Hepatitis C virus (HCV) infection is a major health problem in Europe and the Mediterranean countries in particular, where prevalence rates range from 0.31 to 0.42%.
Patients with chronic HCV infection have a higher prevalence of tuberculosis (TB) compared to the general population. The epidemiological context of both diseases is similar in certain cases, such as in people suffering from intravenous drug addiction, prisoners and the homeless. HCV treatment with peginterferon and ribavirin was previously associated with a greater risk of TB reactivation,1,2 but experience relating to the reactivation of infections with new direct-acting antivirals (DAAs) is limited.
We present the case of a 59-year-old man who was born in Guinea and has been living in Spain since the age of 10. He presented with liver cirrhosis (LC) due to hepatitis B virus (HBeAg negative, HBV DNA 1753IU/ml) and HCV (genotype 2a, HCV RNA 2470203IU/ml); Child–Pugh A (5 points); negative HIV antibodies; a FibroScan® score of 69.1kPa; and no oesophageal varices. He also had grade 1 ascites a few months prior, which resolved after diuretic therapy and a low-sodium diet. Since there was only a small amount of ascitic fluid, it was not possible to obtain a sample for analysis. The patient started entecavir at the beginning of December 2015. In mid-December, he began treatment with sofosbuvir and ribavirin.
He was admitted to hospital in February 2016 with a one-month history of asthenia, anorexia, 10kg weight loss and febrile episodes of up to 38.7°C, mainly at night. His only diagnostic sign was persistent constipation in the few weeks prior. The cardiovascular and respiratory examination was unremarkable and his abdomen showed signs of grade 2 ascites. The patient's laboratory tests revealed leucocyte levels of 3850×109, Hb 146g/l; Platelets 213×109, AST/ALT 65/57U/l, bilirubin 0.8mg/dl; INR 1.1. ESR 52mm/h, CRP 7.9mg/dl, CA-125 115.6U/ml; HCV RNA undetectable and HBV DNA <20IU/ml. A chest X-ray showed minimal right-sided pleural effusion with no consolidation. A series of blood cultures were negative.
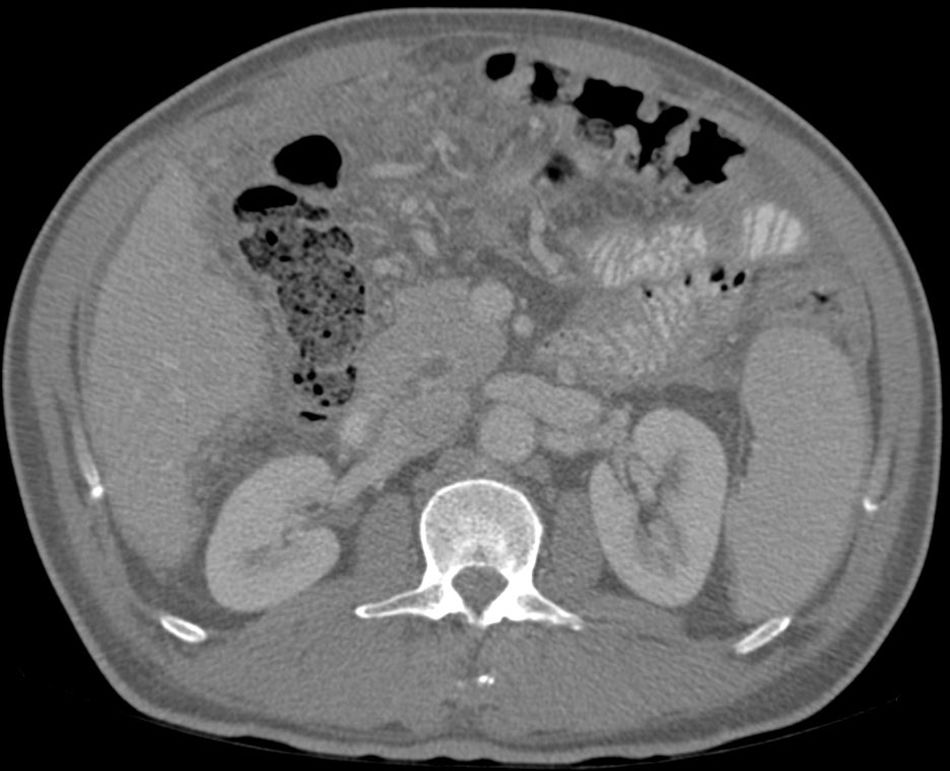
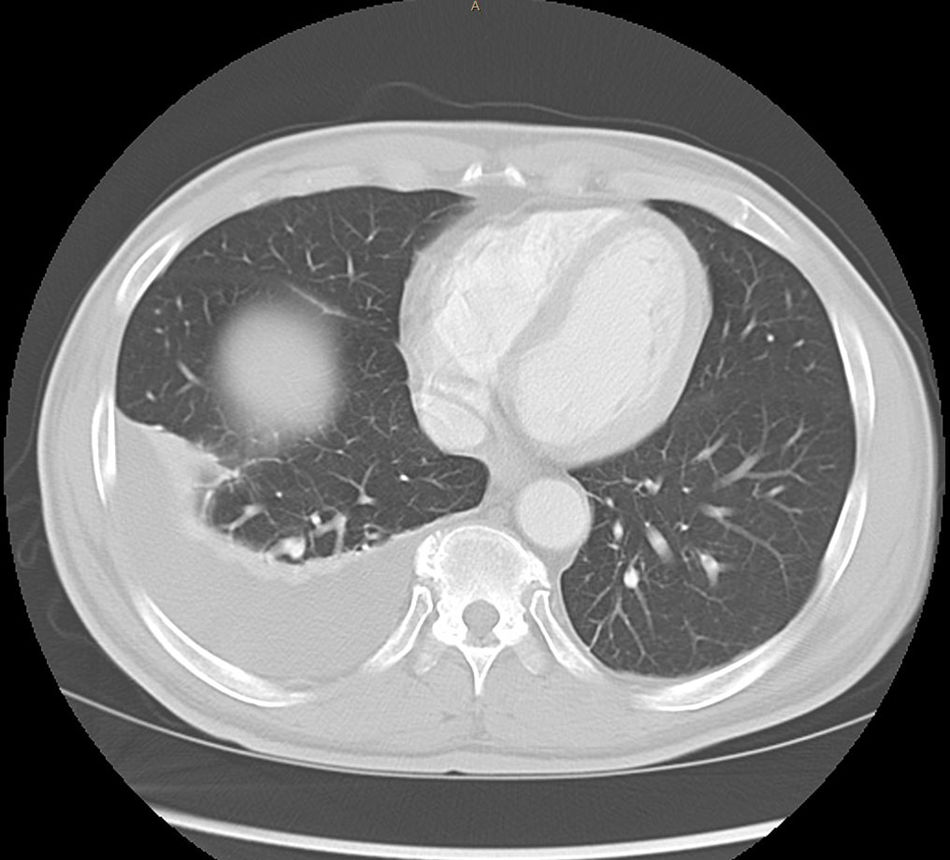
Abdominal paracentesis was carried out, removing 3.5l of ascitic fluid with 400leukocytes/mm3 (95% lymphocytes). Proteins, albumin and adenosine deaminase (ADA) were not determined at any time due to suspected decompensated LC. A CT scan of the chest and abdomen was performed, revealing multiple 2–3mm subpleural nodules, free perihepatic and perisplenic fluid, as well as peritoneal thickening and a diffuse increase in mesenteric density. Right-sided pleural effusion (Figs. 1 and 2).
Thoracentesis was also performed, which showed pleural fluid with protein levels of 58g/l, leukocytes of 1325/mm3 (99% lymphocytes), ADA of 42.3U/l (upper limit of normal) and pleural fluid CRP positive for Mycobacterium tuberculosis. QuantiFERON-TB® >4IU/ml.
Given the above results, the case was deemed a reactivation of abdominal and pleural TB in the context of DAA treatment in a patient with cirrhosis due to HBV and HCV.
DAA treatment was stopped on 18/02/2016 and anti-TB therapy initiated, with rifampicin (RIF), isoniazid (INH) and ethambutol (for two months) plus four further months of RIF+INH. The patient made good clinical progress thereafter, with his fever disappearing and toxic symptoms and constipation resolving (normal outpatient colonoscopy). The ascites also disappeared without diuretic treatment.
Regarding the HCV infection, he had a sustained virologic response despite only receiving 12 weeks of treatment. A CT scan of the chest and abdomen six months after finishing anti-TB therapy showed that the right-sided pleural effusion of peritoneal fluid and peritoneal fat thickening had resolved, and no pulmonary micronodules were observed.
Reactivations of infections following the use of DAAs are uncommon, although cases are described in relation to the HBV and herpes simplex virus (HSV), among other infections.3–5 Reported cases of TB reactivation are rare and in relation to dual therapy with interferon and ribavirin or triple therapy with the addition of boceprevir or telaprevir. To date, only one case of miliary TB reactivation has been reported during DAA treatment. Said patient had received treatment with sofosbuvir/ledipasvir and ribavirin.6 There are various factors which may have led to TB reactivation in the patient, the first being underlying cirrhosis, which has been linked to dysfunction of the neutrophils, lymphocytes and macrophages and to decreased IFN-α and TNF-α production.7–9 Conversely, the immune system disorders caused by DAAs are not fully understood. Various studies show reduced lymphocyte activation and normalised natural killer cell function.10–13 HSV and HBV reactivations have been reported, as well as a potential increase in hepatocellular carcinoma recurrences in patients treated with DAAs. As in the case described above, establishing a causal relationship with DAA treatment is difficult and the timing of the two infections could simply be a coincidence. This could potentially be the second case of TB reactivation during DAA treatment. Given the low incidence of reported cases of TB reactivation with DAAs to date, we cannot make recommendations in favour of patients being screened prior to beginning treatment.
Please cite this article as: Pedrosa M, Nogales S, Vergara M, Miquel M, Casas M, Dalmau B, et al. Reactivación de tuberculosis peritoneal y pleural durante el tratamiento de la hepatitis C con antivirales de acción directa. Gastroenterol Hepatol. 2019;42:174–175.