The bridge of this project is a reinforced concrete structure of 1980m divided in 40 spans. The deck is a single-cell box girder and a total width of 13m including sidewalks and shoulders. After 25 years in marine environment, the structure showed reinforcement corrosion, especially in piles and deck bottom.
It was repaired with four types of intervention: conventional reparation with patches, corrosion inhibitors, cathodic protection with sacrificial anodes and an hybrid cathodic protection system with an initial impressed current and galvanic current. In this research it is described the refurbishment and comparative results of the evolution of the repairs and therefore data of the corrosion progression and its effect in service life. The hybrid system, by the moment, is the system with the best results.
El puente de este proyecto es una estructura de hormigón armado de 1.980m dividida en 40tramos. La cubierta es una viga de cajón unicelular y tiene una anchura total de 13m, incluyendo calzadas y aceras. Después de 25años en un entorno marino, la estructura mostraba corrosión de las armaduras, especialmente en pilares y base de plataforma.
Se reparó con cuatro tipos de intervención: reparación convencional con parches; agentes anticorrosivos; protección catódica con ánodos galvánicos, y un sistema de protección catódica híbrido con una corriente impresa inicial y una corriente galvánica. En esta investigación se describe la remodelación y los resultados comparativos de la evolución de las reparaciones y, por tanto, los datos de la progresión de la corrosión y su efecto en la vida útil. De momento, el sistema híbrido es el que presenta los mejores resultados.
The bridge of this research links the island of Arousa with the coast of Galicia in the NW of Spain. It is a reinforced concrete structure, planned and executed in the mid-eighties of the twentieth century (year of commissioning: 1985).
The bridge is 1980m long and it was designed in a circumference of 2.5km radius. It consists of 40 spans, 38 of which are 50m span, while the 2 ends with 40m span. It includes joints only in the two brackets.
The deck consists of a single box girder, 2.3m height, with two transversal projections. The total width of the roadway is 13.00m, divided into two sidewalks of 1.50m, two shoulders of 1.5m and two lanes of 3.5m.
The bridge was inspected and it was decided that it was required a full intervention. The pathologies detected were: corrosion damage in deck and piles, shear cracks and of compatibility in the deck, some nests of gravel in piles and abutments, corrosion of the bearing plates, corrosion due to deficient surface drainage, deterioration of neoprene pot bearings, and damage in lighting boxes.
The structural condition of the bridge was good with some problems of durability, that was expectable considering the elapsed time from construction and the aggressiveness of the marine environment. This ambient saturated with chloride ions (Cl−) produced corrosion of the reinforcement in some areas. Although this attack does not affect the structural safety, there are large areas damaged visible at plain sight that affects the aesthetics of the bridge.
Within the field of concrete repair, the usual treatment is to apply localized repairs in the concrete. This technique was applied with satisfactory results. However, given the aggressiveness of the ambient, it was decided to use also cathodic protection methods in conjunction with the localized patches repair [1].
2Corrosion of reinforced concrete2.1Corrosion fundamentalsCorrosion is defined as the deterioration of a material under attack by an electrochemical environment [2]. More generally, it can be understood as the general tendency of material to find its most stable or lower internal energy form. Provided that the corrosion is caused by an electrochemical reaction (oxidation), the rate at which occurs will depend to some extent on the temperature, the salinity of the fluid in contact with the metal and the properties of the metals in question [3].
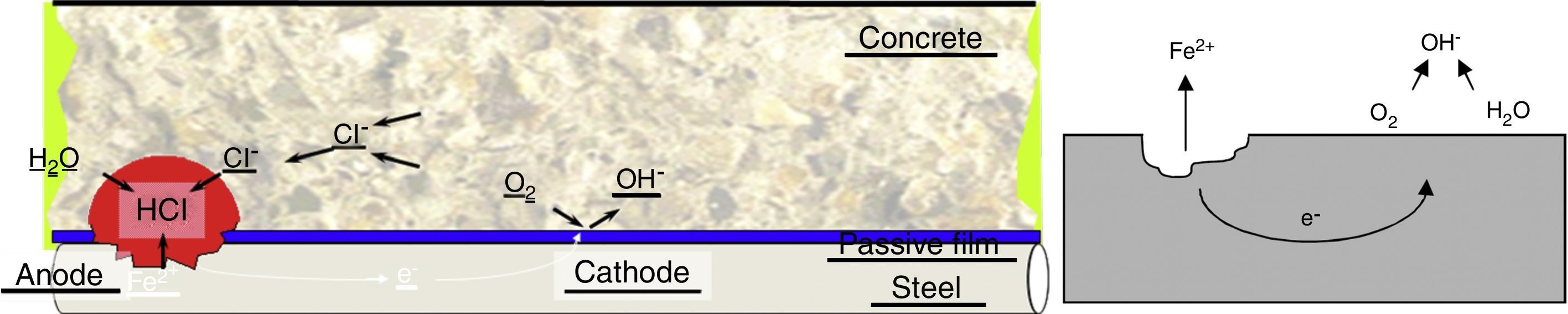
There are major differences between what is known as generalized corrosion and corrosion by chlorides. In the case of chloride corrosion, the chlorine acts as a catalyst for the reaction, causing it to accelerate at certain points producing what is named pit corrosion [4].
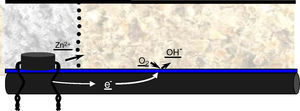
Chloride corrosion, which is generated from a reaction in chain, is usually located in a very limited area of the steel. This is started by the precipitation of particles of hydrochloric acid in that area, the acid dissolves the metal and accelerates the electrochemical reaction (Fig. 1). This phenomenon causes pathology in certain areas of the steel, with a very focused attack in the pit point. This makes it difficult to detect and there is abundant casuistry of failure of structures due to this type of corrosion.
2.2Chloride attack in reinforced concrete: repairing methodsThere are several repair systems that have traditionally been used with corroded reinforced concrete with chloride attack [5]. However, the technology has evolved with the experience and the monitoring of the evolution of the repaired structures:
- •
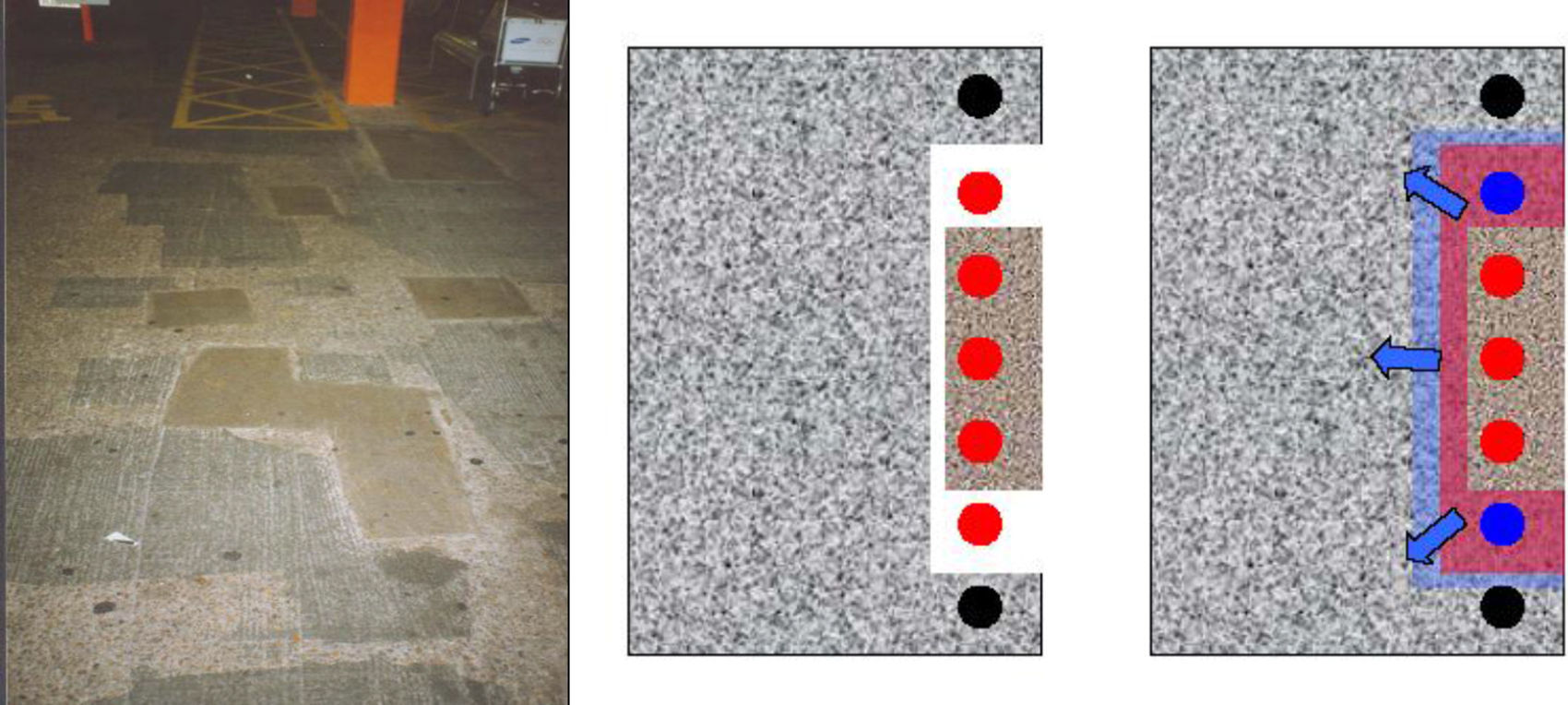
Conventional reparation in patches. This is the most traditional repair system. It consists of the clean up of the affected areas, uncovering the rebar and applying new mortar to restore the affected area to its original state. This is the fastest and most economical repair option. In case of severe corrosion, this type of repair can produce new corrosion in the perimeter of the repaired area (Fig. 2). Also, there is a “radial expansion” over time. This phenomenon is known as incipient anode.
- •
Repair with mortar with corrosion inhibitors. A breakthrough to conventional patch repair is the use of hydraulic mortars modified including migratory corrosion inhibitors. These are polymeric products, which create a protective film around the steel; with this, there is a reduction of the possibility of corrosion by chemical attack. The fact that they are migratory products makes these able to penetrate into the concrete mass located on the perimeter of the patch. The distance that these products can penetrate in the concrete mass is based on Fick's law.
- •
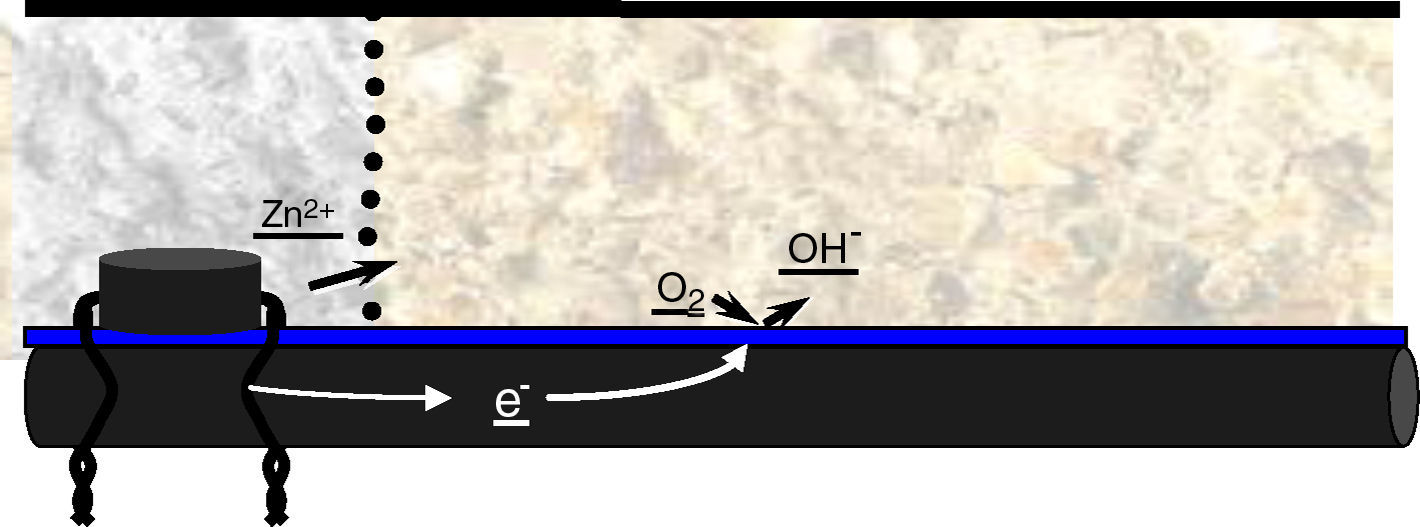
Cathodic protection with anodes of sacrifice. This method uses the knowledge of the scheme corrosion of steel in reinforced concrete, and in particular, the scheme of chloride attack. It applies sacrificial anodes in the perimeter of the repair; with this it prevents the creation of incipient anodes in the outside of the repair patch. Also, it is in compliance with the electrochemical scheme already established in the steel rebars. As stated previously, the corrosion of the sacrifice metal itself is at the same time, protecting the adjacent steel (Fig. 3). This is because the corrosion acidification leads to the re-alkalinization of the contiguous steel, protecting it thanks to the hydroxyl groups that migrate to these areas. This is the principle of cathodic protection, allow controlled corrosion (in this case the anode, not the steel bars), and the re-alkalinization and protection of steel in the vicinity of the anode.
- •
Cathodic protection with impressed current. Impressed current protection is another example of cathodic protection. The technique of impressed current is the use of an external power distributed by wiring to the entire structure and including a network of titanium anodes. With this it is achieved a distributed protection along the whole structure by means of the re-alkalization produced around the steel.
The motivation of the repair project was to accomplish the structural repairs that would allow extending the service life of the bridge. The intervention is divided into two parts: the deck repair and the reparation of piles. The division is made not only by the difference in the types of elements, but also by the different degrees of aggression that have the affected areas and treatments needed to be performed.
The sanitizing carried out in the damaged areas was performed with two methods, one manually by chipping hammer and the other by hydro-demolition machine, which proved to be the most effective method, both produced acceptable quality and cleanliness of the demolish (Fig. 4).
The repair system applied was one of cathodic protection of the steel structure. The type of cathodic protection selected was sacrifice anodes of zinc. This metal, thanks to its lower galvanic potential draws to itself the aggressive agents (chlorides and oxygen). Furthermore, this metal presents another useful feature, when oxidized it does not suffer a significative increase in volume (this is the biggest problem of corrosion of reinforcing steel embedded in concrete, which increase about 6–10 times its volume when oxidized).
One of the decisions taken at the beginning of repair, given the volume of work that had to be faced, was to use various products and patents on the market in time. This permitted to evaluate each of the products efficiency and applicability.
3.1Repairs in the deckThe major structural repairs on the deck were of a greater magnitude in areas close to the supports of the piles, at the junction flange-web of the deck and the junctions between vertical sides and the bottom of the girder.
It was detected that many existing corrosion problems had been caused by defects in the concrete placement. In addition, in the project of the bridge it was established to use continuous plastic along the rebars. This type of spacer for reinforcing bars was a novelty at the time and it favoured substantially the entry of aggressive agents more directly and quickly (Fig. 5).
The repair was carried out on the deck consisted of sanitizing each damaged area, replacement of steel in areas where the loss of section was excessive (Fig. 6). After this, placing discrete zinc anodes in the area of repair, next the application of thixotropic mortars. And finally, the entire surface of the deck (repaired and unrepaired areas) was painted with a special anti-chloride paint.
3.2Repair of the bridge pilesThe area of the piles the area of tidal and splash zone and therefore suffered a much higher risk of corrosion. In the project it was indicated the need of cathodic protection as in the deck but with a different approach. After inspection, it was clearly observed the need for a global repair instead of repair by batches as was performed in the deck.
The repair of the piles was structured and planned as follows:
- •
Conservation of one of the piles with conventional repair and without cathodic protection. This will serve as reference and permit comparison for the deterioration of the bridge without cathodic protection.
- •
Conventional repair+cathodic protection jacket system (Fosroc). This system consists of placing some jackets of fibreglass mesh+zinc batteries. Filling the gap between the pile and the jacket with an special concrete that allows the current flowing between the mesh of zinc and the reinforcement (Fig. 7). This system was used in a limited group of piles because of the difficulties of the works in the areas hit by the sea. All circuits for the reinforcement and the zinc anodes was conducted to the deck and connected in electric boxes protected inside de girder box. This makes monitoring over time easy.
- •
Conventional reparation+cathodic protection system with the hybrid system (CPT). The functioning of this technique stems from the introduction of sacrificial anodes in small holes in the structure and all embedded in a mortar of activation. The arrangement of sacrificial anodes was made based on a grid designed according to the detected damage and location relative to the different zones: tidal, splash, etc. These anodes are connected through titanium wire that closes the circuit against reinforcement steel bars, which in turn are connected in a connection box within the board for later supervision (Fig. 8).
The network of anodes and wiring permits the application of impressed current for a short period of time. This was performed initially after the repair works, however it can also be applied after a period of time since the intervention with a similar effect. What is seek is to produce the re-alkalization of the structure, with the consequent prevention of chloride attack. This delayed treatment would be possible simply using external batteries and for a very short period of application, between 1 and 2 weeks. Thereafter the system is disconnected from the battery, starting its operation as conventional sacrificial anodes. The new electrochemical equilibrium starts from steel in a substantially improved situation from its initial point due to the process of re-alkalization and previous chloride extraction technique.
3.3Control of corrosion evolutionAs mentioned above, the repair performed included innovative solutions in many aspects, which include monitoring by monitor the cathodic protection. It is possible to measure the connections between the sacrifice anodes and the steel reinforcement, they are accessible in boxes for collecting data that allows the tracking of the installed systems (Fig. 9).
The monitor is designed to take data selectively from different areas of the bridge (north side, south side, splash zone, tidal zone). In this way, it is planned a detailed monitoring of the installed systems. It would permit to compare different electric consumption in the cathodic protection of the different zones depending on the aggressiveness of the areas.
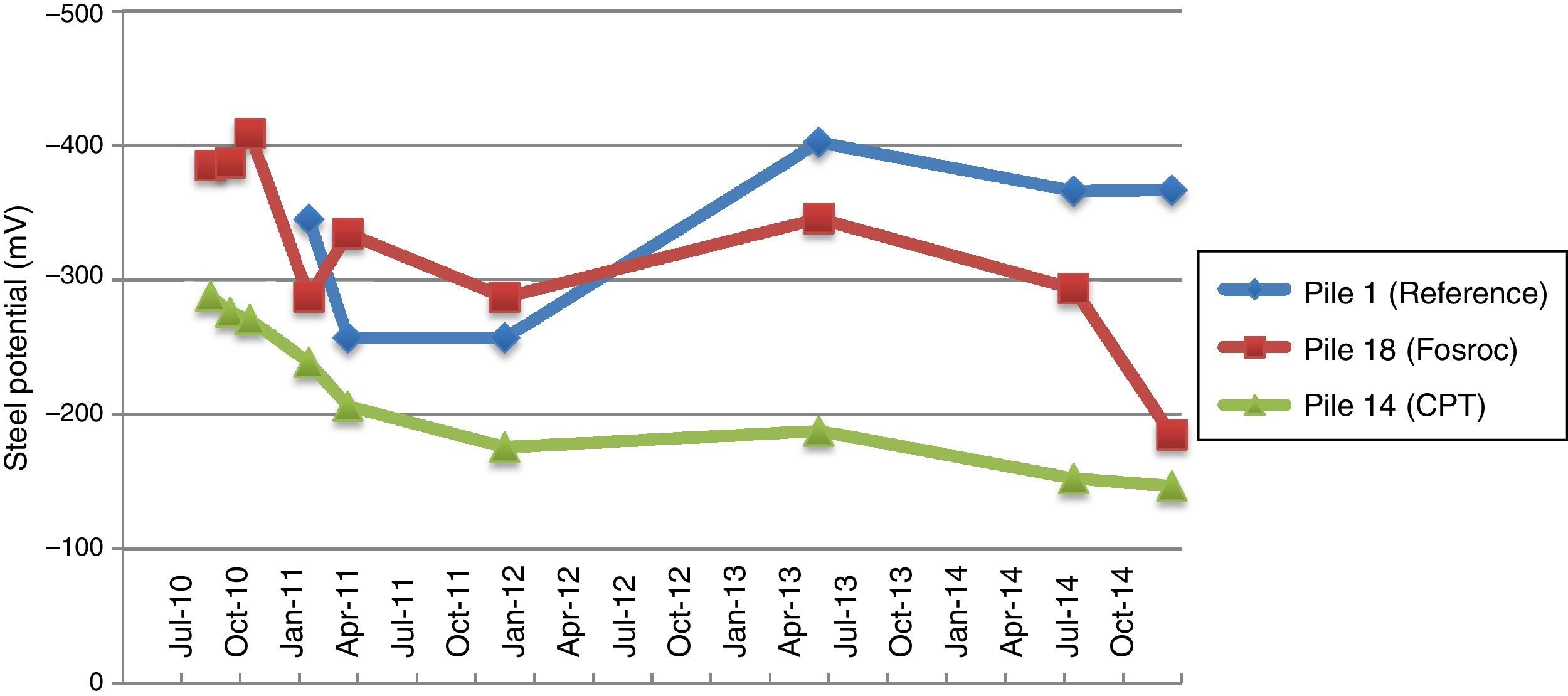
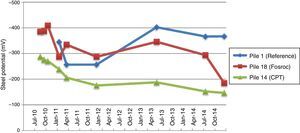
4Results of the evolution of the corrosionThe follow-up visits have produced extensive measurement campaign data. This enables to assess the correct behaviour of the cathodic protection. Ensuring that they fulfil the criteria established by UNE 12696: 2000 and EN 12696 [7]: 2012. Also, it has been made, in first instance, corrosion potential measurements according to ASTM C876-09 [8]. In Fig. 10 are represented results of pile potential in different areas of the bridge repair.
In the displayed graphic is observable the different behaviours on different cathodic protection systems employed in the repair of the bridge. As we can see in the case of pile 1, where it was performed a conventional repair, the corrosion potentials were after two years of the repair, below -350mV. With the predefined criterion of corrosion (less than −200mV), we would be in a case of highly probable corrosion [6].
In the case of the pile 14, in which it was used the system of discrete anodes with CPT hybrid cathodic protection, we see as a major change in the corrosion potentials, led by the first contribution impressed current applied at the initial time [9]. We see potential are above −200mV almost from the start, and the trend is almost horizontal with over the years.
The next case is the one of pile 18. In this pile it was used for galvanic protection the system of Fosroc GRP shirts that were covered with zinc anodes. In this case, the corrosion potentials have a different behaviour than the CPT system. As it can be seen, in this case the corrosion potentials increase rapidly after the repair work. With time, the protection system becomes more positive, slowly coming closer to the values given by the CPT system values. The fundamental difference is that this system does not give an initial contribution of impressed current, so that the potential of corrosion of steel are essentially the same as existed prior to the repair. Over time, the potential becomes increasingly positive; turning to over −200mV positive values, which indicates almost certainly the passivity of steel in reinforced concrete.
5ConclusionsThe paper summarizes the most common repair techniques used for repair of reinforced concrete problems with reinforcement corrosion due to chloride attack.
Among the major repair techniques used, they were discussed the following:
- •
Conventional repair by patches. This is the traditional solution. It consists of the sanitizing of the affected areas, uncovering the steel bars and applying a mortar to restore the affected area to its original state. This is the fastest and most economical repair option. However it is not effective in the long term, it can produce corrosion in the surroundings of the repaired zone.
- •
Corrosion inhibitors. A breakthrough to conventional patch repair is the use of hydraulic mortars modified with migratory corrosion inhibitors (MCI type). These are polymeric products that create a protective film around the steel that reduces the possibility of chemical attack.
- •
Cathodic protection with sacrificial anodes. The main problem posed by the conventional patch repair is the emergence of the phenomenon known as “incipient anodes”. This is the onset of corrosion phenomena in the perimeter of the repair once this. The option of placing sacrificial anodes on the perimeter of the repair avoids creating incipient anodes. This is the principle of cathodic protection, essentially produce corrosion (in this case focused and limited to the anode, not to the steel), and as side-effect the re-alkalinization and protection of the steel in the vicinity of the anode.
- •
Hybrid Cathodic protection with impressed current. This technique uses an external power distributed by wiring to the entire structure, as result, it induces the corrosion of sacrificial anodes protecting the reinforcement steel that behaves as cathode. It is achieved thereby an even distribution of protection along the whole structure and also it is provided with the re-alkalization around the steel.
Among the techniques used in the project Repair Repair Bridge Illa de Arousa, it was used primarily cathodic protection with sacrificial anodes on the deck (using galvanic current), and in the piles it was chosen to perform a test with the different protection systems. In this case, one of the piles was repaired with the conventional repair, to serve as control of the evolution of the corrosion over time. The result after a few years of this repair indicates possibility of corrosion due to the values of electrochemical potential measured.
For the rest of systems analyzed, we see the “CPT” hybrid system presented very favourable results from the beginning. The first phase of impressed current represents an advantage over other systems, the values of potential achieve higher values (above −200mV) faster than the Fosroc system. In this system, although corrosion potentials take longer to get in than −200mV values (which is the barrier taken as synonym for passivity of steel in concrete), it does not mean that the system is not working. The trend of the potential indicates that the system is operating, and there are more forms of assessment and other measurement techniques to make these checks.