The aim of the study is to evaluate the clinical and biochemical response of inflammatory bowel disease (IBD) patients treated with vedolizumab, 16 weeks after transitioning from intravenous (iv) to subcutaneous (sc).
MethodsAn observational, prospective, single-center cohort study was performed. Patients with IBD and maintenance treatment with vedolizumab, stable for at least 4 mo, were offered to switch to sc formulation. At the same time of treatment administration a blood test was performed, with vedolizumab levels and fecal calprotectin.
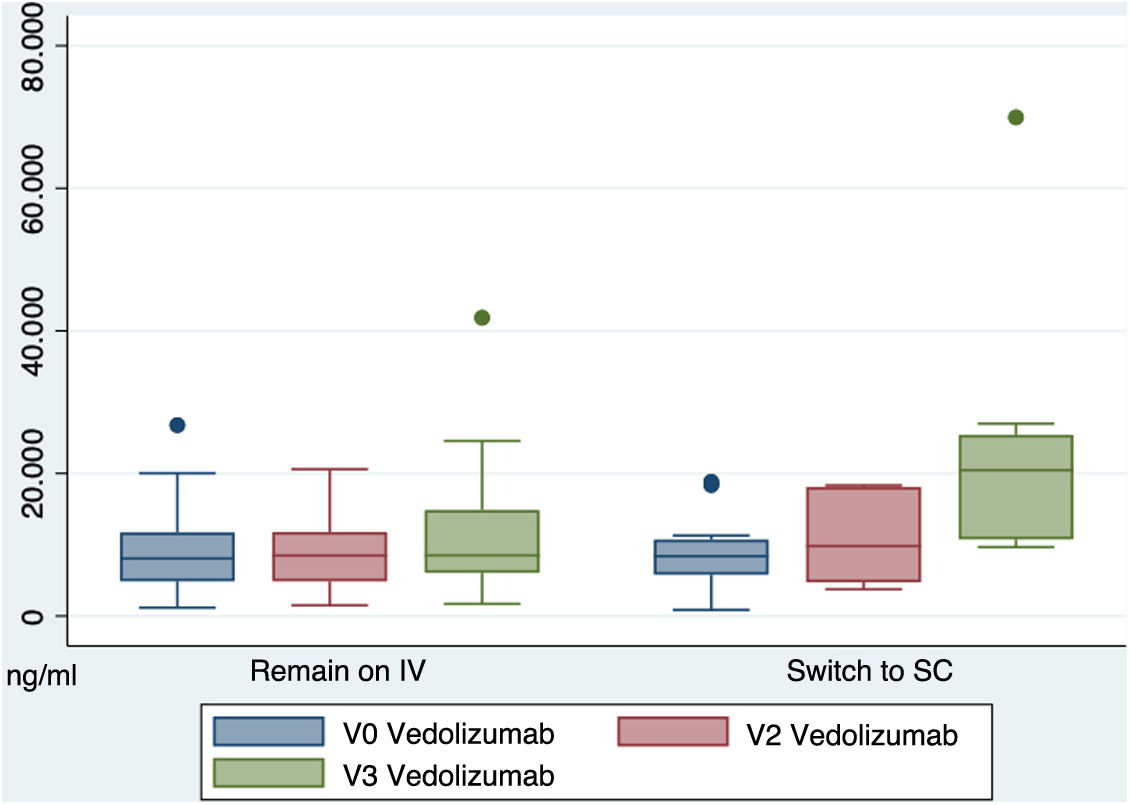
ResultsForty-three patients were included, 12 of them (27.9%) chose to transition to sc formulation. All included patients remained in remission during follow-up. At week 16 (w16), no significant differences were found in terms of calprotectin levels in patients on iv treatment (mean 146.6 ± SD 45.9) vs. sc (159.26 ± 53.9) (p 0.9). Vedolizumab serum levels at w16 were higher in the sc group (22364.3 ± 5141.6) vs. iv (11425.9 ± 1514.2) (p 0.009). At w16, 9 (75%) of the patients in the SC group were highly satisfied with the medication and 11 (91.7%) considered it easy to administer. 4 patients (12.9%) in the iv group and 2 (16.6%) in the sc group presented mild adverse effects. The 2 cases (100%) of the sc group the adverse event was local inflammation at the injection site.
ConclusionIn our experience, vedolizumab sc is a convenient alternative to iv administration. Vedolizumab serum levels in patients who transitioned to sc were higher than iv formulation.
El objetivo del estudio es determinar la evolución clínica y bioquímica de pacientes con enfermedad inflamatoria intestinal (EII) tratados con vedolizumab, 16 semanas después de cambiar de la vía intravenosa (iv) a subcutánea (sc).
MétodosSe llevó a cabo un estudio de cohortes prospectivo, observacional y unicéntrico. Se ofreció a pacientes con EII y tratamiento de mantenimiento con vedolizumab, estable durante al menos 4 meses, cambiar a sc. Al mismo tiempo de la administración del tratamiento, se realizó un análisis de sangre, midiendo los niveles de vedolizumab y calprotectina fecal.
ResultadosSe incluyeron 43 pacientes, de los cuales 12 (27.9%) optaron por cambiar a sc. Todos los pacientes incluidos permanecieron en remisión durante el seguimiento. En la semana 16 (s16), no se encontraron diferencias significativas en los niveles de calprotectina entre el grupo iv (media 146.6 ± DE 45.9) y sc (159.26 ± 53.9) (p 0.9). Los niveles séricos de vedolizumab en la s16 fueron más altos en el grupo sc (22364.3 ± 5141.6) vs. iv (11425.9 ± 1514.2) (p 0.009). En la s16, 9 (75%) de los pacientes en el grupo sc estaban muy satisfechos con el medicamento y 11 (91.7%) lo consideraron fácil de administrar. 4 pacientes (12.9%) en el grupo iv y 2 (16.6%) en el grupo sc presentaron efectos adversos leves. En los 2 casos (100%) del grupo sc, el evento adverso fue inflamación local en el sitio de inyección.
ConclusiónEn nuestra experiencia, vedolizumab sc es una alternativa eficaz. Los niveles séricos valle de vedolizumab en el grupo sc fueron mayores que en el grupo iv.