The efficacy of bright light therapy (BLT) in ameliorating depression has been validated. The present study is to investigate the changes of depressive symptoms, cognitive function and cerebellar functional connectivity (FC) following BLT in individuals with subthreshold depression (StD).
MethodParticipants were randomly assigned to BLT group (N = 47) or placebo (N = 41) in this randomized controlled trial between March 2020 and June 2022. Depression severity and cognitive function were assessed, as well as resting-state functional MRI scan was conducted before and after 8-weeks treatment. Seed-based whole-brain static FC (sFC) and dynamic FC (dFC) analyses of the bilateral cerebellar subfields were conducted. Besides, a multivariate regression model examined whether baseline brain FC was associated with changes of depression severity and cognitive function during BLT treatment.
ResultsAfter 8-week BLT treatment, individuals with StD showed improved depressive symptoms and attention/vigilance cognitive function. BLT also increased sFC between the right cerebellar lobule IX and left temporal pole, and decreased sFC within the cerebellum, and dFC between the right cerebellar lobule IX and left medial prefrontal cortex. Moreover, the fusion of sFC and dFC at baseline could predict the improvement of attention/vigilance in response to BLT.
ConclusionsThe current study identified that BLT improved depressive symptoms and attention/vigilance, as well as changed cerebellum-DMN connectivity, especially in the cerebellar-frontotemporal and cerebellar internal FC. In addition, the fusion features of sFC and dFC at pre-treatment could serve as an imaging biomarker for the improvement of attention/vigilance cognitive function after BLT in StD.
Subthreshold depression (StD)/ subsyndromal depression exhibits 2–4 standard depressive symptoms that persist for 2 weeks or more, with at least one core symptom (depressed mood or anhedonia), but does not meet the diagnostic criteria for major depressive disorder (MDD) (Rodriguez, Nuevo, Chatterji & Ayuso-Mateos, 2012). Population-based studies have shown that StD is widespread among college students (Jiang et al., 2019; Mikolajczyk, Maxwell, Naydenova, Meier & El Ansari, 2008), and is a threatening precursor to MDD (Tuithof et al., 2018). Individuals with StD are at risk for developing other disorders, including substance use disorder, anxiety disorders, and even suicidal tendencies (Cuijpers et al., 2021). Accordingly, early and effective treatment of StD is crucial, and to prevent the onset of MDD.
There is a high prevalence of cognitive impairments among people with MDD, even after remission (Bo et al., 2019; Monzon et al., 2010). Meta-analyses reported that deficits of cognitive functions were identified in patients with first-episode drug-naïve MDD (Lee, Hermens, Porter & Redoblado-Hodge, 2012) and their first-degree relatives (MacKenzie, Uher & Pavlova, 2019). Cognitive deficits in MDD have been found in many domains of function, including attention, verbal learning/memory and executive function. Moreover, even subthreshold depressive symptoms are recently reported associated with multiple negative outcomes, including cognitive dysfunction (Dotson et al., 2020; Hybels, Blazer & Pieper, 2001). Cognitive dysfunction had been sporadically emphasized in individuals with StD, (Dotson et al., 2020; Hwang et al., 2015; Hybels et al., 2001; Lv et al., 2023) involving impairments in cognitive processing (memory, decision making, cognitive inflexibility) and cognitive biases (negative thoughts) (Hwang et al., 2015; Lv et al., 2023). However, the available evidence is constrained, and the associations between specific dimensions of cognitive dysfunction and StD remains under-elaborated.
The efficacy of antidepressant medication in relation to placebo is positively correlated with the severity of depression symptoms, and it is possible that the benefits are negligible or absent in patients with mild or moderate symptoms, on average (Fournier et al., 2010). Numerous studies have shown that antidepressants are limited-effect in StD (Barbui, Cipriani, Patel, Ayuso-Mateos & van Ommeren, 2011; Fournier et al., 2010), and antidepressants should not be routinely used to treat StD (Barbui et al., 2011; Fournier et al., 2010). Additionally, psychological intervention and stepped-care approaches have the disadvantages of slow onset, low remission rate and high cost (Kroenke, 2017). Bright light therapy (BLT) is a kind of physical therapy with early effect, few adverse reactions, patient friendliness and low cost. In recent years, more and more evidence has confirmed the effectiveness of BLT for seasonal depression (Pjrek et al., 2020), bipolar depression (Hirakawa & Terao, 2020), juvenile depressive disorder (Krysta, Krzystanek, Janas-Kozik & Krupka-Matuszczyk, 2012), and senile depressive disorder (Zhao, Ma, Wu, Chi & Bai, 2018). BLT has been proven to have the same therapeutic effect as antidepressants and is well tolerated in the treatment by patients (Jiang et al., 2020; Lam et al., 2016). Previous work of our team found that BLT was effective in relieving depressive and anxiety symptoms in individuals with StD by increasing the functional connectivity (FC) between the midbrain and frontal cortex (Chen et al., 2023; Jiang et al., 2020). In addition, the effects of light therapy go beyond mood improvement to improve cognition functions (Huang, Tao & Ren, 2023; Riemersma-van der Lek et al., 2008), including alertness (Mu et al., 2022; Pachito et al., 2018), attention (Liu, Liou & Jou, 2021), memory (Huang et al., 2021), and executive function (Killgore, Vanuk, Shane, Weber & Bajaj, 2020) as well. For example, a RCT study found that blue wavelength light therapy in relation to placebo can improve subjective and objective attention/alertness cognitive function in patients recovering from mild traumatic brain injury (Killgore et al., 2020). Another study on older adults with dementia reported that BLT showed greater improvements in attention and calculation comparing to general lighting (Liu et al., 2021). To date, clinical studies are lacking to investigate whether BLT would improve cognitive function in MDD or StD. And the neurobiological mechanism by which BLT alleviate symptoms of depression and cognitive dysfunction in non-seasonal StD is unclear.
The cerebellum has a well-established role in motor coordination and also plays a critical role in non-motor functions of emotion and cognition (Carta & Chen, 2019). The cerebellum exhibits intrinsic connectivity with the cerebrum and cerebellar axons form synaptic connections directly with dopamine neurons (Carta & Chen, 2019; Schmahmann, 2019). Cerebellar impairment leads to abnormal dopamine levels and function, which can affect mood and cognitive function (Carta & Chen, 2019; Schmahmann, 2019). Previous meta-analyses have shown that the severity of depressive symptoms in patients with MDD is related to abnormal functional activities and structure of the cerebellum (Arnone et al., 2016; Gong et al., 2020; Jiang et al., 2017). Functional MRI evidence reported that improvement of depressive mood by light was accompanied by a strong activation response of cerebellum in patients with MDD (Fan-peiGloriaYang et al., 2020). The default mode network (DMN) is a core brain network that is widely disrupted in MDD and StD (Clemm von Hohenberg et al., 2018; Hwang et al., 2016; Yokoyama et al., 2018). Previous resting-state functional magnetic resonance imaging (rs-fMRI) studies have shown that the DMN are strongly connected with the bilateral cerebellar Crus I, Crus II, and lobular IX subregions (Buckner, Krienen, Castellanos, Diaz & Yeo, 2011; Diedrichsen, Balsters, Flavell, Cussans & Ramnani, 2009; Halko, Farzan & Eldaief, 2014). The abnormalities of resting-state static functional connectivity (sFC) and dynamic functional connectivity (dFC) in the cerebellum-DMN circuits were reported in MDD (Ding et al., 2022; Wang et al., 2023; Zhu et al., 2020) and bipolar depression (Chen et al., 2019; Luo et al., 2018). Moreover, it was found that patients with MDD showed improved FC in the cerebellum-DMN circuits after electroconvulsive therapy (Wang et al., 2018) and antidepressant therapy (Wang et al., 2020). However, the role of the cerebellum-DMN circuits in the improvement of emotion and cognition in StD by BLT remains unclear.
In this randomized placebo-controlled trial study, we investigated the impact of BLT on mood, cognition, and brain function in young individuals with StD. We hypothesized that BLT would result in greater improvement in depression symptom severity and cognitive dysfunctions in individuals with StD compared with placebo. We additionally hypothesized that BLT treatment would change static and dynamic FC within the cerebellum-DMN circuits of StD, and that these effects would be associated with improvement in mood and cognitive functions. Finally, we also explored whether the fusion of pre-treatment sFC and dFC of the cerebellum would predict BLT treatment outcomes.
Methods and materialsParticipantsAll StD participants were recruited through leaflet and advertisements at Jinan University in Guangzhou, China, from March 2020 to June 2022. All participants have 2–4 standard depressive symptoms that persist for 2 weeks or more, with at least one core symptom (depressed mood or anhedonia). These symptoms have no seasonal pattern. In addition, the climate of Guangzhou, China, is not clearly divided into seasons. The inclusions were as follows: (1) age 18–28 years; (2) right-handed, Han nationality; (3) Center for Epidemiologic Studies Depression Scale (CESD) score ≥16; this cutoff has been used in previous studies of clinical depression (Buntrock et al., 2016; Jiang et al., 2019). (4) 8 ≤ 24-item Hamilton Depression Rating Scale (HDRS-24) score≤20. The exclusion criteria were as follows: (1) Diagnosed with MDD, bipolar disorder, schizophrenia, or another mental disorder according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-5); (2) Received any form of depression interventions within a year; (3) had MRI contraindications; (4) Any presence of a concurrent and major physical illness; (5) Suicidal ideation or behavior; (6) Lactating or pregnant.
The study was approved by the ethics committee of the First Affiliated Hospital of Jinan University and registered prior to the onset of the study (trial registration: ChiCTR2000032633). All participants provided written informed consent after receiving a complete description of the study.
Study designTo evaluate the effect of BLT on the improvement of clinical symptoms and cognitive functions of StD and on the cerebellar sFC and dFC based on randomized controlled trials, StD participants were randomly allocated to BLT group and the placebo group with randomization codes. The BLT group was treated with 5000 lux for 8 weeks, and placebo group was treated with an inactive device (emitting weak light < 5 lux) for 8 weeks (Chen et al., 2023; Jiang et al., 2020). Clinical symptoms and cognitive functions were assessed at baseline and 8 weeks after treatment, and structural and functional MRI data were also collected. The researchers responsible for clinical symptoms and cognitive functions assessment, MRI data collection and analysis did not know the intervention grouping. Non-blind researchers assigned participants, handed out light boxes, and provided instructions on how to use them.
InterventionsThe intervention consisted of daily exposure to a custom-made lightbox (LED) for 30 min in the morning (before 12 p.m.). The light intensity was set at 5000 lux. The devices measured 60 × 30 × 1.5 cm, with small blue-white LEDs inside, 5000-Kelvin color temperature, and 100 % UV filter. The participants were instructed to place the device on a desk 50 cm away and fully expose their faces to the light without staring at it. The status of light (on and off) was monitored online to ensure adherence of the participants. The placebo treatment device was identical to that of the intervention group but was deactivated to emit weak light (<5 lx). The two switch lights were on for both intervention and placebo groups, suggesting that the light was power-on and turn-on. The participants in the intervention and control groups used the light box at their homes and were provided with same standardized verbal and written instructions.
Assessment of mood scaleHDRS-24 (Hamilton, 1960, 1967), CESD (Radloff et al., 1997), Positive and Negative Affect Scale (PANAS) (Watson, Clark & Tellegen, 1988) were used to assess mood. HDRS-24 score was used to assess the changes in depression symptoms from pre- to post-treatment (Jiang et al., 2020; Rolle et al., 2020). Remission is defined as having an HDRS score of ≤7 at post-treatment (Dunlop et al., 2023).
Assessment of cognitive functionCognitive appraisal was conducted using the Chinese version of Measurement Consensus Cognitive Battery (MCCB) (Chen et al., 2022; Nuechterlein, Green & Kern, 2023; Shunkai et al., 2023). MCCB is an accurate and effective measure of cognitive deficits that has established reliable clinical validity and retest reliability in patients with depression (Huang, Lai et al., 2023; Shi et al., 2015). The MCCB assessment process takes approximately 70 min. MCCB contains seven domains of cognition functions, including processing speed, attention/vigilance, working memory, verbal learning, visual learning, reasoning problem-solving, social cognition, and overall composite scores (detailed in supplementary materials). MCCB assessment was conducted by two trained graduate students, and the correlation coefficient between two raters was greater than 0.8.
MRI data acquisitionThe rs-fMRI and three-dimensional brain volume imaging (3D-BRAVO) data were obtained using an 8-channel phased array head coil on the GE Discovery MR750 3.0T system (supplementary materials).
Data preprocessingThe preprocessing was carried out using Data Processing Assistant for Resting-State fMRI (DPABI_V3.0, http://restfmri.net/forum/DPABI) (C. Yan, Wang, Zuo & Zang, 2016) which is based on Statistical Parametric Mapping (SPM12, http://www.fil.ion.ucl.ac.uk/spm/) (supplementary materials).
Seed-based sFC and dFC analysisTo calculate the sFC and dFC between the cerebellar subdivision and the whole brain voxels, six cerebellar subdivisions from the cerebellum were defined by DPABI_V3.0 (http://restfmri.net/forum/DPABI) as seeds for sFC and dFC analysis. We extract bilateral cerebellar subdivision Crus I, Crus II, and lobule IX from the probabilistic cerebellar atlas with a minimum probability threshold of 75 % (Diedrichsen et al., 2009) (Fig. 2A). The cerebellar probabilistic atlas (spatially unbiased infratentorial template, SUIT) has been widely applied in bipolar disorder (Chen et al., 2019), persistent postural perceptual dizziness (Lee, Lee & Kim, 2018), and Alzheimer disease (Zheng, Liu, Song, Li & Wang, 2017).
For sFC analysis, the Pearson's correlation coefficients between the seed point reference time series of each cerebellar subdivision and all the other brain voxels. Then, to improve the normality, Fisher's r-to-z transformation was used to convert the obtained correlation maps into z-values maps.
For dFC analysis, the dFC variability characteristics of the cerebellar subdivisions were calculated using the sliding-window method based on the Temporal Dynamic Analysis (TDA) toolkits integrated in the DPABI software (http://rfmri.org/DPABI). More details on dFC analyses were added in the supplementary materials.
Statistical analysisSample size was estimated in supplementary materials. Demographics (for age and education), mood scale and cognitive function (pre-treatment, post-treatment, and the changes from pre- to post-treatment) were compared between BLT and placebo groups by independent-sample t-tests or chi-squared tests (for sex) using IBM SPSS Statistics 22 (Armonk, NY, USA). The Kolmogorov–Smirnov test was used for data normality verification. Effect sizes (Cohen's d) of depression scores were calculated for the mean differences between pre-treatment and post-treatment in BLT group. Benjamini-Hochberg false discovery rate (BHFDR) was conducted for the multiple comparison correction for the comparisons of mood scale and cognitive function. The statistical threshold was set at p < 0.05, two-tailed.
Statistical analysis of sFC and dFCA voxel-wise linear mixed analysis model was used to evaluate the interaction effects of the whole brain sFC and dFC differences in each ROI seed between BLT and placebo groups (treatment × time). The linear mixed analysis model included age, sex, education, and mean FD as nuisance covariates. The interaction F statistical maps for each seed were corrected for multiple comparisons with Gaussian random field (GRF) correction (voxel pvalue < 0.005; cluster pvalue < 0.05/6 = 0.0083). To identify the group differences underlying the significant interaction effects, the mean sFC and dFC values were extracted from clusters identified by voxel-wise analysis using DPABI. Then, the sFC and dFC in clusters of effect was compared between baseline BLT and placebo as well as between post-treatment BLT and placebo using independent t-tests (inter-group). Then, post-hoc analysis compared the pre- and post- in each treatment group (intra-group).
Correlation between the cerebellar subdivision FC (sFC and dFC) changes and mood scale and cognitive function changesWhen different sFC and dFC values of interaction effect clusters between pre- and post-treatment were observed in the StD BLT group, Pearson's correlation analysis was used to examine the correlations between the sFC and dFC changes and mood scale changes and cognitive function changes within groups (p < 0.05, two-tailed).
Prediction of mood scale and cognitive function changesA multivariate regression model was utilized to predict the mood scale and cognitive function changes by the fusion of sFC and dFC at pre-treatment in the BLT group (supplementary materials).
ResultsParticipantsA total of 153 participants were assessed for eligibility during the baseline visit, and 136 (89 %) young volunteers were invited to participate in this randomized controlled trial. The eligible participants were included in the trial after providing a written informed consent and then were randomly assigned to either 68 BLT or 68 placebo group. 106/136 (78 %) participants, including 55 BLTs and 51 placeboes, provided clinical assessment and underwent fMRI scanning before and after treatment. Among these completers, 36 of 55 (65.5 %) individuals treated with BLT and 11 of 51 (21.57 %) individuals treated with placebo achieved remission. No difference was observed in baseline demographics and clinical characteristics between the BLT and placebo groups (Table 1). For rs-fMRI data, 8 participants in the BLT group and 10 participants in the placebo group were excluded because of poor quality of rs-fMRI data, excessive head movement, or lack of coverage of the whole brain. Among those randomized, 47 participants in the BLT and 41 in the placebo group were included in the final rs-FC analysis (Fig. 1). The comparison of participants’ demographics and treatment outcomes between 47 BLTs and 41 placeboes is shown in Table S1.
Participant demographics, comparisons of mood scale and cognitive function between BLT and placebo.
Mean (with standard deviation in parentheses) are reported unless otherwise noted. BLT, bright light therapy; HDRS, Hamilton Depression Rating Scale; CESD, Centre for Epidemiologic Studies Depression Scale; PANAS, Positive and Negative Affect Scale; FDR, false discovery rate. †, The p value for gender distribution was obtained by chi-square test. a, the p values were obtained by independent-sample t-tests. *, p < 0.05, **, p < 0.01, ***, p < 0.001.
As expected, the post-treatment group exhibited significantly greater decreases in depression scores than the pre-treatment in BLT group (p < 0.001 [effect size (Cohen’ s d) = 1.63]). BLT group displayed a significant decrease in HDRS (t = 2.997, FDR-p = 0.015), CESD (t = 2.317, FDR-p = 0.038), PANAS-N (t = 2.208, FDR-p = 0.038) scores from pre-treatment to post-treatment compared to placebo. No difference was observed in PANAS-P (p > 0.05) scores between the BLT and placebo groups. In addition, a significant increase in attention/vigilance cognitive function from pre-treatment to post-treatment compared to placebo (t = 2.567, p = 0.012), but did not survive FDR correction. No difference was observed in changes of other cognitive function between BLT and placebo groups (p > 0.05) (Table 1).
Seed-based sFCLeft cerebellar Crus I. Whole-brain analyses showed a significant interaction between treatment and time in sFC between the left cerebellar Crus I and left cerebellum posterior lobe (CPL) (F = 17.636, p < 0.0083). Post-hoc analyses indicated that BLT group had lower sFC than placebo group in post-treatment. The post-treatment sFC was increased compared with pre-treatment in placebo group, while no differences were detected between pre- and post-treatment in BLT group (Fig. 2B and Table 2). Additionally, the post-treatment sFC of left cerebellar Crus I-left CPL was decreased compared with pre-treatment in BLT remission group while it was not significant in the non-remission group (Fig. S1A and Table S2).
The whole brain static and dynamic FC differences in the cerebellar subdivisions between BLT and placebo groups. (A) The subdivision of the cerebellum. (B) The whole brain and the post-hoc comparisons of sFC and dFC in the cerebellar subdivisions between BLT and placebo groups. sFC, static functional connectivity; dFC, dynamic functional connectivity; CPL, cerebellum posterior lobe; TP, temporal pole; mPFC, medial prefrontal cortex; BLT, bright light therapy; L, left; R, right.
Significant interaction effect of sFC and dFC of subregion in the cerebellum between BLT and placebo at pre and post-treatment.
sFC, static functional connectivity; dFC, dynamic FC; BLT, bright light therapy; BA, Brodmann region; MNI, Montreal Neurological Institute; SFG, superior frontal gyrus; L, left; R, right.
Left cerebellar Crus II. Whole-brain analyses showed a significant interaction between treatment and time in sFC between the left cerebellar Crus II and the bilateral CPL (F = 22.367, p < 0.0083). Post-hoc analyses indicated that BLT group had lower sFC than placebo group in post-treatment. The post-treatment sFC was decreased compared with pre-treatment in BLT group while opposite treatment change was observed in the placebo group (Fig. 2B and Table 2). Additionally, the post-treatment sFC of left cerebellar Crus II-bilateral CPL was decreased compared with pre-treatment in BLT remission group while it was not significant in the non-remission group (Fig. S1B and Table S2).
Right cerebellar lobule IX. Whole-brain analyses showed a significant interaction between treatment and time in sFC between the right cerebellar lobule IX and left CPL (F = 20.314, p < 0.0083) and between the right cerebellar lobule IX and left TP (F = 14.827, p < 0.0083) (Fig. 2B and Table 2).
For the right cerebellar lobule IX-left CPL sFC, post-hoc analyses indicated that compared to the placebo group, the BLT group had higher sFC at pre-treatment and lower sFC at post-treatment. Post-treatment sFC was decreased compared with pre-treatment in BLT group while opposite treatment change was observed in the placebo group (Fig. 2B and Table 2). Additionally, decreased sFC of right cerebellar lobule IX-left CPL was observed in the post-treatment BLT remission group and non-remission group when compared with pre-treatment (Fig.S1C and Table S2).
For the right cerebellar lobule IX-left TP sFC, post-hoc analyses indicated that compared to the placebo group, the BLT group had lower sFC at pre-treatment and higher sFC at post-treatment. Post-treatment sFC was increased compared with pre-treatment in BLT group while opposite treatment change was observed in the placebo group (Fig. 2B and Table 2). Additionally, increased sFC of right cerebellar lobule IX-left TP was observed in the post-treatment BLT remission group while it was not significant in the non-remission group (Fig.S1D and Table S2).
Seed-based dFCRight cerebellar lobule IX. Whole-brain analyses showed a significant interaction between treatment and time in dFC between the right cerebellar lobule IX and left medial prefrontal cortex (mPFC) (F = 19.983, p < 0.00625). Post-hoc analyses indicated that compared to the placebo group, the BLT group had higher dFC at pre-treatment and lower dFC at post-treatment. Post-treatment dFC was decreased compared with pre-treatment in BLT group while opposite treatment change was observed in the placebo group (Fig. 2B and Table 2). Additionally, decreased dFC of right cerebellar lobule IX-left mPFC was observed in the post-treatment BLT non-remission group while it was marginal significantly decreased in the remission group (Fig.S1E and Table S2).
Additionally, decreased dFC of right cerebellar lobule IX-left mPFC was observed in the post-treatment BLT non-remission group while it was marginal significantly decreased in the remission group (Fig.S1E and Table S2).
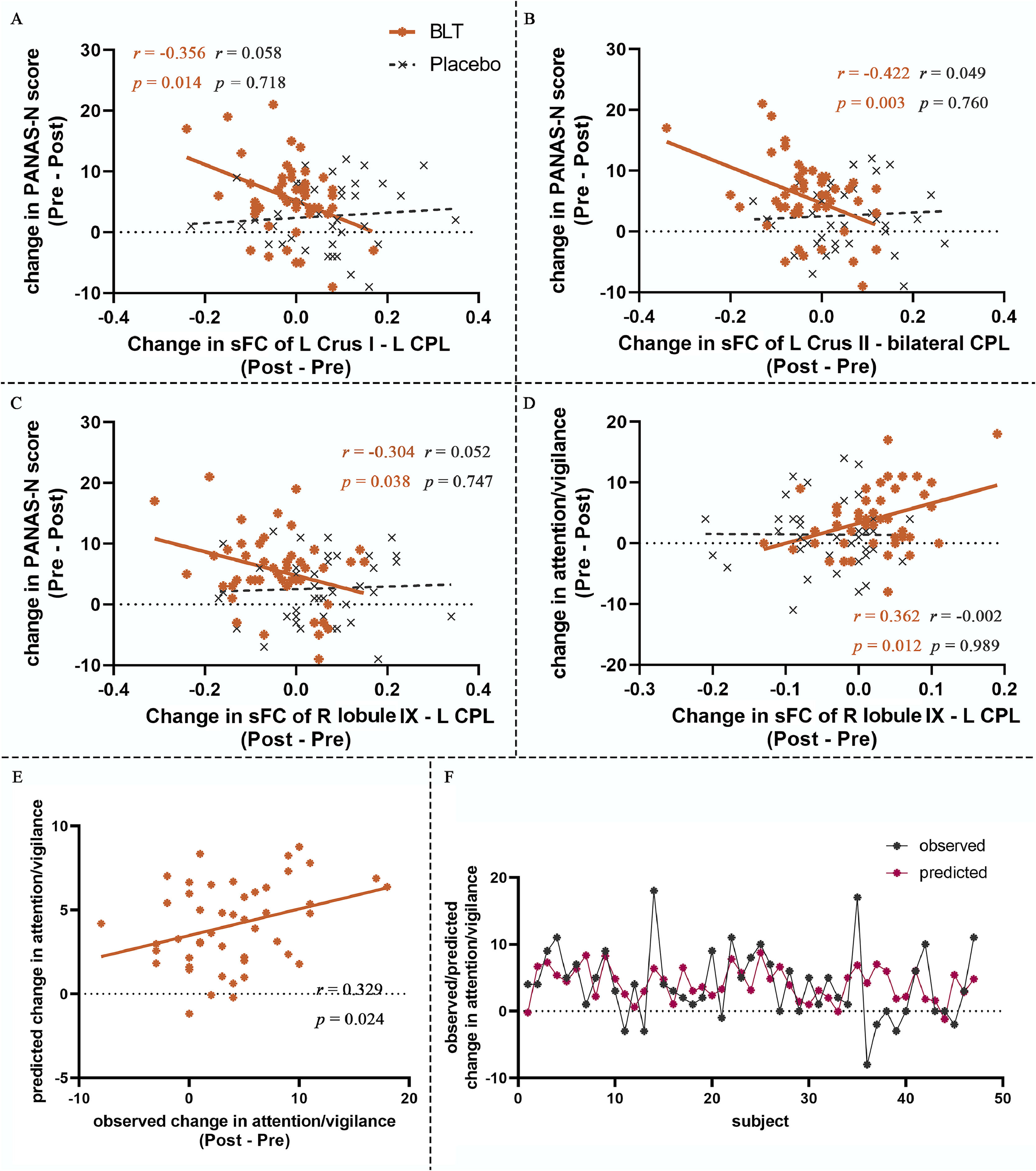
Correlation between FC changes, mood scale changes and cognitive function changes in BLTFor sFC, in the BLT group, we observed a significant positive correlation between the changes of PANAS-N and the changes of left cerebellar Crus I-left CPL sFC (r = 0.356, p = 0.014), the changes of left cerebellar Crus II-bilateral CPL sFC (r = 0.422, p = 0.003), and the changes of right cerebellar lobule IX-left CPL sFC (r = 0.304, p = 0.038), respectively (Fig. 3A-C). A significant correlation was observed between the changes of attention/vigilance score and the changes of right cerebellar lobule IX-left TP sFC (r = 0.346, p = 0.023) in the BLT group (Fig. 3D). No significant correlations were detected between all sFC changes, mood scale changes and cognitive function changes in the placebo group (p > 0.05). And no significant correlation was detected between dFC changes, mood scale change and cognitive function changes in BLT or placebo group (p > 0.05).
The correlation between static and dynamic FC changes and mood scale and cognitive functions changes as well as prediction of attention/vigilance changes. (A) The correlation between sFC changes (left cerebellar Crus I – left CPL) and PANAS-N changes in BLT. (B) The correlation between sFC changes (left cerebellar Crus II − bilateral CPL) and PANAS-N changes in BLT. (C) The correlation between sFC changes (right cerebellar lobule IX − left CPL) and PANAS-N changes in BLT. (C) The correlation between sFC changes (right cerebellar lobule IX − left CPL) and attention/vigilance changes in BLT. (E-F) Prediction of attention/vigilance changes from fusion sFC and dFC at pre-treatment of cerebellar subdivisions. sFC, static functional connectivity; dFC, dynamic functional connectivity; CPL, cerebellum posterior lobe; PANAS, Positive and Negative Affect Scale; BLT, bright light therapy; L, left; R, right.
We found that the fusion of sFC and dFC in brain regions with significant treatment-time interactions at baseline in the BLT group could predicted the changes in attention/vigilance in individuals with StD after BLT (r = 0.317, p = 0.0146) (Fig. 3E-F), but could not predict changes of mood scales and other cognitive functions (p > 0.05). Separate sFC and dFC could not predict changes of mood scales and cognitive functions (p > 0.05). No significant prediction was observed in the placebo group either (p > 0.05).
DiscussionTo the best of our knowledge, this is the first study to explore the effect of BLT on improving mood and cognition, and the corresponding cerebellar FC changes among young individuals with StD using rs-fMRI. After the 8-week BLT treatment, StD individuals showed improved clinical symptoms of depression and attention/vigilance. The rs-fMRI results showed that, BLT increased the sFC between the right cerebellar lobule IX and left TP, as well as decreased short range sFC within the posterior lobes of cerebellum, and decreased dFC between the right cerebellar lobule IX and left mPFC. The altered sFC within the cerebellum was associated with improved negative affect, and the changed sFC of the cerebellar lobule IX -TP was associated with improved attention/vigilance. Moreover, in BLT-treated (but not placebo-treated) StD individuals, the fusion of BLT-related sFC and dFC of the cerebellum at baseline predicted the improvement of attention/vigilance.
Compared to placebo, BLT increased sFC between the right cerebellar lobule IX and left TP in StD. The TP have been linked to many higher-order cognitive-affective functions, but indeed the region's precise functions remain largely undiscovered (Herlin, Navarro & Dupont, 2021). Several studies demonstrated that temporal pole may be in “transition zones” bridging between networks involved in internally-focused cognitive states (i.e., DMN) and emotion processing (i.e., limbic areas) (Stern et al., 2022; Uddin, Yeo & Spreng, 2019; Yeo et al., 2011). Even in individuals with StD, reduction of gray matter volume (Webb, Weber, Mundy & Killgore, 2014) and functional activity (Zhang et al., 2020) were observed in the TP, which was associated with the severity of depressive symptoms (Webb et al., 2014). Functional connectivity strength increases in the cerebellum and TP were also reported in depressive patients (Wang et al., 2016). Treatments of ECT and probiotics would increase the functional connectivity of TP that correlated with the reduction of depressive symptoms (Argyelan et al., 2016; Liu et al., 2015; Yamanbaeva et al., 2023). It was suggested that the antidepressant effect of treatments may be mediated by regulation of functional activity and connectivity (Argyelan et al., 2016). Therefore, these findings suggested that BLT may effectuate by restoring dysfunctional cerebellum-TP connectivity in StD.
Furthermore, we found that a significant increase in cerebellar lobule IX-TP connectivity was associated with the enhanced attention/vigilance ability in the BLT treatment group. Cerebellar injury causes abnormal levels and functions of dopamine in the brain, resulting in cognitive dysfunction such as attention and social behavior (Carta & Chen, 2019). Since the cerebellum has efferent pathways to the nigra, and depression is associated with a lack of biomonoamine, Schutter et al. found that stimulation of the cerebellum by repetitive transcranial magnetic stimulation (rTMS) increased attention and alertness in depressed patients, possibly stimulating the release of dopamine and leading to changes in temporal cortex activity (Schutter, van Honk, d'Alfonso, Peper & Panksepp, 2003). In addition, a functional fMRI meta-analysis found that patients with attention deficit showed hypoactivation in the TP, which considered as an important region associated with attention deficit (Cortese et al., 2016). A single-photon emission computed tomography imaging study also reported that hypometabolism in the TP was associated with the decreased performance on tests of attention and memory (Willeumier, Taylor & Amen, 2012). A previous study demonstrated that the effects of medication treatment effects could modify functional connectivity of the TP in patients with bipolar depression during the execution of attentional paradigm task (Li et al., 2022). Taken together, after BLT, the changes of FC between the cerebellum and TP plays an important role in the improvement of attention and emotion in individuals with StD.
In the study, BLT reduced the short range sFC within the cerebellum in individuals with StD, including sFC between the left cerebellar Crus I and left CPL, the left cerebellar Crus II and bilateral CPL, and the right cerebellar lobule IX and left CPL. Previous rTMS studies have shown that treating the cerebellum as a therapeutic target can improve depressive mood in patients with MDD (Davis et al., 2023; Schutter & van Honk, 2005; Schutter et al., 2003). A longitudinal rs-fMRI study of ketamine in the treatment of MDD suggested that post-ketamine cerebellar global sFC reduced following successful treatment (Abdallah et al., 2017). A recent study on rats revealed that chronic light deprivation leads to abnormal increased cerebellar glutamate and cortisol levels, causing the accumulation of inflammatory cells in the cerebellum, and successful treatments can reverse pathological changes in the cerebellum (Sarena et al., 2022). Moreover, we also found that changes of sFC within the cerebellum were correlated with the improved negative affect in the BLT group. Negative affective states, including behavioral despair, anhedonia, heightened stress reactivity, decreased motivation, and dysphoria are symptom clusters observed in a plethora of neuropsychiatric disorders, including depression (Tejeda & Bonci, 2019). An emotion task fMRI study indicated that changes of cerebellar activation were associated with negative emotional improvement in patients with MDD following antidepressant treatment (Cullen et al., 2016). Therefore, alterations in cerebellar FC may serve as crucial pathophysiological mechanism underlying the improvement of negative affect states following BLT in StD.
In addition, BLT decreased dFC between the right cerebellar lobule IX and left mPFC in StD, suggesting BLT intervention could reduce FC variability. The dFC analysis methods were used to measure the spatial variability of FC, to assess the time-varying characteristics of brain region connections throughout the scanning period (Hutchison, Womelsdorf, Gati, Everling & Menon, 2013; Liao et al., 2019). The dFC has been widely regarded as a powerful supplement to sFC (Kam, Zhang, Jiao & Shen, 2020), and the combination of sFC and dFC can help to better depict a full picture of rs-FC abnormalities related to mental disorders (Liao et al., 2018; Rashid et al., 2016). An animal study based on optogenetic technology found that cerebellar axons directly form synaptic connections with midbrain dopamine neurons, and then project to the prefrontal cortex (Carta & Chen, 2019). The mPFC is a core region of the DMN, dysfunctions of connectivity between mPFC and multiple regions (including the cerebellum) may best explain the breadth of mood and cognitive impairments observed in depression (Chen et al., 2019; Price & Duman, 2020; Yan et al., 2023). A recent dynamic amplitude of low-frequency fluctuation (dALFF) study reported increased dALFF in the CPL and prefrontal cortex in first-episode, drug-naïve MDD, suggested dynamic spontaneous neural activity changes in frontal cortex-cerebellum-cortical circuit may occur early in the course of MDD (Zheng et al., 2021). One task based fMRI study found that patients with MDD relative to healthy controls showed increased FC between the mPFC and cerebellar subregions during the social cognition task (Tepfer, Alloy & Smith, 2021). A multicenter study reported that mPFC-based dFC across the whole brain were significantly correlated with depressive rumination of patients with MDD, and the cerebellar-mPFC dFC stood out as particularly important for this model (Kim et al., 2023). Another multicenter study with large sample found that MDD patients on medication displayed reduced FC of the DMN, including the mPFC, when compared to first-episode MDD patients who had not yet received any medication treatment (Yan et al., 2019). It was suggested that the findings of reduced DMN FC in MDD could be attributed to treatment effect, which may alleviate depressive symptoms by reducing DMN FC (van Wingen et al., 2014; Yan et al., 2019). In short, our findings provided evidence that, along with improvements of depressive symptoms and cognitive function, the cerebellum-DMN dFC variability of StD could change following BLT intervention.
Notably, the present study initiatively identified improvement of not only mood but also cognitive functions in individuals with StD following BLT. A complete set of cognitive function tests were conducted, including processing speed, attention/vigilance, working memory, verbal learning, visual learning, reasoning problem-solving, and social cognition. We had identified specific cognitive improvement in attention/vigilance domain in individuals with StD following BLT. More recently, a study on patients with depression found that impaired attention/vigilance have a negative impact on quality of life (Lu et al., 2023). Vigilance is sometimes called "sustained attention" and refers to the ability to maintain a readiness to respond for a period of time, attention/vigilance is a crucial cognitive ability in community and work functioning (Green, Kern, Braff & Mintz, 2000). More importantly, the current study found that fusion of sFC and dFC in brain regions with significant treatment-time interactions before treatment in the BLT group could significantly predicted increased attention/vigilance in individuals with StD after BLT. It was generally accepted that investigating from both static and dynamic perspectives could yield more complementary information compared to relying on a single method, ultimately enhancing predictive accuracy (Fu et al., 2019). Several fMRI studies had proven that the combination of sFC and dFC could predict depressive symptoms (Kang et al., 2023; Li et al., 2023; Pang et al., 2020) and therapeutic effect (Li et al., 2023) in patients with depression. Therefore, the static and dynamic FC of cerebellum to predict attention/vigilance could somehow guide the treatment strategy for individuals with StD.
There were several limitations in the study. First, the participants exhibit a relatively high level of education and are confined to a cohort within a narrow age range. Thus, the current findings might not be replicable for other age cohorts. Second, the inclusion criteria for StD in current study did not exclude the possibility of dysthymia (persistent depressive disorder). In addition, anxiety and depression are highly comorbid. Therefore, these confounding factors can't be completely avoided. Third, the current study did not investigate whole brain FC of all cerebellar subregions, which might overlook certain results. Fourth, considering that our study is exploratory, our results of correlation analysis should be interpreted cautiously since the results cannot survive multiple comparison corrections. Finally, BLT cannot confirm treatment compliance. Nevertheless, we monitored the status of the lighting equipment (on and off) to ensure participant compliance. When the lighting equipment is turned off, we remind participants to turn on the equipment and encourage them to comply.
ConclusionsIn young individuals with StD, BLT compared to placebo not only improved depressive symptoms, but enhanced attention/vigilance. Importantly, successful BLT treatment results in changed cerebellum-DMN connectivity, especially in the cerebellar-frontotemporal and cerebellar internal FC. Static and dynamic FC of the cerebellum at pre-treatment has been potential to be an imaging biomarker for the improvement of attention/vigilance cognitive function after BLT in individuals with StD. In the future, studies with a larger sample from multi-centers are required to assess the feasibility and acceptability of the BLT in order to establish the clinical utility of this treatment.
Author contributionsGuanmao Chen: Data curation, Formal analysis, Investigation, Writing - original draft. Zixuan Guo: Data curation, Investigation, Formal analysis, Writing - original draft. Pan Chen: Data curation, Formal analysis, Visualization, Methodology, Writing - original draft. Zibin Yang: Investigation, Methodology. Hong Yan: Investigation, Methodology. Shilin Sun: Investigation, Resources. Wenhao Ma: Data curation, Investigation, Resources. Yuan Zhang: Data curation, Investigation, Resources. Zhangzhang Qi: Data curation, Investigation. Wenjie Fang: Data curation, Investigation, Resources. Lijun Jiang: Data curation, Investigation, Resources. Qian Tao: Project administration, Supervision, Validation, Writing - review & editing. Ying Wang: Software, Project administration, Supervision, Validation, Writing - review & editing.
The study was supported by grants from the National Natural Science Foundation of China (81971597 and 82172530); National Key Research and Development Project (2020YFC2005700); Science and Technology Projects in Guangzhou (2024B03J1299).