screening can be scientifically justified. However, in high immunological risk situations (such as history of pregnancy, history of previous blood transfusions, history of previous transplantation) and in patients with graft failure of unknown origin, anti-HLA antibodies assessment by single-bead antigen assay, wherever available, should be recommended.
FundingJV is supported by Swiss National Science Foundation (SNSF) grant P2LAP3_181318. JC is supported by Carlos III Institute of Health (ISCIII, Ministry of Economy and Competitiveness, Spain) grant PI18/01125 and by Agència de Gestió d’Ajuts Universitaris i de Recerca (AGAUR, grant SGR 2017). These funding bodies had no role in the design and writing of the manuscript.
Conflict of interestThe authors have no competing interests to declare.