The ENEIDA registry, promoted by the Spanish Working Group on Crohn's Disease and Ulcerative Colitis (GETECCU), was created in 2005 by a group of gastroenterologists interested in improving the management of patients with inflammatory bowel disease. The main objectives of the registry were to facilitate the collection of clinical data of interest for clinical care practice, as well as to carry out collaborative studies using clinical data and biological samples. In its 15 years of existence, ENEIDA has evolved in many aspects, from its content or technological support to the number of participating centres, to become one of the reference registries for the study and care of patients with inflammatory bowel disease, with a continuous and high quality scientific production that has positioned it as an example of collaborative scientific exploitation at an international level. This article reviews the objectives, design, structural characteristics, monitoring and scientific exploitation of the ENEIDA registry.
El registro ENEIDA, promovido por el Grupo Español de Trabajo en Enfermedad de Crohn y Colitis Ulcerosa (GETECCU), fue creado en 2005 por un grupo de gastroenterólogos interesados en mejorar el manejo de los pacientes con enfermedad inflamatoria intestinal. Los objetivos principales del registro fueron facilitar la recogida de datos clínicos de interés para la práctica clínica asistencial, así como la elaboración de estudios colaborativos a partir de datos clínicos y muestras biológicas. En sus 15 años de existencia, ENEIDA ha evolucionado en múltiples aspectos, desde su contenido o su soporte tecnológico hasta el número de centros participantes, para convertirse en uno de los registros de referencia para el estudio y cuidado de los pacientes con enfermedad inflamatoria intestinal, con una producción científica continua y de alta calidad que lo ha situado como ejemplo de explotación científica colaborativa en el ámbito internacional. En este artículo se revisan los objetivos, el diseño, las características estructurales, la monitorización y la explotación científica del registro ENEIDA.
The Grupo Español de Trabajo en Enfermedad de Crohn y Colitis Ulcerosa [Spanish Working Group on Crohn's Disease and Ulcerative Colitis] (GETECCU) is a non-profit association founded 30 years ago whose mission is to improve the lives of people with inflammatory bowel disease (IBD) by promoting excellence in healthcare, teaching and research and having an influence on political and social efforts. The GETECCU currently has almost 1000 members and is the leading scientific association for IBD in Spain, as well as a seedbed for training professionals who care for patients with IBD. Following its early years, when it focused on training and teaching, the GETECCU turned its attention to clinical research. The Estudio Nacional en Enfermedad Inflamatoria intestinal sobre Determinantes genéticos y Ambientales [Spanish National Study on Genetic and Environmental Determinants of Inflammatory Bowel Disease] (ENEIDA) registry, designed in 2005 by a group of gastroenterologists belonging to the GETECCU who were interested in improving the management of patients with IBD, was created to facilitate the collection of clinical data that could be useful in clinical practice and to conduct collaborative studies based on clinical data and biological samples.
Beginning of the projectThe ENEIDA project was approved in 2006 by the Ethics Committee of the Hospital Clínic i Provincial de Barcelona [Clinical and Provincial Hospital of Barcelona]. Its overall objective was to establish the infrastructure and operating procedures needed to conduct ongoing collaborative multicentre studies on factors involved in the aetiology and pathophysiology of IBD, including genetic and environmental factors and epidemiological studies. From the outset, the principles governing participation in and use of the registry were established and have been constantly upheld. First, participation and data recording are voluntary and non-remunerated; therefore, the degree to which variables are filled in depends on the commitment and the will of the investigators affiliated with each site, in the absence of an obligation to record data. Second, the registry claims to have a potential threefold function: to be a tool for healthcare (a source of rapid searches for clinical data to prepare reports or for in-person or remote healthcare), to be a registry for use by scientific centres all over the world and to be a collaborative multicentre registry for scientific use regulated by the GETECCU.
Initially, participating sites had to have a specific ENEIDA software programme in which they entered information locally. Every so often, they had to upload this anonymised local information to the main database from a remote access point so that the data could be used in collaborative research projects. At that time, sites that wanted to adopt the registry had to contend with certain limitations that impacted the database's growth. First of all, there was the mistrust likely generated in some sites by participating in a non-remunerated collaborative initiative, as this participation required selfless sharing of patient data. Second, a lack of staff with enough time to dedicate to IBD (which represented a considerable additional workload) and a database that was technically and visually difficult and complex to manage further limited the growth of the database. Finally, the technological limitations of that time should be borne in mind, since many sites lacked the Internet access that the application required, meaning that investigators often had to send data from their local databases by their own means.
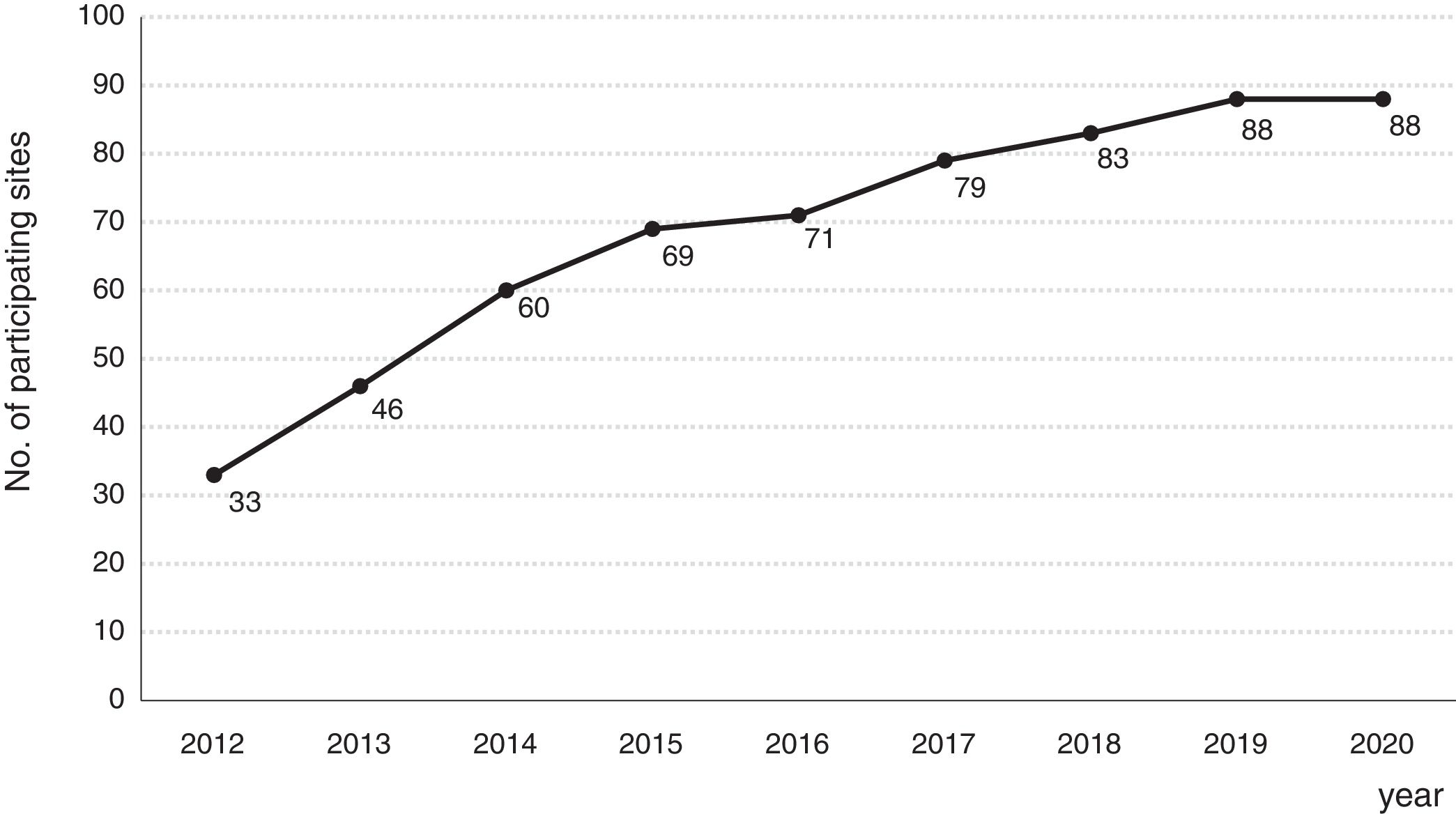
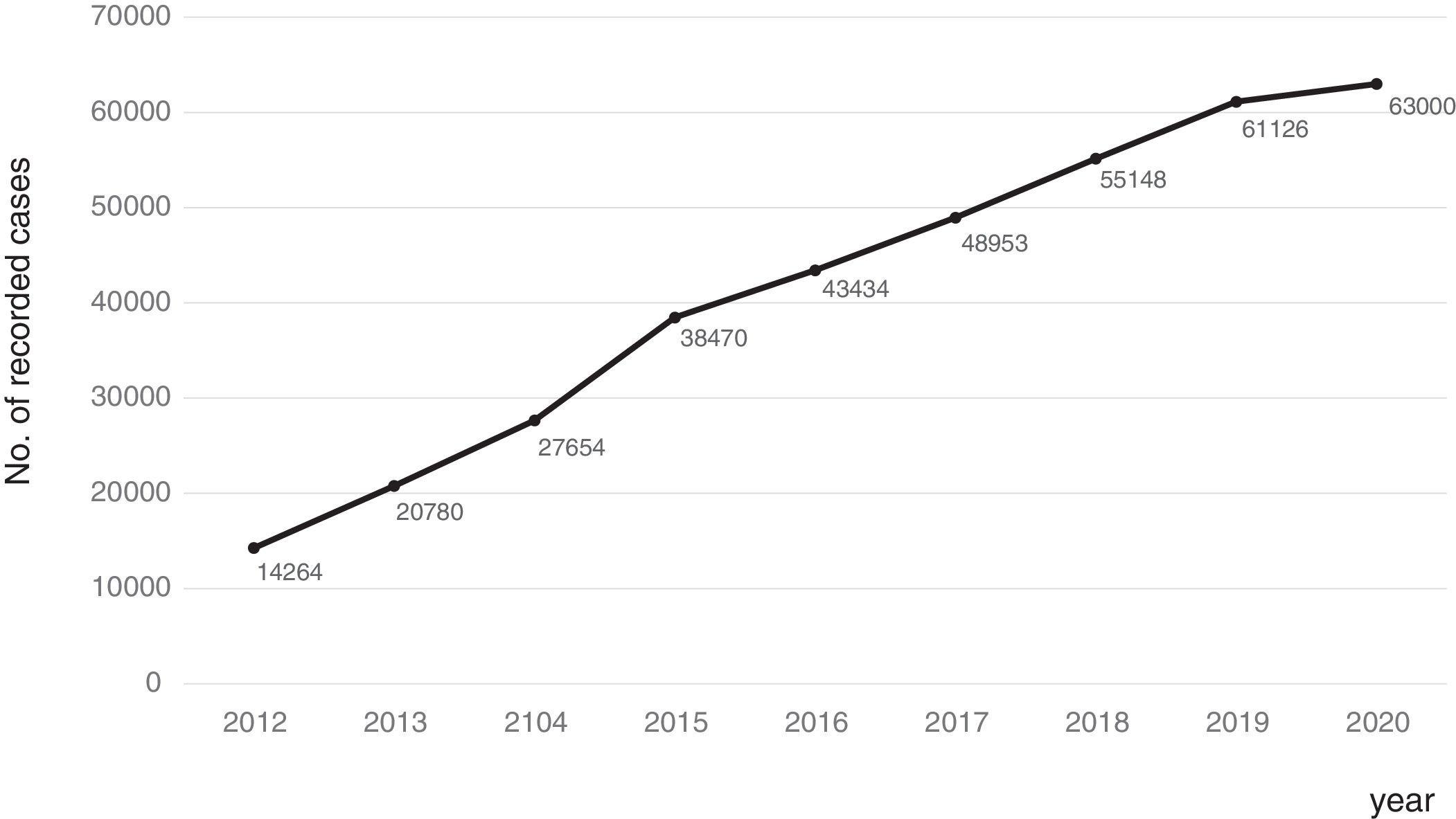
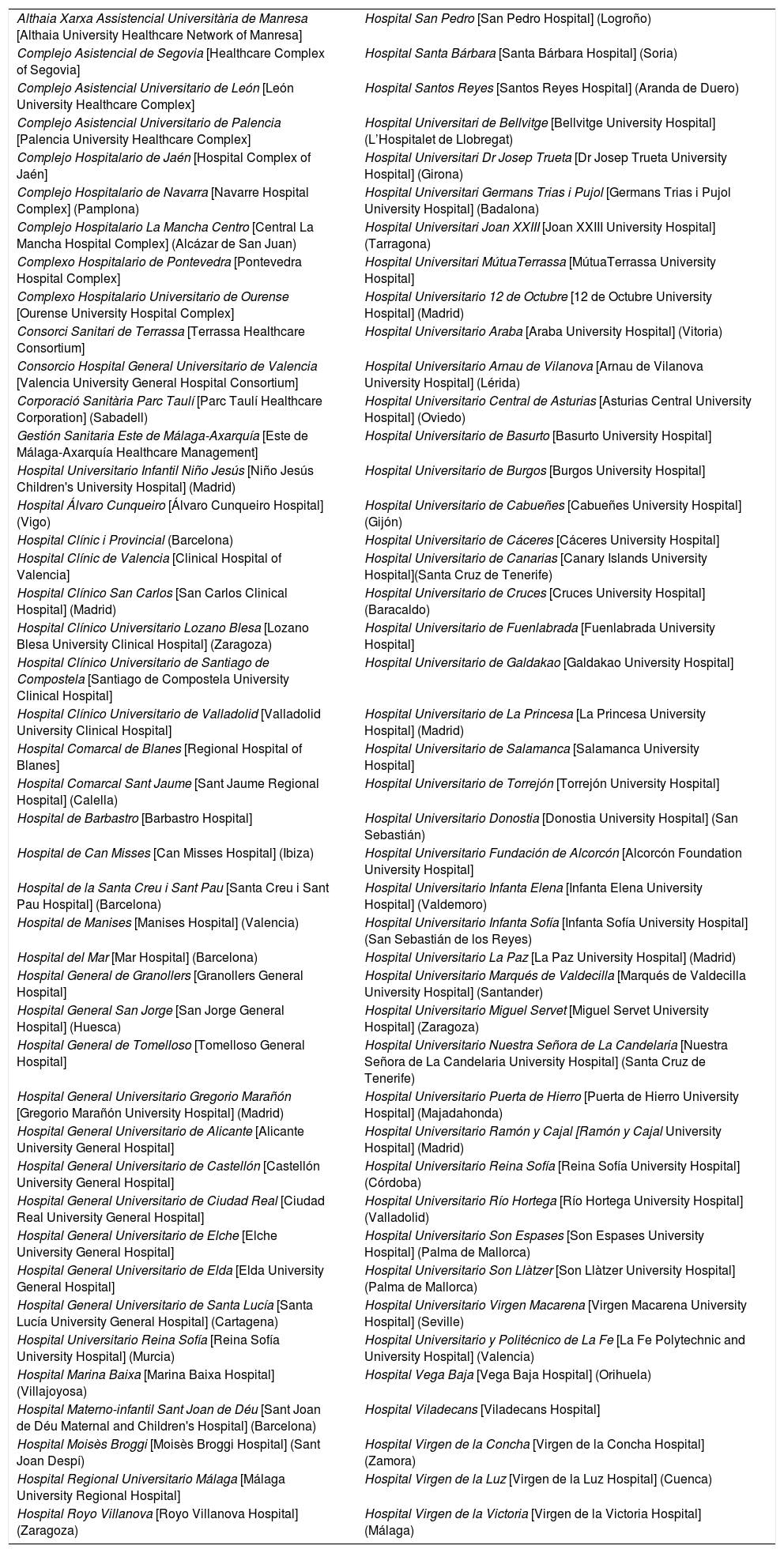
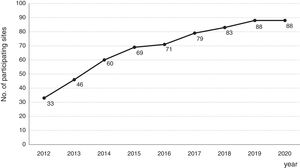
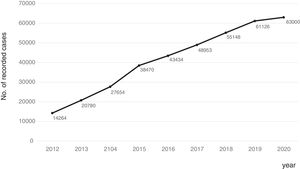
Consolidation of the registryThe GETECCU's registry took its definitive place as a leading registry with the creation of the online version in 2012, with no need for a local application and subsequent periodic data uploads. The change in system made the registry simpler and more dynamic. Each site uploaded all the patients included in the local database to the new central database in a single operation, and from then on the data were entered directly in the online database. The need for signed agreements between the GETECCU and the management of the different hospitals involved in the project, as well as the approval of each institution's ethics committee, hampered the project's expansion somewhat. At present, more than 80 sites are participating in the project (Table 1) and more than 60,000 patients have been included (Figs. 1 and 2). Finally, the project now includes a collection of more than 8000 DNA samples. One final technological change was made to the database in 2020, when it was migrated to a new platform with superior technical features.
List of hospitals participating in the ENEIDA registry.
| Althaia Xarxa Assistencial Universitària de Manresa [Althaia University Healthcare Network of Manresa] | Hospital San Pedro [San Pedro Hospital] (Logroño) |
| Complejo Asistencial de Segovia [Healthcare Complex of Segovia] | Hospital Santa Bárbara [Santa Bárbara Hospital] (Soria) |
| Complejo Asistencial Universitario de León [León University Healthcare Complex] | Hospital Santos Reyes [Santos Reyes Hospital] (Aranda de Duero) |
| Complejo Asistencial Universitario de Palencia [Palencia University Healthcare Complex] | Hospital Universitari de Bellvitge [Bellvitge University Hospital] (L’Hospitalet de Llobregat) |
| Complejo Hospitalario de Jaén [Hospital Complex of Jaén] | Hospital Universitari Dr Josep Trueta [Dr Josep Trueta University Hospital] (Girona) |
| Complejo Hospitalario de Navarra [Navarre Hospital Complex] (Pamplona) | Hospital Universitari Germans Trias i Pujol [Germans Trias i Pujol University Hospital] (Badalona) |
| Complejo Hospitalario La Mancha Centro [Central La Mancha Hospital Complex] (Alcázar de San Juan) | Hospital Universitari Joan XXIII [Joan XXIII University Hospital] (Tarragona) |
| Complexo Hospitalario de Pontevedra [Pontevedra Hospital Complex] | Hospital Universitari MútuaTerrassa [MútuaTerrassa University Hospital] |
| Complexo Hospitalario Universitario de Ourense [Ourense University Hospital Complex] | Hospital Universitario 12 de Octubre [12 de Octubre University Hospital] (Madrid) |
| Consorci Sanitari de Terrassa [Terrassa Healthcare Consortium] | Hospital Universitario Araba [Araba University Hospital] (Vitoria) |
| Consorcio Hospital General Universitario de Valencia [Valencia University General Hospital Consortium] | Hospital Universitario Arnau de Vilanova [Arnau de Vilanova University Hospital] (Lérida) |
| Corporació Sanitària Parc Taulí [Parc Taulí Healthcare Corporation] (Sabadell) | Hospital Universitario Central de Asturias [Asturias Central University Hospital] (Oviedo) |
| Gestión Sanitaria Este de Málaga-Axarquía [Este de Málaga-Axarquía Healthcare Management] | Hospital Universitario de Basurto [Basurto University Hospital] |
| Hospital Universitario Infantil Niño Jesús [Niño Jesús Children's University Hospital] (Madrid) | Hospital Universitario de Burgos [Burgos University Hospital] |
| Hospital Álvaro Cunqueiro [Álvaro Cunqueiro Hospital] (Vigo) | Hospital Universitario de Cabueñes [Cabueñes University Hospital] (Gijón) |
| Hospital Clínic i Provincial (Barcelona) | Hospital Universitario de Cáceres [Cáceres University Hospital] |
| Hospital Clínic de Valencia [Clinical Hospital of Valencia] | Hospital Universitario de Canarias [Canary Islands University Hospital](Santa Cruz de Tenerife) |
| Hospital Clínico San Carlos [San Carlos Clinical Hospital] (Madrid) | Hospital Universitario de Cruces [Cruces University Hospital] (Baracaldo) |
| Hospital Clínico Universitario Lozano Blesa [Lozano Blesa University Clinical Hospital] (Zaragoza) | Hospital Universitario de Fuenlabrada [Fuenlabrada University Hospital] |
| Hospital Clínico Universitario de Santiago de Compostela [Santiago de Compostela University Clinical Hospital] | Hospital Universitario de Galdakao [Galdakao University Hospital] |
| Hospital Clínico Universitario de Valladolid [Valladolid University Clinical Hospital] | Hospital Universitario de La Princesa [La Princesa University Hospital] (Madrid) |
| Hospital Comarcal de Blanes [Regional Hospital of Blanes] | Hospital Universitario de Salamanca [Salamanca University Hospital] |
| Hospital Comarcal Sant Jaume [Sant Jaume Regional Hospital] (Calella) | Hospital Universitario de Torrejón [Torrejón University Hospital] |
| Hospital de Barbastro [Barbastro Hospital] | Hospital Universitario Donostia [Donostia University Hospital] (San Sebastián) |
| Hospital de Can Misses [Can Misses Hospital] (Ibiza) | Hospital Universitario Fundación de Alcorcón [Alcorcón Foundation University Hospital] |
| Hospital de la Santa Creu i Sant Pau [Santa Creu i Sant Pau Hospital] (Barcelona) | Hospital Universitario Infanta Elena [Infanta Elena University Hospital] (Valdemoro) |
| Hospital de Manises [Manises Hospital] (Valencia) | Hospital Universitario Infanta Sofía [Infanta Sofía University Hospital] (San Sebastián de los Reyes) |
| Hospital del Mar [Mar Hospital] (Barcelona) | Hospital Universitario La Paz [La Paz University Hospital] (Madrid) |
| Hospital General de Granollers [Granollers General Hospital] | Hospital Universitario Marqués de Valdecilla [Marqués de Valdecilla University Hospital] (Santander) |
| Hospital General San Jorge [San Jorge General Hospital] (Huesca) | Hospital Universitario Miguel Servet [Miguel Servet University Hospital] (Zaragoza) |
| Hospital General de Tomelloso [Tomelloso General Hospital] | Hospital Universitario Nuestra Señora de La Candelaria [Nuestra Señora de La Candelaria University Hospital] (Santa Cruz de Tenerife) |
| Hospital General Universitario Gregorio Marañón [Gregorio Marañón University Hospital] (Madrid) | Hospital Universitario Puerta de Hierro [Puerta de Hierro University Hospital] (Majadahonda) |
| Hospital General Universitario de Alicante [Alicante University General Hospital] | Hospital Universitario Ramón y Cajal [Ramón y Cajal University Hospital] (Madrid) |
| Hospital General Universitario de Castellón [Castellón University General Hospital] | Hospital Universitario Reina Sofía [Reina Sofía University Hospital] (Córdoba) |
| Hospital General Universitario de Ciudad Real [Ciudad Real University General Hospital] | Hospital Universitario Río Hortega [Río Hortega University Hospital] (Valladolid) |
| Hospital General Universitario de Elche [Elche University General Hospital] | Hospital Universitario Son Espases [Son Espases University Hospital] (Palma de Mallorca) |
| Hospital General Universitario de Elda [Elda University General Hospital] | Hospital Universitario Son Llàtzer [Son Llàtzer University Hospital] (Palma de Mallorca) |
| Hospital General Universitario de Santa Lucía [Santa Lucía University General Hospital] (Cartagena) | Hospital Universitario Virgen Macarena [Virgen Macarena University Hospital] (Seville) |
| Hospital Universitario Reina Sofía [Reina Sofía University Hospital] (Murcia) | Hospital Universitario y Politécnico de La Fe [La Fe Polytechnic and University Hospital] (Valencia) |
| Hospital Marina Baixa [Marina Baixa Hospital] (Villajoyosa) | Hospital Vega Baja [Vega Baja Hospital] (Orihuela) |
| Hospital Materno-infantil Sant Joan de Déu [Sant Joan de Déu Maternal and Children's Hospital] (Barcelona) | Hospital Viladecans [Viladecans Hospital] |
| Hospital Moisès Broggi [Moisès Broggi Hospital] (Sant Joan Despí) | Hospital Virgen de la Concha [Virgen de la Concha Hospital] (Zamora) |
| Hospital Regional Universitario Málaga [Málaga University Regional Hospital] | Hospital Virgen de la Luz [Virgen de la Luz Hospital] (Cuenca) |
| Hospital Royo Villanova [Royo Villanova Hospital] (Zaragoza) | Hospital Virgen de la Victoria [Virgen de la Victoria Hospital] (Málaga) |
The project has more than surpassed its objective of generating scientific knowledge about IBD. The first collaborative article based on ENEIDA data was published in 2011; to date, a total of 29 articles have been published,1–29 not to mention the studies conducted by different sites using the registry's data. It is important to emphasise that its output has been not only copious, but also of the highest scientific quality, reflected in the fact that 30% of its total output has been published in first-decile journals in the speciality and 89% thereof has been published in first-quartile publications. All completed and ongoing studies involved in the ENEIDA project can be consulted on the GETECCU website, as can the links to the respective publications (http://geteccu.org/eneida/estudios-aprobados-en-eneida). The quality of the scientific output from the ENEIDA registry is precisely what has cemented the unanimous and international recognition of the GETECCU as a scientific association. On several occasions, the European Medicines Agency has recommended the use of the ENEIDA registry as a database of safety records of new drugs in clinical practice. Finally, we would like to emphasise the diversity in the leadership of the scientific use made of the ENEIDA registry, which the GETECCU has promoted from the outset. The leadership of 9 different research groups and 18 different lead authors of the 29 published articles stands as proof of this diversity.
Design, content and monitoring of the ENEIDA registryThe ENEIDA registry is a registry of patients diagnosed with IBD with prospective follow-up. Once a site decides to participate in the database, it usually includes all the patients that it has in active follow-up. This means that when the site begins to participate, patient inclusion is essentially retrospective, though from then on follow-up is always prospective. The same applies when a patient begins follow-up at a participating site after having being diagnosed at a different one. However, a few months after each site begins to participate, inclusion is essentially prospective.
It allows for selection of patients by any of the multiple variables that it contains. Therefore, it is possible to identify patients who have been followed up entirely on a prospective basis and those whose date of addition to the registry coincides with their date of diagnosis.
In addition to the date of addition to the registry (which is automatically entered by the system when the patient's data are first entered in the registry, and cannot be modified), the researcher must update the date of last visit each time the patient is contacted. If the patient is lost to follow-up for any reason (death, change of address or any other reason), this must be recorded. Since the project's main objectives include epidemiological and environmental studies, a decision was made to limit the scope of the database to Spain, meaning that sites in other countries are not included.
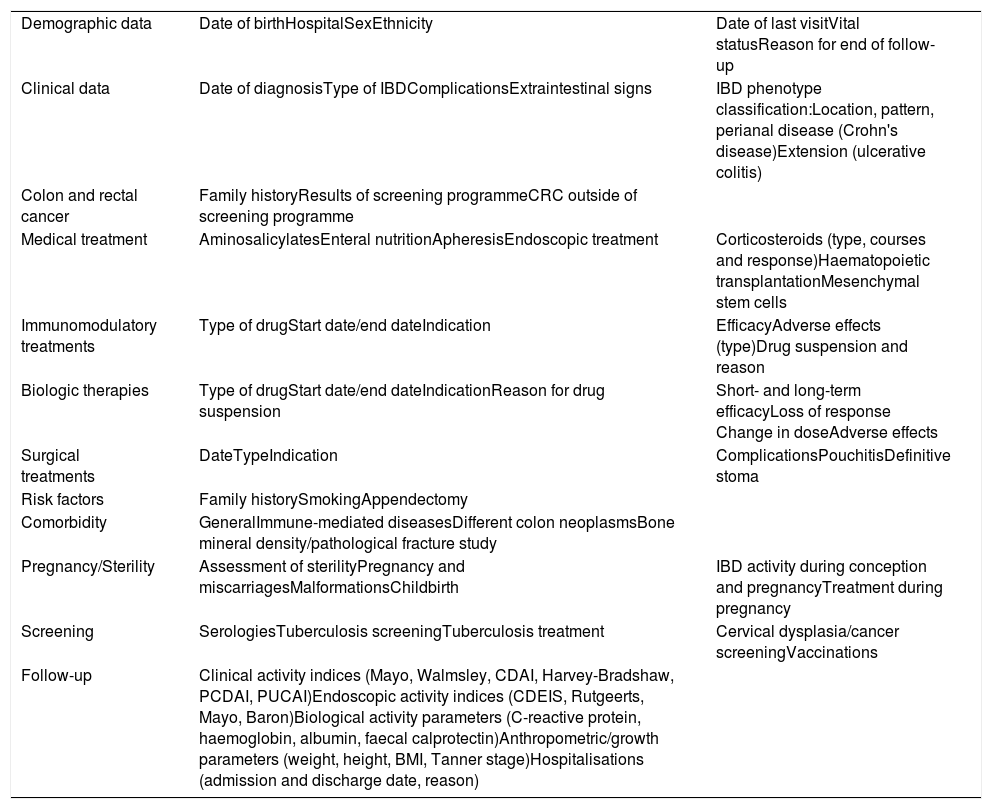
The variables collected in the ENEIDA registry have been modified several times in the course of its existence, basically to add new variables, as a result of advances made in the knowledge of IBD and the availability of new diagnostic and follow-up tools and new treatments. These variables have always been modified at the discretion of an expert group including members from the research area and the chairperson of the GETECCU board, as well as any GETECCU members appointed by the board at any time. The need for these modifications is clearly demonstrated by the approval of new drugs; since the registry's beginnings, all new treatment options placed on the Spanish market, including biosimilars, have been added. In this regard, new variables have always been added almost immediately in order to guarantee the data's prospective nature. The variables are grouped on 11 forms: demographic data; clinical characteristics of the disease (based on the Montreal classification30); colon neoplasm diagnosis and screening; non-immunosuppressant medical treatments; treatments with immunosuppressant drugs; treatments with biologics; surgical treatments; familial and environmental risk factors; general, immune-mediated and neoplastic comorbidities; pregnancy and fertility; and infection screening and follow-up (clinical and endoscopic indices, laboratory test results and hospitalisations). Table 2 summarises the current variables.
Main variables included in the ENEIDA registry.
| Demographic data | Date of birthHospitalSexEthnicity | Date of last visitVital statusReason for end of follow-up |
| Clinical data | Date of diagnosisType of IBDComplicationsExtraintestinal signs | IBD phenotype classification:Location, pattern, perianal disease (Crohn's disease)Extension (ulcerative colitis) |
| Colon and rectal cancer | Family historyResults of screening programmeCRC outside of screening programme | |
| Medical treatment | AminosalicylatesEnteral nutritionApheresisEndoscopic treatment | Corticosteroids (type, courses and response)Haematopoietic transplantationMesenchymal stem cells |
| Immunomodulatory treatments | Type of drugStart date/end dateIndication | EfficacyAdverse effects (type)Drug suspension and reason |
| Biologic therapies | Type of drugStart date/end dateIndicationReason for drug suspension | Short- and long-term efficacyLoss of response Change in doseAdverse effects |
| Surgical treatments | DateTypeIndication | ComplicationsPouchitisDefinitive stoma |
| Risk factors | Family historySmokingAppendectomy | |
| Comorbidity | GeneralImmune-mediated diseasesDifferent colon neoplasmsBone mineral density/pathological fracture study | |
| Pregnancy/Sterility | Assessment of sterilityPregnancy and miscarriagesMalformationsChildbirth | IBD activity during conception and pregnancyTreatment during pregnancy |
| Screening | SerologiesTuberculosis screeningTuberculosis treatment | Cervical dysplasia/cancer screeningVaccinations |
| Follow-up | Clinical activity indices (Mayo, Walmsley, CDAI, Harvey-Bradshaw, PCDAI, PUCAI)Endoscopic activity indices (CDEIS, Rutgeerts, Mayo, Baron)Biological activity parameters (C-reactive protein, haemoglobin, albumin, faecal calprotectin)Anthropometric/growth parameters (weight, height, BMI, Tanner stage)Hospitalisations (admission and discharge date, reason) |
BMI: body mass index; CDAI: Crohn's Disease Activity Index; CDEIS: Crohn's Disease Endoscopic Index of Severity; CRC: colorrectal cancer; IBD: inflammatory bowel disease; PCDAI: Paediatric Crohn's Disease Activity Index; PUCAI: Paediatric Ulcerative Colitis Activity Index.
To create a new patient record, demographic data must be filled in first, as without them the system will not allow the user to enter any other variables. The patient's data can only be viewed locally for healthcare purposes, and all records are anonymised centrally in accordance with the confidentiality regulations established by Spanish law. Similarly, each investigator only has direct access to his or her own site's data. Since participation in the registry is voluntary, an indirect system for monitoring record completion was established. For this purpose, it was decided that certain variables were necessary in order to determine that a case had the minimum information required to be included in studies. Every month, each site receives a detailed report about the patients for whom these variables are incomplete; their completion is left up to the investigator. This makes it possible to exclude incomplete cases from studies and to select sites with good completion records if more than 80% of their cases feature complete information.
Dynamics of the ENEIDA registryOnce the ethics committee of the participating site has approved the project and the management has signed the agreement with the GETECCU pertaining to compliance with data protection regulations and regulations governing clinical trials with drugs for human use, the GETECCU provides the site's principal investigator and team of investigators with the passwords that they need to access the registry locally. Each patient must sign the relevant informed consent form for clinical data entry. The GETECCU, as the owner of the ENEIDA registry, avails itself of a technology vendor for data storage and protection with application of the required security measures. The ENEIDA registry currently uses the ClinicaaL® (Persei vivarium S.L., Madrid, España) eHealth platform.
There is the option of collecting not only clinical variables but also, with the signing of the relevant informed consent form, a blood sample for subsequent DNA extraction at the Institut d’Investigació Biomèdica August Pi i Sunyer [August Pi i Sunyer Biomedical Research Institute] (IDIBAPS) biobank, where the sample will be stored to ensure optimal storage conditions. Delivery of biological samples to the biobank is governed by a standard operating procedure (SOP) available to all ENEIDA registry investigators.
Scientific use of and dissemination of information from the ENEIDA registryAlthough each investigator is free to use their local ENEIDA registry data for research purposes with no need for additional approval, use of central data is regulated by a specific SOP. All the participating investigators who submit clinical information from at least 100 patients can ask the GETECCU for permission to use the information contained in the registry. Moreover, investigators who have provided at least 50 biological samples for the project can also request studies based on these samples. Only GETECCU members whose sites are participating in the ENEIDA registry can request a study with ENEIDA data; independent investigators, other scientific associations and public and private enterprises cannot. Once the study has been approved by the GETECCU, information about the variables selected in anonymised fashion is delivered to the investigator for subsequent use, analysis and publication. Anyone who requests genetic studies will receive, in addition to specific clinical variables, the DNA samples from the patients included in the study as indicated in another specific SOP for sample transfer. There is a clearly defined authorship policy (http://geteccu.org/eneida/politica-de-autorias), meaning that all ENEIDA project investigators who have helped with the data for a patient from their site for each specific study are entitled to authorship. Both articles and authorship must be approved by the GETECCU research area, the ENEIDA coordinator and the principal investigators from the sites involved. All these processes are managed and overseen by a scientific secretariat, the ENEIDA coordinator and the voting members of the research area of the governing board of the GETECCU.
With a view to disseminating the knowledge generated through the ENEIDA registry, all investigators receive a quarterly bulletin which reports on communications at congresses and publications resulting from approved studies, as well as any changes made to the database itself, the SOPs or any other matter related to the project. Every six months, coinciding with the annual meetings of the Asociación Española de Gastroenterología [Spanish Association of Gastroenterology] and the GETECCU, the ENEIDA coordinator (appointed every 2 years by the governing board of the GETECCU) presents an update of the registry and its scientific output.
ConclusionsThe ENEIDA project is the largest prospective clinical registry of IBD patients in the world and constitutes a paradigmatic example of a collaborative effort in clinical investigation within the framework of a scientific association. Moreover, it highlights the importance of selfless participation of patients and investigators with high-quality scientific output that has conferred worldwide visibility and prestige on the GETECCU as a firmly established group.
Conflicts of interestThe authors declare that they have no conflicts of interest.
The authors would like to thank the chairpersons of the GETECCU; Dr Miquel Àngel Gassull, Dr Antoni Obrador and Dr Joaquín Hinojosa, for their contribution to the project concept; Dr Joan Clofent, Dr Antonio López Sanromán, Dr Natalia Borruel, Dr Francisco Guarner, Dr Belén Beltrán, Dr Ana Gutiérrez, Dr Miguel Mínguez and Dr María Chaparro, prominent GETECCU members, for having belonged at some point to the different committees and working groups tied to the ENEIDA registry; Maica Massamunt and Anna Casas, for their efficient work at the head of the ENEIDA registry's scientific secretariat; and the pharmaceutical industry, for its financial support which has made this project possible without interfering with its contents or use. Finally, the authors would like to thank all the investigators for their work and all the patients for their cooperation in facilitating advances in knowledge of inflammatory bowel diseases.
Please cite this article as: Zabana Y, Panés J, Nos P, Gomollón F, Esteve M, García-Sánchez V, et al. El registro ENEIDA (Estudio Nacional en Enfermedad Inflamatoria intestinal sobre Determinantes genéticos y Ambientales) de GETECCU: diseño, monitorización y funciones. Gastroenterol Hepatol. 2020;43:551–558.