Individuals with subclinical depression are prone to major depression and experience emotional responses and attentional biases to negative stimuli.
MethodIn a randomized controlled study (N = 42) using functional magnetic resonance imaging (fMRI), we examined the neurocognitive mechanisms behind mindfulness-based cognitive therapy (MBCT) combining loving-kindness meditation (LKM) on a group with subclinical depression compared with the relaxation group across emotional face n-back (EFNBACK) tasks and resting state. We also collected behavioral and self-reported data to confirm neurocognitive results.
ResultsDuring EFNBACK, the MBCT+LKM group showed greater activation in the left lingual gyrus and right inferior lateral occipital cortex. During rest, the MBCT+LKM group demonstrated increased connectivity of the anterior cingulate cortex and right inferior lateral occipital cortex, right anterior insula and left precentral gyrus. From amplitude of low frequency fluctuation (ALFF) data, activity in brain regions associated with cognitive control decreased and activity in brain regions associated with sensorimotor increased.
ConclusionThese results suggest that MBCT+LKM alleviate depression for subclinical individuals through improving executive function when they face negative stimuli.
Subthreshold depression (StD) refers to depressive clinical symptoms that do not meet the major depressive disorder (MDD) standard diagnostic criteria or that reach the depression scale cutoff but are not confirmed by a diagnostic interview (Cuijpers et al., 2014; Cuijpers & Smit, 2004; Hwang et al., 2015). In China, 65.55% of first-year students experienced subclinical depression, making them prone to depression (Lu et al., 2015). Depressive individuals at risk of depression show impaired executive function (LeMoult & Gotlib, 2019). Working memory and inhibition are two interacting executive functions. Only by inhibiting interference from irrelevant information can individuals free up cognitive space in the working memory system to store useful information. Additionally, individuals can effectively engage in inhibition only by storing information about inhibiting a specific stimulus in working memory (Diamond, 2013). When depressed individuals experience impaired ability to inhibit attention towards negative information, these information enters working memory, intensifies rumination, and gradually transforms into long-term memory, contributing to the development of a depressive mood (Joormann, 2010). Therefore, stopping negative information from interfering with working memory may prevent depression from worsening.
Mindfulness-based cognitive therapy (MBCT) can help participants recognize automatic thoughts and detach from ineffective processes by shifting attention to their current thoughts, emotions, and physical sensations; notably, mindfulness can reduce depression (van der Velden et al., 2015) and improve executive function, including inhibitory function and attentional ability (Im et al., 2021). Depressed individuals are highly susceptible to negative stimuli and tend to ruminate (Rnic et al., 2023). Based on these previous studies, we proposed that improving executive function after MBCT could inhibit negative stimuli and reduce rumination.
Monitor and acceptance theory (MAT) suggests that attention-monitoring skills play a role in how mindfulness improves cognitive function and increases affective reactivity. Acceptance skills reduce affective reactivity from negative stimuli and self-criticism (Lindsay & Creswell, 2017). Loving-kindness meditation (LKM) is a psychological intervention for developing unconditional kindness towards all people, which may reduce negative affectivity and elevate acceptance and empathy (Hofmann et al., 2011). Subclinical depression interventions that combine LKM with MBCT may more effectively reduce the interference of negative stimuli, improve executive function, and alleviate depression than MBCT-only interventions.
To reduce the interference of negative stimuli to working memory, individuals need a core skill that is more alert to the presence of negative stimuli than inhibiting it to shift attention to current working memory (Dotson et al., 2020; Joormann & Tanovic, 2015). Hence, we focused on the neural networks related to visual attention and cognitive control. The lingual gyrus, occipital lobe, and other visual processing areas are the primary sensory cortices involved in visual processing. Depressed individuals demonstrate poor visual cognitive processing and struggle to filter out irrelevant information (Desseilles et al., 2009). Moreover, abnormal functional connections between the prefrontal and visual cortex impair working memory updates (Le et al., 2017). The prefrontal cortex of the central executive network (CEN) and the anterior cingulate gyrus (ACC) of the salience network (SN) are important brain regions in response inhibition (Disner et al., 2011; Menon & D'Esposito, 2022; Tomassini et al., 2022). The anterior insula of the SN plays a key role in awareness and emotional regulation (Tan et al., 2022). CEN–SN interactions improve executive control in individuals with depression (Ho et al., 2021; Liu et al., 2021; Papakostas & Culpepper, 2015). Changes in the functioning of these networks predict the development of depressive symptoms (Marwood et al., 2018; McGrath et al., 2013). In particular, the CEN and SN regulate the primary sensorimotor system and improve the negative attention bias of depressed individuals (Almdahl et al., 2023; Bi et al., 2022; Disner et al., 2011).
Existing studies on mindfulness-based intervention (MBI) for depression primarily used questionnaires to explore the mechanism of mindfulness (van der Velden et al., 2015), although some have explored neural mechanisms (Tang et al., 2015). However, most studies were not strictly randomized controlled trials, did not include participants with subclinical depression, and did not use multiple indicators. In response, we conducted a randomized controlled design using fMRI to investigate the effect of MBCT combined with LKM on negative stimuli's impact on working memory in individuals with subclinical depression using EFNBACK tasks. Task- and resting-state brain imaging scans were performed before and after the intervention. We also collected behavioral data and a series of psychological indicators (e.g., mindfulness, depression, compassion, and rumination) through questionnaires to provide more evidence regarding the effectiveness of the intervention.
Materials and methodsStudy designWe conducted a single-blinded and randomized controlled trial to compare the neural mechanism of MBCT + LKM with relaxation to reduce interference from negative information on working memory in subclinical depression.The CONSORT flow diagram see Fig. 1.
ParticipantsWe recruited participants by putting up posters in universities with the theme of "Relieving negative emotion.” The participants accessed the PHQ-9 and BDI-2 questionnaires using the QR code on the poster. The inclusion criteria were subjects with scores greater than 5 on the PHQ-9 and 14 on the BDI-2 (Dozois et al., 1998; Kroenke & Spitzer, 2002). We excluded subjects (1) with depression who met the criteria for clinical diagnosis or were currently experiencing a depressive episode, (2) had suicidal thoughts or behaviors within the last month, (3) had experience with mindfulness or relaxation techniques, (4) had a psychotic episode in the past year and psychological or drug treatment in the last month, and (5) with drug or alcohol dependence.
This study was approved by the university's academic ethics committee (No. H22037). The registration number is ChiCTR2300074371.
InstrumentsAn EFNBACK task was used to measure the interference of negative stimuli to working memory (Ladouceur et al., 2009). EFNBACK involves a visual display of a pseudo-random sequence of letters and participants respond with a pre-specified letter appearing on a computer screen. The memory load condition involved a 2-back task in which participants press the button whenever the current letter is identical to the letter presented two trials back (e.g. M–X–M). Four different emotional distraction stimuli (positive, negative, neutral, and non-stimulating) were on either side of the letters. The goal was to block out emotional distractions to finish the working memory task. (see Fig. 2).
ProcedureComputer-generated random numbers were used to assign participants to an experimental group, which completed a 14-day intensive MBCT+ LKM intervention (n = 22), or an active control group, which completed a relaxation intervention (n = 20).
Both the experimental and control groups attended seven classes,with one class held every other day, each lasting 2.5 h. Participants completed the homework assigned by the certified trainer the day before using the electronic platform to ensure that they practiced for more than one hour daily. The MBCT+LKM and relaxation interventions involved the same course structure and duration and were led by the same trainer certified in mindfulness training. We reminded all participants to complete daily homework.
The intervention protocol of MBCT+LKM primarily includes three aspects:cognitive exercises,awareness, and acceptance. Awareness is further divided into two skills, while acceptance is divided into three skills. The main purpose of MBCT+LKM is to help subclinical depressed individuals become aware of their negative automatic thoughts and habitual reactive tendencies, and to maintain soft awareness and acceptance of their negative emotions and body feelings. Relaxation training also involves three aspects of practice, with the main goal of regulating breathing and relaxing the body to reduce sympathetic nervous system activation but did not include a conscious awareness component,unlike the MBCT+LKM intervention. The programs are further described in the Supplementary Materials.
Participants completed all baseline and post-intervention measurements within two days before the intervention and within two days after the intervention.
Self-report measures and analysisDepressive symptoms were assessed before and after treatment using the BDI-2 and PHQ-9 (Dozois et al., 1998; Kroenke & Spitzer, 2002). Mindfulness was assessed using the Five Facet Mindfulness Questionnaire (Baer et al., 2006), and Mindfulness attention awareness scale (Brown & Ryan, 2003); rumination was assessed using the Rumination Response Scale (RRS) (Nolen-Hoeksema & Morrow, 1991); worry was assessed using the Penn State Werry Questionnaire (Neff, 2003); compassion was assessed using the self-compassion scale (Neff, 2003); positive and negative emotions were assessed using Positive and Negative Affect Schedule (Watson et al., 1988); and emotion regulation was assessed using Emotion Regulation Questionnaire (Gross & John, 2003). We used SPSS 22 to conduct repeated-measures analysis of variance (ANOVA) to analyze the data.
Neuroimaging data acquisition and analysisTask-state dataA Siemens MAGNETOM Prisma 3T scanner was used to obtain MRI images of the participants' brains, including functional and structural images. The resting-state scan lasted 5 min, followed by three run task-state scans. The structural images were scanned last. Preprocessing of the resting-state and task-state images was performed using the DPARSFA toolkit in Matlab 2018 (Yan et al., 2016).The following parameters were used: repetition time (TR) =2000 ms, echo time (TE) = 30 ms, flip angle (FA) =75°, field of view (FOV) =220 × 220 mm2, acquisition matrix size = 64 × 64, and voxel size = 3.4 × 3.4 × 3 mm3. Spinecho sequences were used to scan the T1w structural image data of the participants' brains. The following parameters were used: TR = 1900 ms, TE = 2.52 ms, FA = 9°, matrix size = 64 × 64, FOV = 256 × 256 mm2, and voxel size = 1 × 1 × 1 mm3. During preprocessing, the data format was converted from T1 DICOM to NFTI. Because the signal was unstable 10 s before the MRI machine started, we deleted five time points and performed head motion correction, segmentation registration, and standardization processing. The data were smoothed using a 4 mm half-height full width Gaussian smoothing kernel.
The whole-brain voxel analysis was based on a generalized linear model. All statistical results were corrected using multiple comparisons, and a correction method based on clusters was adopted. The overall False Discovery Rate (FDR) was set at p < 0.05.
Resting-state dataWe collected resting-state data for 5 min before the task-state data collection of the three runs. The settings of the relevant parameters and preprocessing were the same as those used for the task-state data. We employed a previously published and commonly used set of brain network maps to create seed regions for the CEN, SN, and visual network (Power et al., 2011). Resting-state functional connectivity (rsFC) was assessed using the CONN toolbox (Whitfield-Gabrieli & Nieto-Castanon, 2012). Based on the seed-to-voxel method, a group (MBCT+LKM, Relaxation) × time (post, baseline) functional connectivity analysis was conducted, and the standard for multiple comparison correction was set as FDR (p < 0.05). The amplitude of low-frequency fluctuation (ALFF) was determined using DPABI (Yan et al., 2016), and the statistical results were corrected using a permutation test (p < 0.05).
ResultsTable 1 shows the demographic and clinical characteristics of the two groups. Depressive level, childhood trauma, and motivation level were not significant (PHQ - 9: t = 0.75, p = 0.46; BDI - 2: t = 0.06, p = 0.95; childhood trauma: t = 0.44, p = 0.66; motivation level: t = 0.62, p = 0.53).
Baseline characteristics.
| MBCT+KLM(n = 22) | Relaxation(n = 20) | |
|---|---|---|
| Sociodemographic characteristics | N = 22 | N = 20 |
| Age | 21.68±2.80 | 21.20±2.71 |
| Sex(female) | 16(73 %) | 14(70 %) |
| Educational level | ||
| Undergraduate | 13(60 %) | 15(75 %) |
| Under postgraduate | 9(40 %) | 5(25 %) |
| Motivation of participance | 4.18 | 4.05 |
| Clinical characteristics | ||
| PHQ-9(total point) | 8.55 | 7.65 |
| BDI-2(total point) | 24.64 | 23.45 |
| Childhood trauma (total point) | 56.61 | 54.03 |
| Antidepressant usage within 3 months | 0 | 0 |
| Experiencing current depressed episode | 0 | 0 |
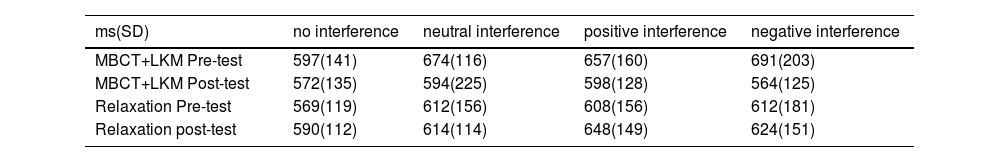
In the pre - and post-tests, the response accuracy of both groups was above 88 %. The main effect of time (F(1,40)= 1.154, p = 0.14) and the interaction (time and group) were not significant (F(1,40) = 3.94, p = 0.06). In this study, we only analyzed the correct responses' response time (RT) and removed values beyond ± 3 SD. Based on the difference score, 2 (pre-test and post-test) × 2 (MBCT+LKM group, relaxation group) × 4 (no interference, neutral interference, positive interference, negative interference) repeated-measures analysis of variance (ANOVA) was used to analyze data. At the baseline level, there was no significant difference in response time between the experimental group and the control group (F(1,40) = 1.169, p = 0.201); also the interaction (time and group) was not significant (F(1,40)=3.75, p = 0.075).However, the post-test response time in the experimental group was lower than the pre-test (F(1,40) = 4.43, p = 0.042, η² = 0.1), while there was no significant difference in response time between the pre-test and post-test in the control group (F(1,40) = 0.27, p = 0.61). We found that in the experimental group, under the negative and neutral interference conditions, the post-test response time was lower than the pre-test (F(1,40) = 7.03, p = 0.011, η² = 0.15; F(1,40) = 6.28, p = 0.016, η² = 0.14). However, in the relaxation group, there were no significant differences among the four interference conditions (Table 2 shows the RTs).
Response time of EFNBACK.
| ms(SD) | no interference | neutral interference | positive interference | negative interference |
|---|---|---|---|---|
| MBCT+LKM Pre-test | 597(141) | 674(116) | 657(160) | 691(203) |
| MBCT+LKM Post-test | 572(135) | 594(225) | 598(128) | 564(125) |
| Relaxation Pre-test | 569(119) | 612(156) | 608(156) | 612(181) |
| Relaxation post-test | 590(112) | 614(114) | 648(149) | 624(151) |
After controlling for the effects of anxiety (measured using the Self-Rating Anxiety Scale) and childhood trauma (measured using the Childhood Trauma Questionnaire) on depression, we used the BDI-2 and PHQ-9 to measure depression,and used FFMQ and MAAS to measure mindfulness in both groups. Meanwhile, there were no significant differences in mindfulness, rumination, compassion, emotional regulation strategies (including expressive suppression and cognitive reappraisal), worry, and positive and negative emotions across the groups at baseline (p > 0.05). The results of the ANOVA revealed a group × time effect for the series of variables above, while the interactive effect of all self-reported variables was not significant (p > 0.05).
Compared to pre-test, both the experimental and control groups showed significant reductions in scores for depression and rumination after the intervention. In the experimental group, post-test scores for cognitive reappraisal, positive emotions, and compassion were significantly higher than pre-test scores,while the opposite pattern was observed in expression suppression and worry.However, there were no significant differences in pre- and post-intervention scores within the control group in these variables. The ANOVA of difference score (post minus pre) indicated significant differences between the two groups in depression (BDI-2: MBCT+LKM: F(1,39)=37.22, p < 0.001, η2 =0.49; relaxation: F(1,39)=16.37, p < 0.001, η2 =0.30; PHQ-9: MBCT+LKM: F(1,39)=24.06, p < 0.001, η2=0.38;relaxation: F(1,39)=6.97, p < 0.05, η2=0.15); rumination (MBCT+LKM: F(1,39) =19.25, p < 0.001, η2=0.33; relaxation: F(1,39)=8.09, p < 0.01, η2=0.17); compassion (MBCT+LKM: F(1,39) =14.00, p < 0.001, η2=0.26; relaxation: F(1,39)=2.93, p > 0.05); expression suppression (MBCT+LKM: F(1,39)=6.21, p < 0.05, η2=0.14;relaxation: F(1,39)=4.10, p = 0.05, η2=0.10); cognitive reappraisal (MBCT+LKM: F (1,39)=3.95, p = 0.05, η2=0.09; relaxation: F(1,39)=2.90, p > 0.05); worry (MBCT+LKM:F (1,39)=4.51, p < 0.05, η2=0.10; relaxation: F(1,39)=0.21, p > 0.05); positive emotion (MBCT+LKM: F(1,39)=11.00, p < 0.01, η2=0.22;relaxation: F(1,39)=3.80,p > 0.05); mindfulness (FFMQ:MBCT+LKM: F(1,39)=3.33, p = 0.076;relaxation: F(1,39)=1.06, p= 0.31; MAAS: MBCT+LKM: F(1,39)=13.76, p < 0.001, η2 =0.26; relaxation: F(1,39)=6.53, p < 0.05, η2=0.14).
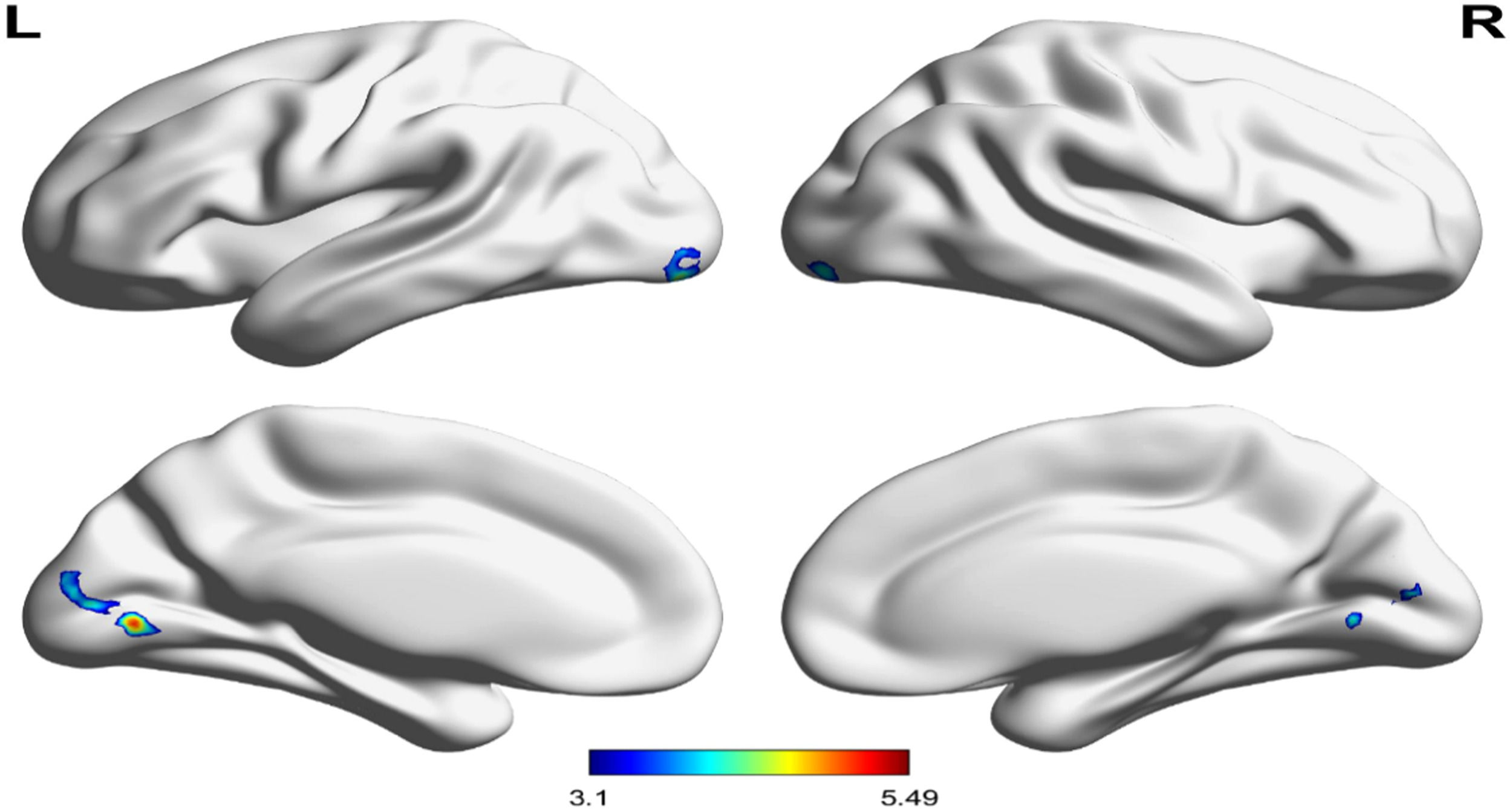
Neural resultTask-State fMRI resultWe found that under negative interference conditions (2-back negative interference-2-back null interference, the difference post minus pre of MBCT+LKM compared relaxation intervention cannot be corrected for multiple comparisons. In the MBCT + LKM group, significantly greater left lingual gyrus (p < 0.05, corrected for FDR, voxels = 593, cluster-threshold k > 97), left inferior lateral occipital cortex (p < 0.05, corrected for FDR, voxels = 199, cluster-threshold k > 97), and right inferior lateral occipital cortex (p < 0.05, corrected for FDR, voxels = 97, cluster-threshold k > 97) activity was observed in the post-test showed compared to the pre-test (see Fig. 3).
Group differences in the left lingual gyrus blood oxygen level-dependent (BOLD) signal during the attentional control of emotion on the 2back: negative-2back:no face contrast. Post measure of MBCT+LKM exhibited significantly greater activity in the left lingual gyrus (peak voxel: −6, −74, 0; t = 5.49), left inferior lateral occipital cortex (peak voxel: −28, −90,−12; t = 4.66), right inferior lateral occipital cortex (peak voxel: 32, −92,−10; t = 4.17) (versus pre measure during the 2-back negative-no face distracter condition).
The functional connections between the SN and the visual network and between the SN and the sensorimotor network were enhanced in the MBCT+LKM group. Specifically, the difference in post-pre of the MBCT+LKM group minus the difference in post-pre relaxation group increased the rsFC between the anterior cingulate cortex (ACC) (MNI = −3, 42, 16) and right inferior lateral occipital cortex (iLOC r) (MNI = 38, −64, −24) (p < 0.001, corrected for FDR, K = 237, t = 4.89). The right anterior insula (MNI =27, 16,−17) showed increased rsFC in the left precentral gyrus (PreCGl) (MNI = −40,−14, 62 (p < 0.001, corrected for FDR, K = 204, t = 4.88). Fig. 4 shows the pre/post changes in connectivity between the ACC and iLOCr, InsulaR, and PreCGl for both groups(see Fig. 4).
Result of amplitude of low-frequency (ALFF)For the result of ALFF, the difference of post-pre of MBCT+LKM group minus the difference of post-pre of relaxation group, we found that ALFF increased in the right thalamus (MNI =18, −27, 6) (corrected for permutation test, cluster size=717,t = 5.44); right lingual gyrus (MNI =15, −60, −6) (corrected for permutation test, cluster size =160, t = 4.71); Conversely, decreased ALFF was observed in the right inferior parietal lobe (MNI = 45, −42, 54) (corrected for permutation test, Cluster size=24, t=−4.06, p < 0.05).Furthermore,we compared the activation of post-intervention minus pre-intervention ALFF in the MBCT+LKM group and found decreased ALFF in the left inferior frontal gyrus (MNI = −42, 33, 6) (corrected for permutation test, Cluster size = 26, t=−5.60) and the right superior frontal gyrus (MNI = 15, 48, 42) (corrected for permutation test, Cluster size = 85, t = −5.13, p < 0.05), both of which are involved in the executive control network. Additionally, increased ALFF was observed in the right insula (MNI = 33, 24, 12) (corrected for permutation test, Cluster size = 13, t = 4.89, p < 0.05) and the left postcentral cortex (MNI = −39, −12, 39) (corrected for permutation test, Cluster size = 20, t = 6.36, p < 0.05), both of which are associated with the sensorimotor network.
DiscussionOur comparison of the experimental and active control groups revealed the following results of MBCT+LKM on subclinical depression. The MBCT+LKM group had a significantly lower RT after the intervention, while the relaxation group's RT did not significantly change, confirming the task-state and rest-state results. Compared to the relaxation group, after the intervention, the MBCT+LKM group demonstrated increased functional connectivity between the ACC and iLOC, insulaR and PreCGl, highlighting that the SN strengthens connectivity among the visual and sensorimotor networks. The self-reported data revealed that both interventions improved depressive symptoms (the effect size of the MBCT+LKM group was larger).
Compared with pre-test, under neutral and negative stimuli, the behavioral data of MBCT+LKM showed response time was significantly lower after the intervention (the difference in effect size was largest under the negative stimuli). The relaxation group showed no significant differences in RT across the four conditions. These results support our hypothesis that MBCT+LKM can reduce the interference of negative stimuli on working memory.
Previous studies have shown that MBIs can improve executive function (Im et al., 2021), increase sensitivity to affective cues, and improve responses to incipient affective cues. This helped signal the need for executive control by cultivating open awareness and non-judgmental acceptance (Joormann & Tanovic, 2015; Teper et al., 2013),and elevating cognitive reappraisal strategies to increase positive emotions and reduce attention to negative stimuli (Garland et al., 2015); Meanwhile, LKM fostered acceptance (Feliu‐Soler et al., 2017), which enhanced the activation of brain areas involved in emotional processing and empathy to reduce affective response of negative stimuli (Hofmann et al., 2011). Therefore, in the EFNBACK task, the experimental group experienced less negative stimuli after the intervention. Our study also found that the post-test response time in the experimental group, following intervention was significantly lower than the pre-test response time under neutral interference stimulus conditions. Research has shown that individuals with severe depression had lower accuracy and slower response times in recognizing neutral faces compared to healthy individuals, suggesting that individuals with depression did not perceive neutral faces as truly neutral but tend to attribute negative interpretations, which influenced cognitive processing (Leppänen et al., 2004). Our study showed that after intervention, the impact of neutral interference stimuli on working memory was reduced, indicating that MBCT+LKM could effectively decrease the negative interpretation bias of individuals with subclinical depression towards neutral stimuli.
From the neural perspective, our task-state results showed that the MBCT+LKM group had greater activation in the left lingual gyrus and left inferior occipital gyrus when completing the EFNBACK tasks under negative interference conditions. The ALFF data confirmed lingual gyrus activation. The FC results also showed that compared with the relaxation group, the functional connectivity of the ACC and left inferior occipital gyrus was enhanced after the intervention in the MBCT+LKM group, which again supported the task-states result. An existing study showed that depressive symptoms were significantly correlated with visual cortex lingual gyrus and occipital gyrus responses to sad faces (Keedwell et al., 2009). Meanwhile, a voxel-based morphometry (VBM) study found that lingual gyrus volume predicted improvements in cognitive function and depressive symptom relief in patients with MDD (Jung et al., 2014). Notably, abnormal function in the lingual gyrus was often related to impaired attention (Yang et al., 2015), and the lingual gyrus regulated cognitive control and depressive symptoms in visual cognitive tasks (Desseilles et al., 2009). We found enhanced functional connectivity between the ACC (SN) and iLOC, which may also be a marker of depressive symptom relief. Earlier studies found that in patients with depression, the functional connection between the SN and occipital cortex was weaker; cognitive deficits were more pronounced (Cera et al., 2019; Liu et al., 2017); the functional connection between the ACC and visual network was enhanced, all of which may be related to attention control and emotional regulation of visual stimuli (van der Werff et al., 2013).
In our study, depressive symptoms decreased after the MBCT+LKM, and visual brain activation, and the functional connection between ACC and the visual network was enhanced, suggesting that MBCT+LKM may reduce negative attention bias, enhance visual cognitive attention ability, and improve working memory updates in individuals with subclinical depression (Le et al., 2017). We also found enhanced functional connectivity between the insula and precentral gyrus in the MBCT+LKM group after the intervention compared with the relaxation group, which suggested that individuals with subclinical depression had elevated receptiveness and emotional awareness to detect emotional stimuli (Simmons et al., 2013; Tan et al., 2022). The increased strength of the FC of the ACC-iLOC and InsulaR-PreCGl in the experimental group suggested that the intervention enhanced the ability to inhibit negative stimuli and that empathy and acceptance reduced emotional responses to negative stimuli. This may be because the precentral gyrus, as the primary motor cortex, controls the voluntary motor (Jin et al., 2022), which may be associated with executive response inhibition. And the InsulaR and ACC are activated by empathy (Hein et al., 2016).
The ALFF results showed that the MBCT+LKM intervention decreased activity in the left inferior frontal gyrus and right superior frontal gyrus (related to cognitive control) and increased activity in left postcentral gyrus and right anterior insula (related to sensorimotor function); these findings are consistent with previous studies (Chiesa et al., 2013; Gard et al., 2012; Hölzel et al., 2011). Additionally, the behavioral data showed that the intervention significantly reduced reaction time. These findings suggested that the experimental group required fewer cognitive resources to overcome the automated behavior pattern to preserve present awareness after the intervention. In addition, the sensorimotor network activation indicated that the experimental group showed improved interception and awareness after the intervention (Tan et al., 2022). The decrease in the activation of cognitive control brain areas and the activation of sensorimotor-related brain areas were both manifestations of the positive effect of mindfulness intervention, indicating that individuals with subclinical depression adopted more bottom-up cognitive regulation strategies after intervention rather than top-down regulation strategies before intervention (Tang et al., 2015).
The strengths of this study lie in its comprehensive demonstration of the effectiveness of MBCT+LKM in alleviating depression in subclinical individuals. This is achieved by improving executive function, as shown through data from self-reports, behavioral assessments, and neural mechanisms.However, some limitations of the study should be noted. Firstly, longitudinal follow-up studies can better illustrate the causal relationship between reducing negative interference and alleviating depression,which is better to prove the psychological mechanism between mindfulness intervention and depression. Secondly, the sample size of the current study was relatively small. Thirdly,the ecological validity of using EFNBACK paradigm to measure the interference of negative stimuli on working memory remains to be further supported.
The study intervention was adapted from an eight-week MBCT course taught by senior mindfulness meditation teacher, Yiwen Chen. Yiwen Chen mainly directed the intervention of the experimental group and the control group in this study. Here, we would like to thank Yiwen Chen and her organization "Just Be" (Wechat official account ID:gh_cc96502ec954) for their contribution to this research.
This work was supported by Fundamental Research Funds for the Central Universities (Dong Yang, grant number SWU2009101); Chongqing Planed Social Science Research Program (Dong Yang, grant number 2020TBWT-ZD07); The National Natural Science Foundation of China (Dong Yang, grant number 71472156).