Spinal cord injury (SCI) patients may have intestinal dysmotility and digestive symptoms that are associated with small intestinal bacterial overgrowth (SIBO). The aim of this study is to describe the prevalence of SIBO in SCI patients and the risk factors of its development.
MethodsTwenty-nine consecutive SCI patients were studied (10 women/19 men; mean age 47 years), 16 with subacute injuries (<9 months) and 13 with chronic injuries (>1 year). Nine patients were affected by tetraplegia and 15 by paraplegia. Each patient underwent a glucose breath test according to the North American Consensus and the presence of abdominal symptoms was evaluated during the test.
The results were compared with 15 non-neurological patients with SIBO.
ResultsSix patients tested positive for SIBO (21%), all of them affected by SCI in the subacute phase, 6/16 vs 0/13 in the chronic phase (p < 0.05) and the majority with tetraplegia, 5/9 vs 1/19 with paraplegia (p < 0.05). No statistically significant relationship was found with other clinical characteristics. All the tests were positive for methane or mixed (methane and hydrogen), while only 67% of the controls had methane-predominant production. (p > 0.05).
ConclusionSCI patients can develop SIBO, more frequently in the subacute phase and in tetraplegic patients, highlighting a high production of methane. This complication should be considered in neurogenic bowel management.
Los pacientes con lesión medular (LM) pueden presentar alteraciones de la motilidad intestinal y síntomas digestivos que se asocian a sobrecrecimiento bacteriano del intestino delgado (SIBO). El objetivo de este estudio es investigar la prevalencia de SIBO en pacientes con LM, y los factores asociados a su desarrollo.
MétodosSe estudiaron 29 pacientes consecutivos con LM (10 mujeres/19 hombres; edad media 47 años), 16 con lesiones subagudas (<9 meses) y 13 con lesiones crónicas (>1 año). Nueve pacientes estaban afectados de tetraplejia y 15 de paraplejia. A cada paciente se realizó un test del aliento con glucosa de acuerdo al Consenso Norteamericano y se evaluó la presencia de síntomas abdominales durante la prueba.
Los resultados se compararon con los de 15 pacientes con SIBO sin enfermedad neurológica.
ResultadosSeis pacientes fueros positivos para SIBO (21%), todos ellos afectados de LM en fase subaguda, 6/16 vs 0/13 en fase crónica (p < 0,05) y la mayoría afectados de tetraplejia, 5/9 vs 1/19 con paraplejia (p < 0,05). No se encontró relación estadísticamente significativa con otros parámetros clínicos. Todos los test fueron positivos para metano o mixto (metano e hidrógeno); mientras que solo el 67% de los controles tenían producción predominante de metano (p > 0,05).
ConclusiónLos pacientes con LM pueden desarrollar SIBO, siendo más frecuente en fase subaguda y en tetrapléjicos, destacando la alta producción de metano. Esta complicación debe tenerse en cuenta en el manejo del intestino neurógeno.
Small intestinal bacterial overgrowth, known by the acronym SIBO, is characterised by the presence of an excessive number of bacteria in the small intestine that are normally found in the large intestine. Most authors consider a diagnosis of SIBO to require a finding of ≥105 colony-forming units/mL in aspiration from the proximal part of the jejunum.1,2
Patients with SIBO may present gastrointestinal symptoms, and even non-gastrointestinal symptoms, due to the negative effects of the excessive number of bacteria on food digestion.1 These include: bloating, flatulence, abdominal pain, nausea, dyspepsia, fatigue; with regard to stooling, diarrhoea is the most common symptom, although those with methane-dominant SIBO are five times more likely to experience constipation, the severity of which will depend on the methane level.3 Reduced nutrient absorption, weight loss, anaemia and vitamin and iron deficiencies are less common symptoms, but are more severe manifestations of SIBO.4
The pathophysiology of SIBO is complex and is associated with multiple causes: reduced gastric acid production (proton pump inhibitors, chronic gastritis, advanced age), altered intestinal motility (primary visceral neuropathy, scleroderma, anticholinergic drugs, morphine derivatives, narcotics and anti-diarrhoeal agents), anatomical alterations to the intestine (intestinal obstruction, diverticula, fistulas, prior ileocaecal resections, adherences), systemic or localised immune alterations, and others. SIBO has also been observed in a range of neurological and non-neurological diseases, such as Parkinson's disease, irritable bowel syndrome, cirrhosis, chronic pancreatitis, obesity, cystic fibrosis, chronic renal failure, coeliac disease, diabetes mellitus, hypothyroidism, hepatic encephalopathy or fibromyalgia.2,4
People who have suffered a spinal cord injury (SCI) experience alterations to mobility, sensitivity, autonomic control and sphincter function, presenting neurogenic bladder and bowel. The degree of involvement will depend on the neurological level of the injury (tetraplegia or paraplegia) and its severity (complete or incomplete). With regard to neurogenic bowel, the two main symptoms are constipation and faecal incontinence. The pathophysiological mechanisms responsible for this dysfunction are slowed colonic transit time, altered anorectal sensitivity, altered voluntary control of the external anal sphincter and perineal musculature, altered extrinsic reflexes (anorectal excitatory reflex and cough reflex) and altered defecation manoeuvre.5,6 To these mechanisms is added a reduction in physical activity, loss of independence and a need for constipation-inducing medication such as anticholinergic or analgesic agents. Constipation and faecal incontinence are accompanied by other symptoms, such as abdominal distension and pain, with a prevalence of 22%-40% and 18%-33%, respectively.7–9
Few studies have been published on SIBO and SCI. Cheng et al.10 investigated the association between SIBO and deep vein thrombosis in patients with SCI, finding a statistically significant association between the two and a SIBO prevalence of 38%. Ojetti et al.11 studied the prevalence of bacterial overgrowth and CH4 production in patients with myelomeningocele, finding SIBO in 39% of children, as well as a correlation between CH4 production, increased intestinal transit time and urinary tract infections.
Given that patients with SCI have risk factors for SIBO and a compatible clinical picture, as well as the scarcity of literature on the subject, this study has been conducted with the following objectives: 1) to assess the prevalence of SIBO in a group of patients with SCI; 2) to determine the factors that might contribute to the presence of SIBO and their relationship with the neurological characteristics of the injury and bowel function, and 3) to compare the results of the breath test in patients with SIBO with the results in patients without neurological injury and SIBO.
Patients and methodsA cross-sectional observational study was conducted in 29 consecutive patients treated at the Institut Guttmann [Guttmann Institute].
Patients under 18 years of age and over 75 years of age were excluded, as we those with cognitive alterations that impeded their comprehension of the study or collaboration.
All of the patients underwent a clinical assessment and a glucose breath test to investigate SIBO.
Fifteen consecutive patients with a positive results for SIBO in the breath test performed at the Gastrointestinal Functional Testing Laboratory at Hospital Germans Trias i Pujol [Germans Trias i Pujol Hospital] who did not have an SCI or other neurological disease were included as a control group, with the objective of comparing the characteristics of the positive test result between the two groups.
Clinical assessmentThe clinical assessment consisted of a personal structured interview and a physical examination.
The structured interview collected data on the patient, SCI (date, aetiology), usual medication and evacuation procedure characteristics: place of evacuation and dependence on another person, method of evacuation (anal digitation, chemical stimulation by suppository and intra-abdominal pressure) and evacuation procedure results: frequency, time taken, stool consistency (Bristol scale), faecal incontinence assessed using the Wexner scale,12 constipation based on the Rome IV criteria,13 abdominal pain and abdominal distension assessed using a numerical scale from 0 to 10 (0 = none/10 = maximum). A subjective assessment of bowel function using a numerical scale from 0 to 10 was also included, as well as neurogenic bowel severity based on the Neurogenic Bowel Dysfunction Score.14
The physical examination assessed the neurological characteristics of the injury, weight, height and umbilical waist circumference. The characteristics of the injury were assessed in accordance with the International Standards for Neurological Classification of Spinal Cord Injury,15 determining the neurological level of the injury (cervical, thoracic or lumbar), and its severity using the American Spinal Injury Association Impairment Scale (ASIA) (A, B, C and D). Patients were grouped by injury level into tetraplegia and paraplegia and by severity into complete motor injury (no voluntary motor activity below the neurological level of the injury, ASIA A or B) and incomplete motor injury (presence of voluntary motor activity below the neurological level of the injury, ASIA C or D).
Breath testAll patients underwent the glucose breath tests following the recommendations of the North American Consensus.16
For two weeks prior to the test, the patients could not take antibiotics, laxatives with fibre content or sugar derivatives, as well as laxative preparations for endoscopy or radiology of the colon. The night before the test, they had to follow a diet free from fermentable foods such as complex carbohydrates.
The test was performed after 8-12 hours of fasting, having performed oral hygiene with an antiseptic and avoiding tobacco and physical activity during the test. Seventy-five grams of glucose diluted in water was administered. Before taking the glucose and every 15 minutes after taking it up to a total of 120 minutes, a sample of expired air was collected in a metallised hermetic bag (Quintron, Milwaukee, USA) for subsequent analysis.
After breathing air into each of the nine bags, the patient had to assess the presence of the following symptoms: abdominal pain, abdominal distension, flatulence, cramps, diarrhoea or other symptoms using a categorical scale (0 = none, 1 = mild, 2 = moderate, 3 = intense).
The samples were analysed within six hours of collection using a gas chromatograph (Quintron, Milwaukee, USA), simultaneously calculating H2, CH4 and CO2 values.
The test was considered positive when there was an increase of ≥20 p.p.m. in H2 or ≥10 p.p.m. in methane above the baseline measurement (prior to the glucose administration) in two consecutive measurements.
The study was approved by the Ethics Committee of Instituto Guttmann and all patients signed an informed consent form before the start of the study.
Data analysisA descriptive analysis of the main characteristics of the study sample with regard to demographic and clinical variables and the results of the breath test was carried out. The categorical variables were described as frequency and percentages. The continuous variables were described as mean and standard deviation.
A bivariate study was performed using Student’s t-test for continuous variables and the chi-squared test for categorical variables. Based on this, contingency tables were created based on patients' characteristics and their bowel function, neurological level and ASIA scale, and time of evolution of the injury, and the results of the breath test (positive/negative) were compared. Finally, the association between the breath test result and the presence or absence of an SCI was studied.
The level of significance considered was p <0.05.
ResultsPatient characteristics and bowel functionThe sample is made up of 10 women and 19 men with a mean age of 47 years (18-75), 16 with an SCI in the subacute phase (<9 months’ evolution [mean 5; range 2-9]) and 13 in the chronic phase (> 1 years’ evolution [mean 14; range 2-29]). The aetiology of the injury was traumatic in 25 cases and non-traumatic in four. Based on the International Standards for Neurological Classification of Spinal Cord Injury, the neurological level of the lesion was tetraplegia in nine patients and paraplegia in 20. Based on ASIA, 19 patients had complete motor injuries (ASIA A or B) and 10 had incomplete motor injuries (ASIA C or D).
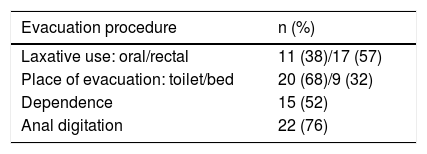
65% percent (n = 19) were taking a medication with a risk of contributing to SIBO, mostly anticholinergic agents, opioids or proton pump inhibitors. The bowel function characteristics of the patients studied can be seen in Table 1.
Bowel function characteristics of the patients with spinal cord injury enrolled in the study.
| Evacuation procedure | n (%) |
|---|---|
| Laxative use: oral/rectal | 11 (38)/17 (57) |
| Place of evacuation: toilet/bed | 20 (68)/9 (32) |
| Dependence | 15 (52) |
| Anal digitation | 22 (76) |
| Evacuation procedure results | n (%)/mean (range) |
|---|---|
| Rhythm: >24-48 h/72 h->72 h | 28 (97)/1 (3) |
| Time taken: <30 min/>30 min | 21 (72)/8 (28) |
| Stool consistency (Bristol scale) | 3.5 (1-6) |
| Faecal incontinence (Wexner scale) | 6.6 (1-14) |
| Constipation (Rome IV criteria) | 23 (79) |
| Neurogenic bowel dysfunction score | 12.7 (1-26) |
| Abdominal distension | 4 (1-10) |
| Abdominal pain | 2.3 (0-10) |
| Subjective assessment of bowel function | 5.5 (0-10) |
The group of patients without neurological injury consisted of four men and 11 women with a mean age of 60.4 years (40-79).
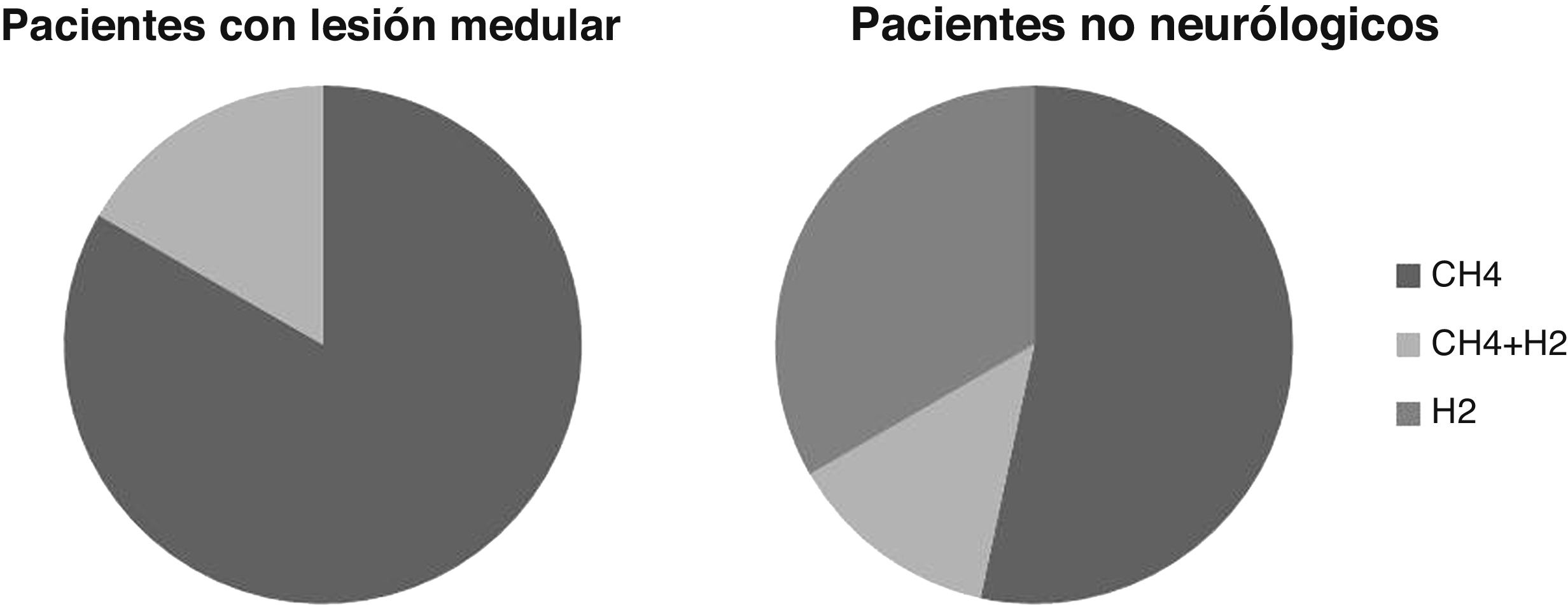
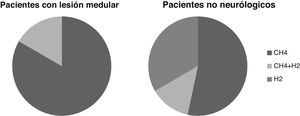
Breath testThe breath test was positive for SIBO in six cases, or 21% of the patients studied. In five cases it was positive due to increased CH4 and in one due to increased CH4 and H2. None were positive due to increased H2 alone.
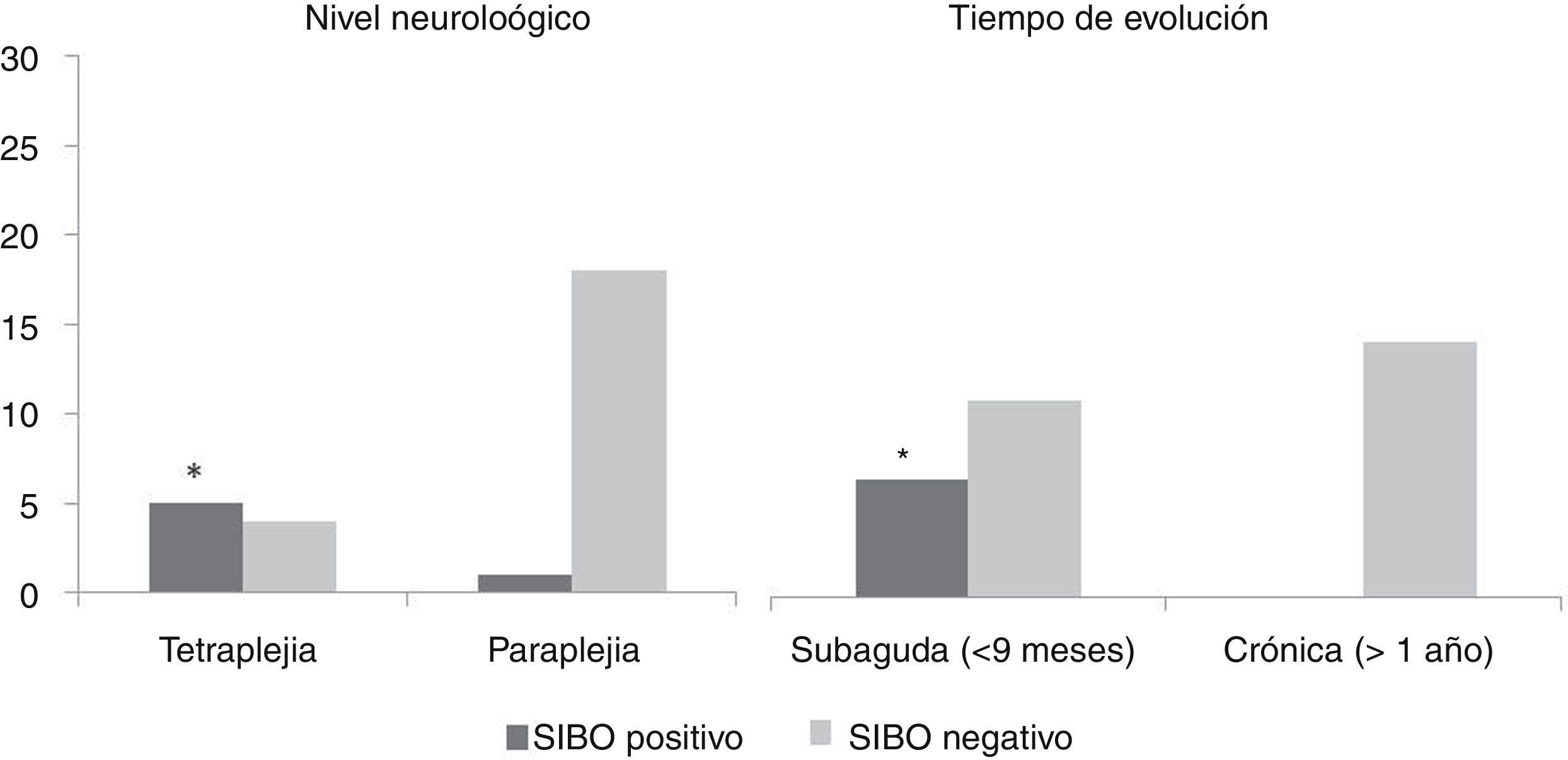
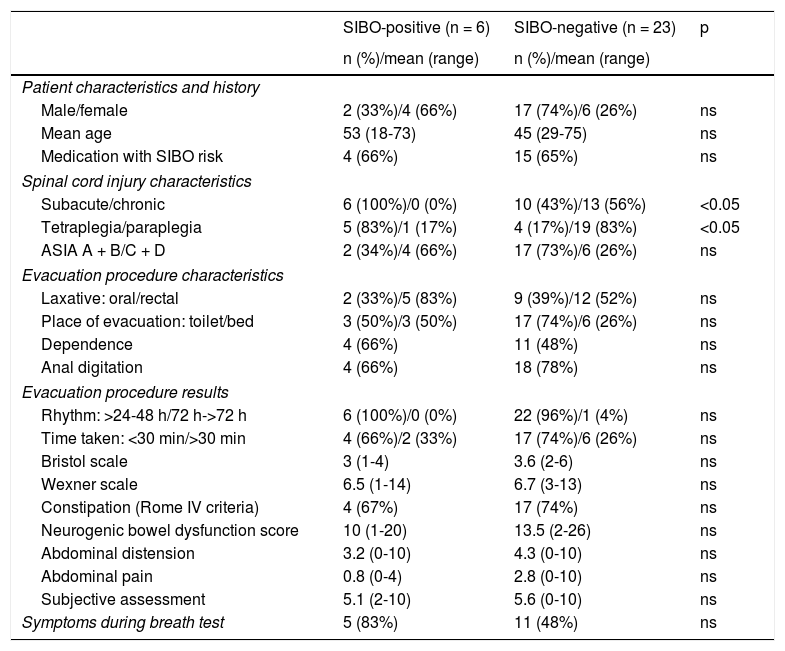
Table 2 shows a comparative analysis between positive and negative cases in the SIBO test against demographic characteristics, SCI characteristics, evacuation procedure and gastrointestinal symptoms. No statistically significant differences were found with regard to gender and age, bowel function characteristics, gastrointestinal symptoms or clinical picture presented during the test. Statistically significant differences (p <0.05) were found in relation to time of evolution of the injury (all patients with a positive test were in the subacute phase of their injury) and the neurological level of the injury (more common in patients with tetraplegia). No link was found with regard to the aetiology of the injury and the ASIA impairment scale (Fig. 1).
Characteristics of patients with spinal cord injury with positive and negative breath tests for SIBO.
| SIBO-positive (n = 6) | SIBO-negative (n = 23) | p | |
|---|---|---|---|
| n (%)/mean (range) | n (%)/mean (range) | ||
| Patient characteristics and history | |||
| Male/female | 2 (33%)/4 (66%) | 17 (74%)/6 (26%) | ns |
| Mean age | 53 (18-73) | 45 (29-75) | ns |
| Medication with SIBO risk | 4 (66%) | 15 (65%) | ns |
| Spinal cord injury characteristics | |||
| Subacute/chronic | 6 (100%)/0 (0%) | 10 (43%)/13 (56%) | <0.05 |
| Tetraplegia/paraplegia | 5 (83%)/1 (17%) | 4 (17%)/19 (83%) | <0.05 |
| ASIA A + B/C + D | 2 (34%)/4 (66%) | 17 (73%)/6 (26%) | ns |
| Evacuation procedure characteristics | |||
| Laxative: oral/rectal | 2 (33%)/5 (83%) | 9 (39%)/12 (52%) | ns |
| Place of evacuation: toilet/bed | 3 (50%)/3 (50%) | 17 (74%)/6 (26%) | ns |
| Dependence | 4 (66%) | 11 (48%) | ns |
| Anal digitation | 4 (66%) | 18 (78%) | ns |
| Evacuation procedure results | |||
| Rhythm: >24-48 h/72 h->72 h | 6 (100%)/0 (0%) | 22 (96%)/1 (4%) | ns |
| Time taken: <30 min/>30 min | 4 (66%)/2 (33%) | 17 (74%)/6 (26%) | ns |
| Bristol scale | 3 (1-4) | 3.6 (2-6) | ns |
| Wexner scale | 6.5 (1-14) | 6.7 (3-13) | ns |
| Constipation (Rome IV criteria) | 4 (67%) | 17 (74%) | ns |
| Neurogenic bowel dysfunction score | 10 (1-20) | 13.5 (2-26) | ns |
| Abdominal distension | 3.2 (0-10) | 4.3 (0-10) | ns |
| Abdominal pain | 0.8 (0-4) | 2.8 (0-10) | ns |
| Subjective assessment | 5.1 (2-10) | 5.6 (0-10) | ns |
| Symptoms during breath test | 5 (83%) | 11 (48%) | ns |
ASIA: American Spinal Injury Association Impairment Scale; SIBO: small intestinal bacterial overgrowth.
In patients without neurological injury, the breath test was positive for SIBO due to increased H2 alone in five patients, CH4 in eight cases and H2 and CH4 in two cases. The percentage of patients positive for increased H2 is greater than in the SCI patients, although these differences are not statistically significant (p >0.05) (Fig. 2).
DiscussionThis study has demonstrated that patients with SCI may present SIBO, especially in the subacute phase of the injury and in patients with tetraplegia.
When an SCI occurs, intestinal motility is affected due to the alteration of its extrinsic nervous regulation (sympathetic/parasympathetic and somatic systems), as well as other factors that can also cause secondary dysmotility such as immobility or the use of certain drugs. The slowing of transit in the small intestine has been described in patients with SCI,17 as well as colonic transit time.5,6 Because intestinal motility is the most important protective mechanism for the prevention of SIBO,1,2 it must be taken into account as a predisposing factor in these patients.
Moreover, it should be taken into account that 83% of SIBO cases correspond to patients with cervical injuries, where the degree of involvement of the musculature that influences bowel functions is very high, these being the patients who also have the greatest effects on gastrointestinal function as a result of the SCI.18
Another finding of this study is the greater presence of SIBO in patients in the subacute phase of their injury. Here, we must remember that the fragile equilibrium of the intestinal microbiota in terms of its composition and growth may have been altered by multiple factors1 that may be present in patients with subacute-phase SCI such as use of antibiotics due to the increase in the number of infections to which they are exposed, alteration of dietary habits during their hospital stay, the stress that subacute-phase patients experience following their SCI, and in particular the alteration to living habits that a cervical injury entails. This factor will have an influence not only on the intestinal microbiota, but there are studies that show an influence on intestinal permeability,19 alteration of gastric acid production and intestinal motility.20 Other factors that may be present in the acute phase of the injury and contribute to the presence of SIBO are immune mechanisms, since a depressed immune system raises the risk of SIBO21 and effects on the gastric barrier/pH, due to the effect of drugs such as proton pump inhibitors.
Moreover, there may be other factors inherent to the patient themselves, such as alterations to endogenous defence mechanisms to prevent bacterial overgrowth: ileocaecal valve dysfunction, alteration of pancreatic or biliary secretions, an others.1,2
There is scant scientific literature on SIBO in patients with SCI, and it does not describe the relationship with the characteristics of the injury as in our work. The two studies published previously, already discussed in the introduction, describe a greater prevalence of SIBO than in our work, at 38% to 39%, and find a relationship between SIBO and symptoms such as abdominal pain,11 abdominal distension and flatulence.10 In contrast, in our case, we did not find a relationship with the gastrointestinal symptoms presented by patients. The study by Cheng et al.10 assessed a sample of acute patients that was much larger than our own (469 patients) and the study by Ojetti et al.11 is in paediatric patients with a congenital lesion, so the results may differ due to the different characteristics of the subjects. Although in our study we did not find a relationship with bowel function characteristics, we think that SIBO may have contributed to the intestinal disorders they presented, even if not as the sole factor, as the pathophysiology of neurogenic bowel is complex and there are many pathophysiological mechanisms involved.5,6
Regardless, Ojetti et al.11 did find high CH4 production and a link to longer intestinal transit time and a higher prevalence of urinary tract infections, aspects that we did not study. Likewise, Cheng et al.10 linked the presence of SIBO with a higher incidence of deep vein thrombosis. It is worth noting that relationship found in both studies between SIBO and common complications in SCI patients (urinary tract infections and deep vein thrombosis).
This study’s limitation are its small sample size, which did not permit us to draw conclusions on SIBO’s effect on the gastrointestinal clinical picture in patients with SCI nor to seek a relationship with other complications present in people with SCI. In contrast, we consider its strengths to be the study of SIBO in relation to SCI characteristics and the comparison with non-neurological patients.
To conclude, patients with SCI may present SIBO during the subacute phase of their injury, especially in tetraplegia, with marked CH4 production. Although no link has been found with bowel function characteristics, the presence of SIBO should be considered in these patients. More extensive studies are required in order to be able to link the presence of SIBO to gastrointestinal symptoms in SCI.
FundingThis study received no specific funding from public, private or non-profit organisations.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Vallès M, Antuori A, Mearin F, Serra J. Sobrecrecimiento bacteriano del intestino delgado en pacientes con lesión medular. Gastroenterología y Hepatología. 2021;44:539–545.