Motor control declines with age and requires effective connectivity between the sensorimotor cortex and the primary motor cortex (M1). Despite research indicating that physical exercise can improve motor control in older individuals the effect of physical exercise on neural connectivity in older adults remains to be elucidated.
MethodsOlder adults with experience in table tennis and fit aerobics and individuals without such experience for comparison were recruited for the study. Differences in motor control were assessed using the stop-signal task. The impact of exercise experience on DLPFC-M1 and pre-SMA-M1 neural connectivity was assessed with transcranial magnetic stimulation. Varied time intervals (short and long term) and stimulus intensities (subthreshold and suprathreshold) were used to explore neural connectivity across pathways.
ResultsThe present study showed that behavioral iexpression of the table tennis group was significantly better than the other two groups;The facilitatory regulation of preSMA-M1 in all groups is negatively correlated with SSRT. Regulatory efficiency was highest in the table tennis group. Only the neural network regulatory ability of the Table Tennis group showed a negative correlation with SSRT; Inhibitory regulation of DLPFC-M1 was positively correlated with SSRT; this effect was most robust in the table tennis group.
ConclusionThe preliminary findings of this study suggest that table tennis exercise may enhance the motor system regulated by neural networks and stabilize inhibitory regulation of DLPFC-M1, thereby affecting motor control in older adults.
Older adults are increasingly at risk of living in a changing environment. This heightened vulnerability stems from the deterioration of motor control, attributed to impairment of the sensorimotor system(Liu et al., 2014). Previous research has shown that exercise can improve motor control in older adults(Christou, 2011; Deprá et al., 2022; Lü et al., 2016; Voss et al., 2010). Exercises can be divided into open-skill exercises and closed-skill exercises based on the external environment in which the skill is performed (Wang et al., 2013). Previous studies have found significant advantages in control ability among participants engaged in open-skill exercises(Wang et al., 2013; Yu et al., 2017), and these advantages are also reflected in neural function(Wang et al., 2020). Other studies have found that compared to closed-skill exercise, open-skill exercise interventions are more effective in improving older adults' coordination ability, agility, reaction speed, and dynamic balance(Tsai et al., 2017; Tsai & Wang, 2015; Wark et al., 2008). However, limited attention has been paid to studies examining the effects of different types of exercise experience on motor control ability and the neural connectivity of the sensorimotor system in older adults.
The motor process is known to be information collected by the sensorimotor-related cortex, transmitted to primary motor cortex (M1) for processing and then to the spinal cord to complete motor execution(Tokuno & Nambu, 2000). Motor control, as an advanced motor process involving cognitive processing components, engages key regions within the sensorimotor-related cortex(Fink et al., 1999; Xu et al., 2016). Previous studies have suggested that the right dorsolateral prefrontal cortex (DLPFC), as a representative cortex of the sensory system, is implicated in cognitive processes that are co-regulated by the left M1(Cao et al., 2022). The pre-supplementary motor area (pre-SMA) is co-activated with the left M1 during motor preparation(Ball et al., 1999)and mediates muscle movements in the process of motor control(Toma et al., 1999), as well as being involved in motor movement planning(Coxon et al., 2009; Nachev et al., 2005). Therefore, the neural connections between pre-SMA, DLPFC, and M1 may directly influence the quality of motor control execution.
Measurements of neural connectivity can be used as a metric for the extent of shared information processing in various brain regions(Goh, 2011). Previous studies have utilized techniques such as functional magnetic resonance imaging (fMRI) and event-related potentials (ERP) to explore changes in brain structure or activation, indirectly inferring potential alterations in neuralconnections(Fox et al., 2012; Tsai et al., 2017). Transcranial magnetic stimulation (TMS), as an electrophysiological technique, offers a more direct approach for assessing neural connectivity(Nakata et al., 2010; Rosenkranz et al., 2007). Dual-site paired-pulse TMS can be employed to investigate the connectivity between brain functional areas and the motor cortex(Ni et al., 2009). In this paradigm, the first pulse of TMS, known as the conditioned stimulus (CS), is applied to different brain functional areas based on the research objectives. The second pulse of TMS, referred to as the test stimulus (TS), is applied to M1 and induces a motor evoked potential (MEP) in the contralateral hand muscles. Previous studies have used different intensities of CS and different time intervals to investigate neural connectivity between M1 and various brain regions(Bäumer et al., 2009; Fiori et al., 2016).
Many studies utilize the stop-signal task (SST) to investigate motor control behavior. During completion of the SST, the involvement of cognitive processing components activates the sensorimotor-related cortex(Aron et al., 2007; Badry et al., 2009; Kwon & Kwon, 2013). Simultaneously, it enables the measurement of both proactive and reactive motor control, as well as reaction speed and accuracy(Logan et al., 1984; Verbruggen & Logan, 2009). Reactive control is quantified by measuring the stop-signal reaction time (SSRT), or the time it takes to inhibit an action after a stop signal is presented, and proactive control is measured by determining the response delay effect (RDE). This study aims to further explore how behavioral changes are correlated with alterations in neural connectivity within the sensorimotor system.
This study aimed to investigate how table tennis experience (open-skill exercise) and fit aerobics experience (closed-skill exercise) affect motor control ability, and neural connectivity of sensorimotor system networks in older adults, using the stop signal paradigm and two-site paired-pulse TMS. We hypothesized that physical exercise has a significant effect in maintaining motor control in older adults, with different exercises having different effects in maintaining neural connectivity in relevant brain regions.
Materials and methodsThis study design is a cross-sectional survey comparing the motor control abilities of older adults with different types of exercises experience. The aim is to assess the relationship among exercise experience, motor control ability, and neural connectivity within the sensorimotor system network. The study was conducted at the Center for Exercise and Brain Science, School of Psychology, Shanghai University of Sport. The research protocol was approved by the Research Ethics Committee of Shanghai University of Sport (Approval No: 102772019RT012) in accordance with the principles outlined in the Helsinki Declaration. Prior to participation in the study, all participants provided written informed consent. This study has been registered prospectively in the Chinese Clinical Trial Registry (ChiCTR2100043616).
ParticipantsWe used G*Power software to determine the sample size for each group. The results indicated that 66 participants (22 per group) would be needed to detect exercise effects on behavioral and neurophysiological outcomes using variance (ANOVA) at a significance level of 0.05 and power of 80 %. To account for potential participant attrition, we increased the sample size by 40 %, for total recruitment of 93 participants (31 per group). Participants with heart disease, neurological disorders, musculoskeletal disorders, and other conditions that may be exacerbated by physical activity, as well as those with Montreal Cognitive Assessment (MoCA) score below 26 or contraindications to TMS, were excluded.
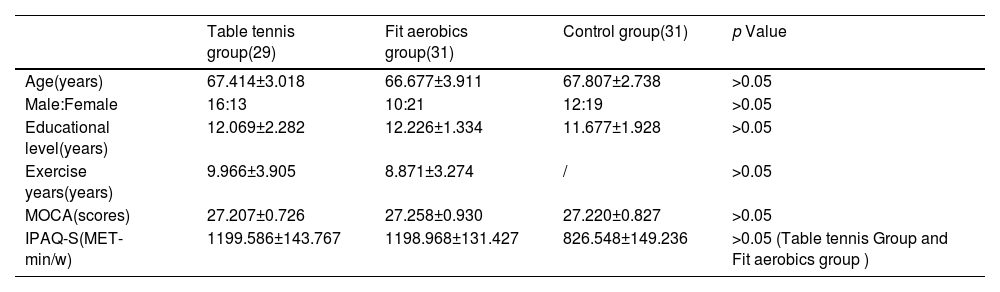
Open-skill exercises require athletes to quickly adapt to changes in the external environment, alter their responses based on current external stimuli, and perform skills in unstable environments with moving objects, where the timing of movements is determined by external conditions. In contrast, closed-skill exercises typically require athletes to maintain high levels of stability and consistency in their movements, adjusting them based on their internal environment. The operator has control over the timing of movements (Chan et al., 2011; Di Russo et al., 2010). Therefore, we have chosen table tennis as the open-skill exercises and fit aerobic exercise as the closed-skill exercises for our study. All participants were between the ages of 60 and 70 years old, right-handed as assessed by the Edinburgh Handedness Inventory, and lived in their own residence. The exercise group was required to engage in a minimum of 40–60 min of exercise at least 3 times per week for a duration of more than two years(Lambourne & Tomporowski, 2010; Lautenschlager et al., 2008; McMorris & Hale, 2012). Participants in the control group had not engaged in regular exercise over the past two years(Sink et al., 2015). To ensure compliance with the study's exercise requirements, we employed the International Physical Activity Questionary-Short Form (IPAQ-S) to monitor participants' physical activity habits. An appendix was also used to gather self-reported data on specific exercise details, such as habits, frequency, and duration, etc. To assess sports skill mastery, participants reported their involvement in competitions and performances over the past two years. Additionally, we screened for potential confounders (e.g., occupation, medical history, medication use) that could influence TMS test results, aiming to minimize their impact (Machii et al., 2006; Poreisz et al., 2007; Valero-Cabre et al., 2017; Wassermann & Neurophysiology, 1998). Please refer to the appendices for more details, with specific results presented in Appendix Tables 1, 2, and 3. Two participants in the table tennis group withdrew from the test midway due to family needs. Final participant information is shown in Table 1. In the demographic survey, no between-group difference was observed for age, gender, years of education, or MOCA score. Exercise years and physical activity were similar between the table tennis and fit aerobics groups.
Demographic variables.
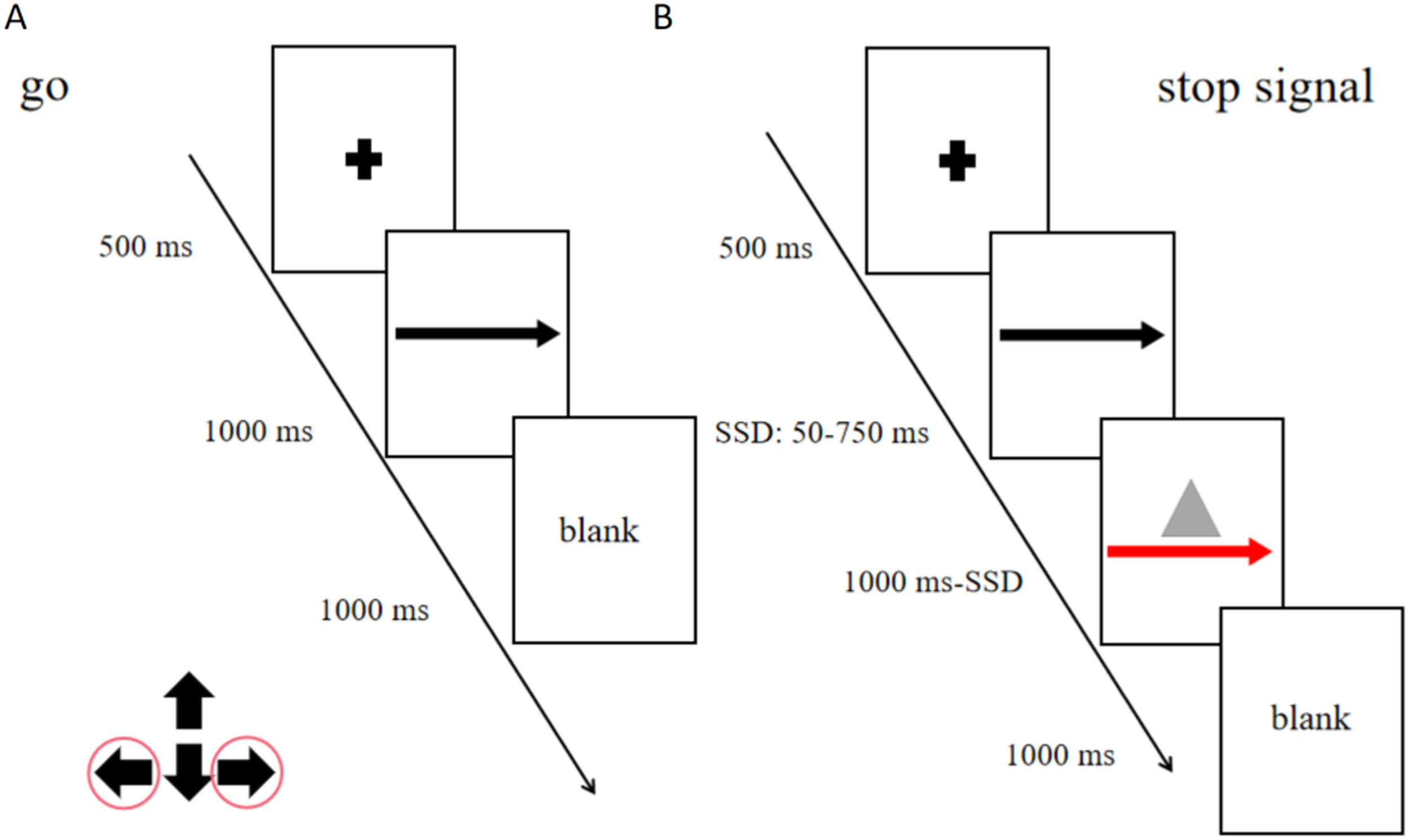
The never stop task (NST), a reaction time task including only go trials, was administered in three blocks (total: 360 trials; Fig. 1A) to measure participants’ general alertness and motor speed. Participants were asked to respond as quickly as possible to the stimuli presented.
Stop signal task flow chart
A: Never Stop Task; B: Stop Signal Task; "+" means the fixation point, the part that requires attention."←" and "→" means to press the left and right buttons according to the arrow.The red arrow and a gray triangle means the participants will press the button to stop or give no response.
The maybe stop task (MST) was administered as a pseudorandom combination of 75 % go trials, 25 % stop trials (total: 480 trials in four blocks; Fig. 1B). In go trials, subjects responded to left- and right-pointing black arrows (displayed for 1000 ms) by pressing corresponding buttons with the right index finger. In stop trials, responses were cued initially by left- and right-pointing black arrows, followed by a red arrow with a gray triangle (requiring nonresponse) after a stop-signal delay (SSD). The SSD (initial duration, 250 ms) was varied from trial to trial to adjust the task difficulty using a stepwise algorithm; to maintain 50 % successful inhibition, SSD was increased by 50 ms following successful nonresponse and decreased by 50 ms following failed nonresponse(Benis et al., 2014). To prevent participants from deliberately slowing down their reaction time to increase the chance of correct stopping (a common strategy used to increase the probability of a successful stop), we were told before the task that the stopping success rate was about 50 % regardless of whether the response time was deliberately delayed. Based on independent horse racing model assumptions, SSRT is estimated using an integral method, which estimates SSRT by subtracting average SSD from mean reaction time (RT) (Mars et al., 2009).
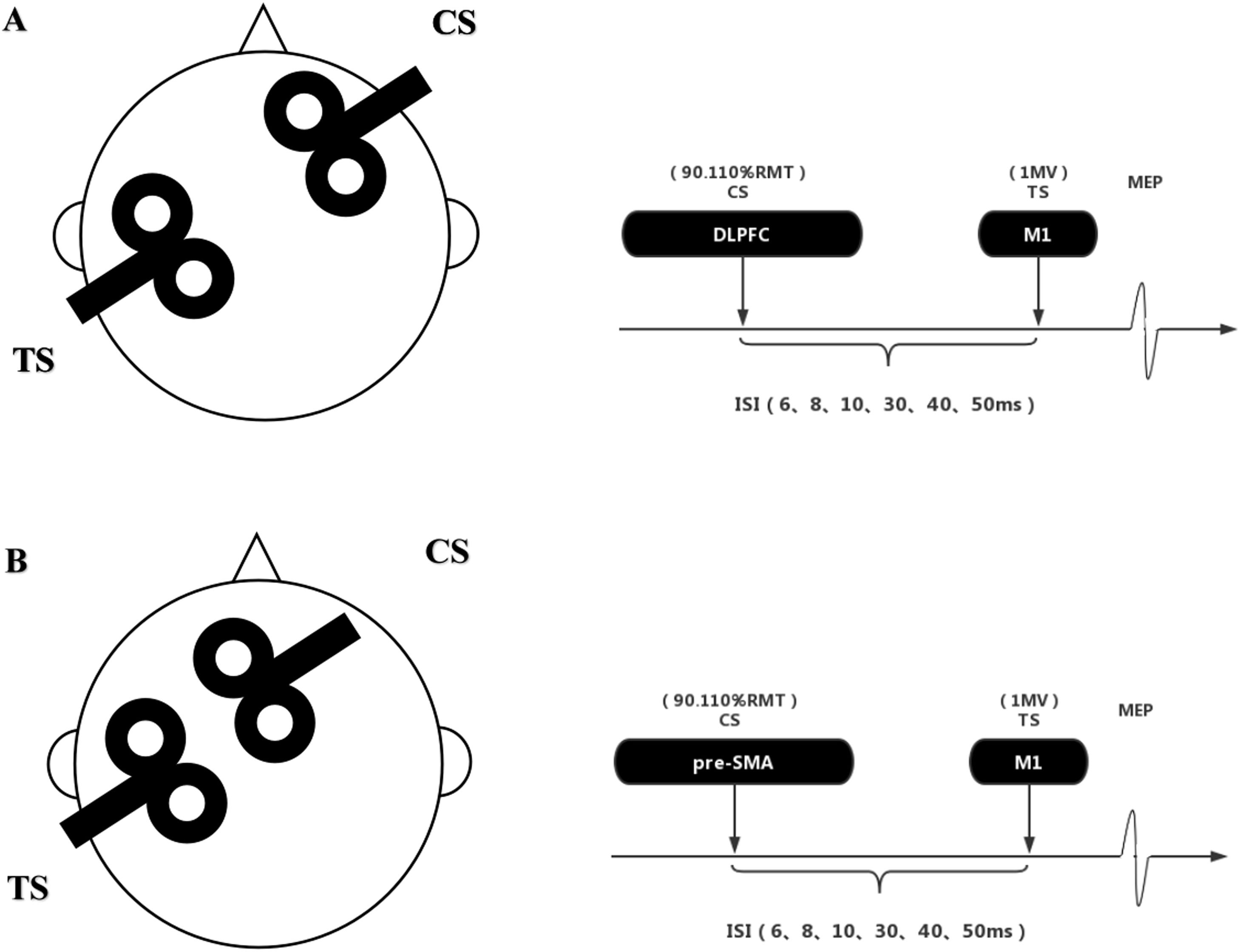
Transcranial magnetic stimulationThe study employed a paired-pulse, dual-site TMS protocol to investigate connectivity between target brain regions, namely the right DLPFC and the pre-SMA, and the left M1. Two figure-eight coils (50 mm, Alpha Branding Iron, Magstim) were connected to two Magstim 200 stimulators. The left and right M1 were defined as the locations where TMS induced the highest peak MEPs in the contralateral first dorsal interosseous muscle (FDI) at a given threshold stimulation intensity. A marker was used to precisely track the outline of the coil on the scalp to ensure accurate capture of the coil's angle and position. The TS coil was applied to the left M1, oriented posterior-anterior at an angle of 30°−45° to the mid-sagittal plane, to generate a focal current. The CS coil was separately positioned over the right DLPFC or pre-SMA and delivered posterior-anterior current(Xia et al., 2022). Similar to previous studies, the right DLPFC was located approximately 5 cm anterior to the right M1, as measured from the scalp(Civardi et al., 2001; Herwig et al., 2001). The pre-SMA was positioned approximately 4 cm anterior to the midline vertex(Buch et al., 2011; Mars et al., 2009). Previous studies have found that target brain regions exert different modulation effects on M1 at stimulus intervals of less than 15 ms vs. greater than 20 ms(Fiori et al., 2016; Mars et al., 2009; Xia et al., 2022). Therefore, in our study, we utilized CS and TS stimulus intervals of 6 ms, 8 ms, 10 ms, 30 ms, 40 ms, and 50 ms (six interstimulus intervals [ISIs]) to investigate both short- and long-term effects on the modulation of M1 by target brain regions.
During the testing procedure, TS intensity was set to elicit a 1.0 mV motor MEP. This intensity was defined as the minimum required to elicit MEPs exceeding 1 mV in at least 5 of 10 consecutive trials in the relaxed FDI muscle. For the paired-pulse condition, different stimulus intensities were used to measure the connectivity between target brain regions and M1. Referring to previous studies, we selected two stimulus intensities as CS, namely suprathreshold and subthreshold, which were 90 % and 110 % of the ipsilateral resting motor threshold (RMT)(Ni et al., 2009; Xia et al., 2022). The RMT was defined as the minimum intensity required to elicit MEPs exceeding 50 μV in at least 5 of 10 consecutive trials in the relaxed FDI muscle. A schematic diagram of the TMS coil and the stimulus intervals is provided in Fig. 2. Each participant completed a total of four testing sessions with a minimum interval of 7 days between sessions. Due to individual differences and factors such as coil placement conformity, a total of 71 participants (23 in the table tennis group, 24 in the fit aerobics group, and 24 in the control group) participated in TMS testing.
Electromyographic recordingElectromyographic (EMG) signals were recorded using disposable surface electrodes positioned in a tendon-belly arrangement over the right FDI muscle. The EMG signal was amplified (×1000) using an Intronix Technologies Corporation Model 2024F amplifier (Bolton, Ontario, Canada), then filtered using a bandpass filter with a range of 20 Hz to 2.5 kHz. The digitized EMG signal was sampled at a rate of 5 kHz using a Micro 1401 data acquisition system (Cambridge Electronics Design, Cambridge, UK) for subsequent offline analysis.
Data analysisDuring behavioral testing, the following measures were collected in the MST: SSRT, SSD, stop accuracy rate (Stop ACC), maybe go response times (MGRT), stop response times (Stop RT), and maybe go accuracy rate (MGACC). In the NST, never accuracy rate (Never ACC) and never response times (Never RT) were collected. RDE was determined by calculating the difference between MGRT and Never RT. SSRT can be used to directly evaluate the responsiveness of control(Kleerekooper et al., 2016), and SSD can be used as a supplementary evaluation index. RDE was used to evaluate active control. Other RT-related indexes were used to evaluate response speed, and ACC-related indexes were used to evaluate response accuracy.
During TMS testing, MEP amplitudes were measured using a peak-to-peak method. The MEP amplitude evoked by the paired-pulse condition was calculated as the ratio of mean MEP amplitude in the paired-pulse condition to that in the TS-alone condition (paired-pulse condition/TS alone condition). A ratio below 1 indicates inhibition, while a ratio above 1 indicates facilitation.
The normality of the data was assessed using the Shapiro-Wilk test, which confirmed a normal distribution. Single-factor ANOVA was used to compare differences in SST measurements of motor control among groups. Three-factor repeated-measures ANOVA was employed to explore the modulation characteristics of the functional network between the target brain regions (DLPFC, pre-SMA) and M1, considering stimulus intensity (90 %, 110 %), stimulus interval (6 ms, 8 ms, 10 ms, 30 ms, 40 ms, 50 ms), and exercise experience (table tennis, fit aerobics, control) in the TMS data. In the case of significant interaction effects, single-factor ANOVA was conducted to examine the differences in functional network modulation among groups at each stimulus interval or intensity. The Greenhouse-Geisser correction was applied to correct for non-sphericity if necessary. The effect of CS on TS in each group was analyzed by paired-sample T-test. We also calculated the slope ([highest-lowest] / coordinate difference) from the most stimulating time point to the most inhibitory time point for each stimulus intensity to express its specific intercortical sensitivity. Finally, we examined the relationship between behavioral performance and neuromodulation using Pearson's correlation. An additional slope was calculated for each group to obtain facilitation to inhibition, and its correlation with SSRT was calculated. For all analyses, the level of statistical significance was set at α = 0.05.
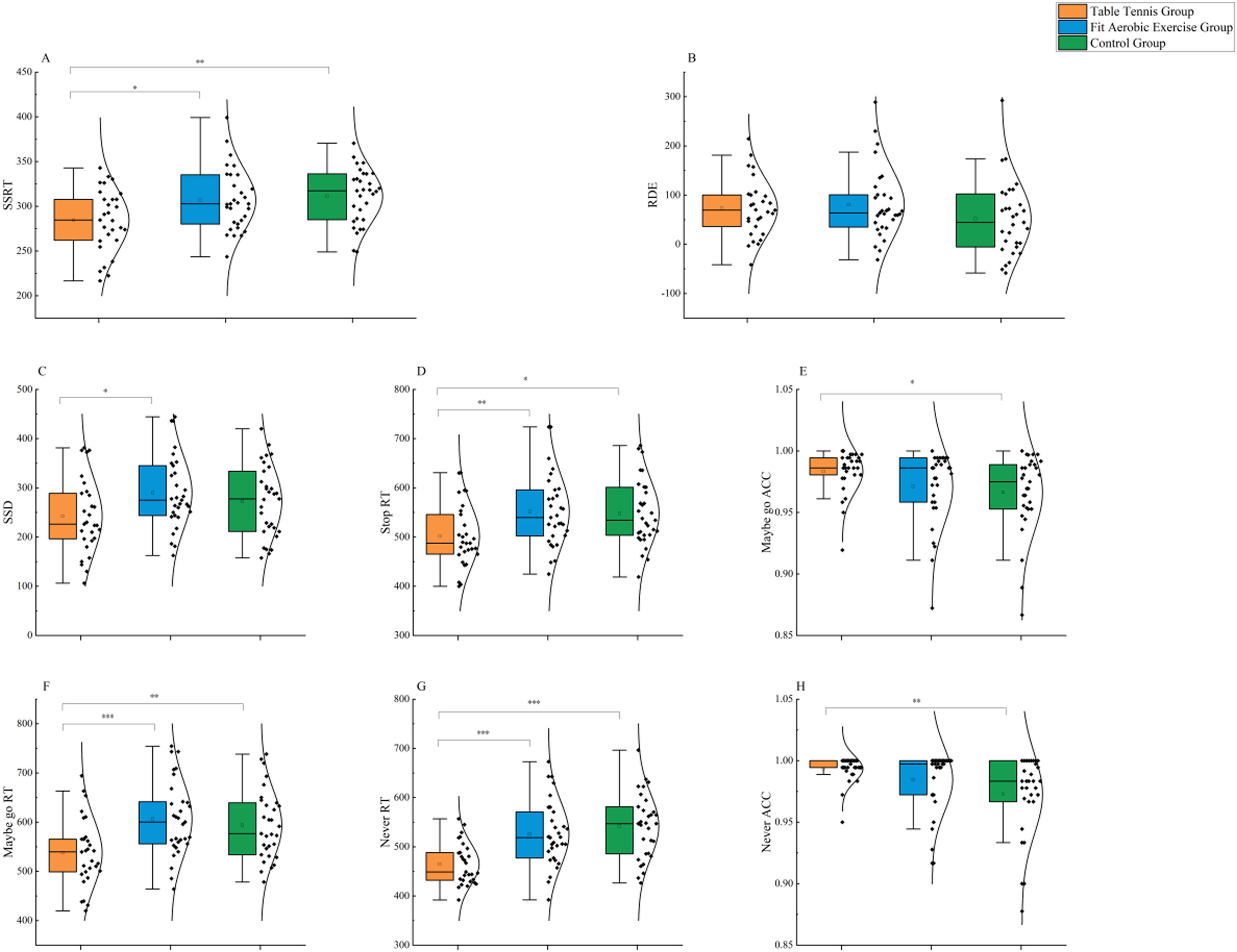
ResultsMotor controlThe results for assessments of reactive control behavior are shown in Fig. 3A. Single-factor ANOVA revealed that different types of exercise experiences influenced the reactive control abilities of older adults (F(2,88) = 6.380, p = 0.003, ηp2 = 0.127). The results demonstrated that the table tennis group performed significantly better than the fit aerobics group (p < 0.05) and the control group (p < 0.01). However, there was no significant difference between the fit aerobics group and the control group (p > 0.05).
The results for assessments of proactive control behavior are shown in Fig. 3B. Single-factor ANOVA revealed that different types of exercise experiences did not have a significant effect on the proactive control abilities of older adults (F(2,88) = 1.445, p = 0.241, ηp2 = 0.032).
Other behavioral measuresIn the MST, as shown in Fig. 3C, single-factor ANOVA revealed that different types of exercise experiences had a significant effect on SSD (F(2,88) = 3.202, p = 0.045, ηp2 = 0.068). The table tennis group showed significant improvement compared to the fit aerobics group (p < 0.05), while there was no significant difference between the control group and either the fit aerobics group or the table tennis group (p > 0.05 for both). Similarly, single-factor ANOVA showed that type of exercise influenced Stop RT in older adults (F(2,88) = 4.920, p = 0.009, ηp2 = 0.101) (Fig. 3D). The table tennis group performed significantly better than the fit aerobics group (p < 0.01) and the control group (p < 0.05), while there was no significant difference between the fit aerobics group and the control group (p > 0.05). Additionally, exercise type had a significant effect on the Maybe go RT of older adults (F(2,88) = 7.765, p = 0.001, ηp2 = 0.150) (Fig. 3E). The table tennis group performed significantly better than the fit aerobics group (p < 0.001) and the control group (p < 0.01), while there was no significant difference between the fit aerobics group and the control group (p > 0.05). Furthermore, single-factor ANOVA indicated that exercise type had an effect on the Maybe go ACC of older adults (F(2,88) = 3.070, p = 0.051, ηp2 = 0.065) (Fig. 3F). The table tennis group performed significantly better than the control group (p < 0.05), while there was no significant difference between the fit aerobics group and either the control group or the table tennis group (p > 0.05 for both).
In the NST, single-factor ANOVA revealed that exercise type had a significant effect on the Never RT of older adults (F(2,88) = 13.954, p = 0.000, ηp2 = 0.241) (Fig. 3G). The table tennis group performed significantly better than the fit aerobics group (p < 0.001) and the control group (p < 0.001), while there was no significant difference between the fit aerobics group and the control group (p > 0.05). Similarly, single-factor ANOVA showed that exercise type influenced the Never ACC of older adults (F(2,88) = 5.043, p = 0.008, ηp2 = 0.097) (Fig. 3H). The table tennis group performed significantly better than the fit aerobics group (p < 0.01) and the control group (p < 0.05), while there was no significant difference between the fit aerobics group and the control group (p > 0.05).
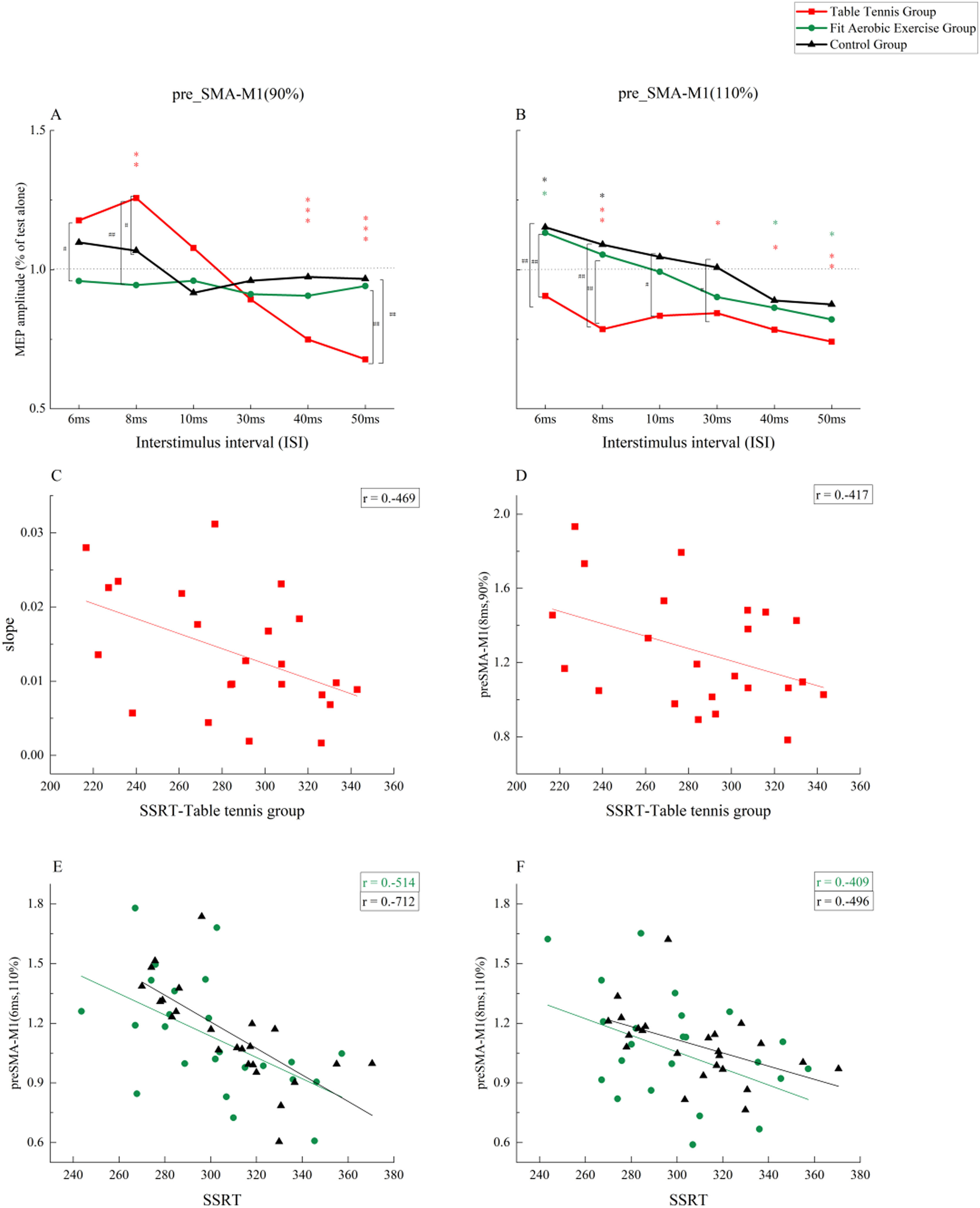
Neural connectivitypre-SMA-M1The results of the three-factor repeated-measures ANOVA showed a significant main effect of time point (F(5, 340) = 15.243, p = 0.000, ηp2 = 0.183), indicating different facilitation and inhibition effects at short vs. long intervals. The group main effect was significant (F(2, 68) = 3.215, p = 0.046, ηp2 = 0.086), indicating a significant difference between groups. The main effect of stimulus intensity was not significant (F(1, 68) = 0.707, p = 0.403, ηp2 = 0.010). No two-factor interaction was found. The interaction between the three factors was significant (F(10, 340) = 4.869, p = 0.000, ηp2 = 0.125). Further analysis showed that when CS was 90 %RMT (Fig. 4A), there were significant differences between groups at time intervals of 6 ms, 8 ms, and 50 ms. Specifically, at 6 ms, the table tennis group was more effective than the fit aerobics group (p < 0.05). At 8 ms, the table tennis group was significantly better than the fit aerobics group (p < 0.01) and the control group (p < 0.05), but there was no significant difference between the fit aerobics group and the control group (p > 0.05). At 50 ms, the table tennis group was significantly inhibited compared with the fit aerobics group (p < 0.01) and the control group (p < 0.01), but there was no significant difference between the fit aerobics group and the control group (p > 0.05). When CS was 110 %RMT (Fig. 4B), there were significant differences between groups at 6 ms, 8 ms,10 ms and 30 ms time intervals. In particular, at the 6 ms time interval, the table tennis group was significantly inhibited compared to the fit aerobics group (p < 0.01) and the control group (p < 0.01), but there was no significant difference between the fit aerobics group and the control group (p > 0.05). At 8 ms, the table tennis group was significantly inhibited compared with the fit aerobics group (p < 0.01) and the control group (p < 0.01). There was no significant difference between the fit aerobics group and the control group (p > 0.05). At 10 ms, the table tennis group was significantly inhibited compared with the control group (p < 0.05). At 30 ms, the table tennis group was significantly inhibited compared with the control group (p < 0.05).
Neural connectivity results(pre-SMA-M1)
A: Neural Connectivity Differences at Different Time Points and Different Groups when CS=90 % RMT; B: Neural Connectivity Differences at Different Time Points and Different Groups when CS=110 % RMT; C: Correlation between Neural Connectivity Decline Slope from 8 ms to 50 ms and SSRT in Table Tennis Group under CS=90 %RMT; D: Correlation between Neural Connectivity at 8 ms and SSRT in Table Tennis Group under CS=90 %RMT; E: Correlation between Neural Connectivity at 6 ms and SSRT in Fit Aerobic Exercise Group and Control Group under CS=110 %RMT; F: Correlation between Neural Connectivity at 8 ms and SSRT in Fit Aerobic Exercise Group and Control Group under CS=110 %RMT.
*: the difference between single MEP and double MEP, *: P ≤ 0.05; * *: P ≤ 0.01, * * *: P ≤ 0.00.
#: difference between groups, #: P ≤ 0.05; ##: P ≤ 0.01, ### : P ≤ 0.00 .
The results of paired-sample t-tests showed that when CS was 90 %RMT (Fig. 4A), the table tennis group showed significant differences with TS at 8 ms, 40 ms and 50 ms, showing significant facilitation (p < 0.01) and significant inhibition (p < 0.01) at 8 ms, 40 ms, and 50 ms. When CS was 110 %RMT (Fig. 4B), the table tennis group showed significant differences from TS at 8 ms, 30 ms, 40 ms and 50 ms, showing overall inhibition (p < 0.05). In the fit aerobics group, there were significant differences from TS at 6 ms, 40 ms, and 50 ms, showing facilitation at 8 ms (p < 0.05) and inhibition at 40 ms and 50 ms (p < 0.05). The control group showed a significant difference from TS at 6 ms and 8 ms, showing overall facilitation (p < 0.05).
Pearson correlations showed significant correlations between slope and SSRT in the table tennis group when CS was 90 %RMT (r =−0.469,p < 0.05). There was a significant negative correlation between MEP amplitude and SSRT when an 8 ms interval was used (r =−0.417, p < 0.05). When CS was 110 %RMT, there was no significant correlation between the decline slope and SSRT in either the fit aerobics group or the control group. MEP amplitude was significantly negatively correlated with SSRT when a 6 ms interval was used in the fit aerobics group (r =−0.514, p < 0.01) and the control group (r =−0.712, p < 0.01). There was a significant negative correlation between MEP amplitude and SSRT when an 8 ms interval was used in the fit aerobics group (r =−0.409, p < 0.05) and the control group (r =−0.496, p < 0.05).
One-way ANOVA was conducted on the slope results for table tennis group (8ms-50 ms under subthreshold conditions) and fit aerobics group and control group (6ms–50 ms under suprathreshold conditions). The results revealed a significant difference (F(2,68) =4.501, p = 0.015, ηp2 =0.143). Post-hoc comparisons showed significantly greater slope in the table tennis group, compared to both the fit aerobics group (p < 0.05) and the control group (p < 0.05). There was no significant difference between the fit aerobics group and the control group (p > 0.05).
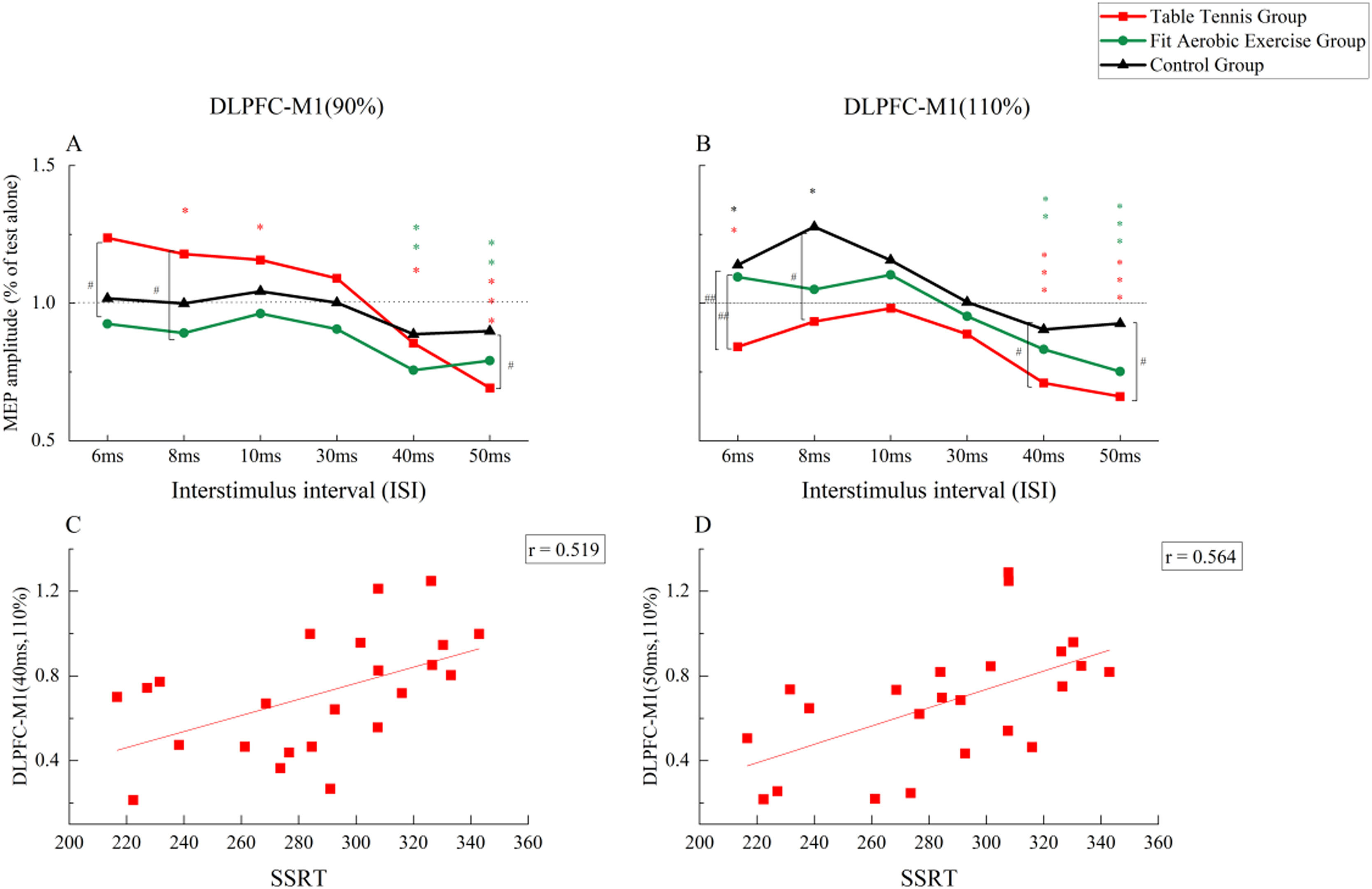
DLPFC-M1The results of three-factor repeated-measures ANOVA showed a significant main effect of time point was significant (F(5, 340) =23.326, p = 0.000, ηp2 =0.255), indicating different facilitation and inhibition effects at short and long intervals. The main effect of stimulus intensity was not significant (F(1, 68) =0.013, p = 0.911, ηp2 =0.000). The main effect of group was not significant (F(2, 68) =1.692, p = 0.192, ηp2 =0.047).The interaction between stimulus intensity and group was significant (F(2, 68) =1.692, p = 0.192, ηp2 =0.047). No other significant interaction effect was observed. Further analysis showed that when CS was 90 %RMT (Fig. 5A), there was a significant difference between the time intervals of 6 ms, 8 ms, and 50 ms. The facilitation effect at 6 ms was significantly greater in the table tennis group than in the fit aerobics group at (p < 0.05). At 8 ms, the table tennis group was more effective than the fit aerobics group (p < 0.05). At 50 ms, the table tennis group was significantly inhibited compared with the control group (p < 0.05). When CS was 110 %RMT (Fig. 5B), there were significant differences among groups at the time intervals of 6 ms, 8 ms, 40 ms, and 50 ms. In particular, at 6 ms, connectivity was significantly inhibited in the table tennis group, compared to the fit aerobics group (p ≤ 0.01) and the control group (p < 0.01), while there was no significant difference between the fit aerobics group and the control group (p > 0.05). At 8 ms, connectivity was significantly inhibited in the table tennis group compared with the control group (p < 0.05). At 40 ms, connectivity was significantly inhibited in the table tennis group compared with the control group (p < 0.05). At 50 ms, connectivity was significantly inhibited in the table tennis group compared with the control group (p < 0.05).
Neural connectivity results(DLPFC-M1)
A: Neural Connectivity Differences at Different Time Points and Different Groups when CS=90 % RMT; B: Neural Connectivity Differences at Different Time Points and Different Groups when CS=110 % RMT; C: Correlation between Neural Connectivity at 40 ms and SSRT in Table Tennis Group under CS=110 %RMT; D: Correlation between Neural Connectivity at 50 ms and SSRT in Table Tennis Group under CS=110 %RMT.
*: the difference between single MEP and double MEP, *: P ≤ 0.05; * *: P ≤ 0.01, * * *: P ≤ 0.00.
#: difference between groups, #: P ≤ 0.05; ##: P ≤ 0.01, ### : P ≤ 0.00 .
The results of paired-sample t-tests showed that when CS was 90 %RMT (Fig. 5A), the table tennis group showed significant facilitation at 8 ms and 10 ms (p < 0.05), and significant inhibition at 40 ms and 50 ms (p < 0.05). The fit aerobics group had significant inhibitory effects at 40 ms and 50 ms (p < 0.05). When CS was 110 %RMT (Fig. 5B), the table tennis group showed significant inhibition at 6 ms, 40 ms, and 50 ms (p < 0.05 for all). The fit aerobic group showed significant inhibition at 6 ms, 40 ms, and 50 ms (p < 0.05 for all). The control group had significant facilitation at 6 ms and 8 ms (p < 0.05 for both).
Pearson correlation analysis (Fig. 5C and D) showed a significant correlation between CS 110 % intensity, 40 ms (r = 0.519, p < 0.05) and 50 ms (r = 0.564, p < 0.05) intervals, and SSRT in the table tennis group.
ANOVA was conducted on the slope results for table tennis (8ms–50 ms under subthreshold conditions) and fit aerobics (6ms–50 ms under suprathreshold conditions), revealing no significant differences.
DiscussionThe results described above show that the table tennis group showed a behavioral advantage in motor control, particularly in reactive control. Electrophysiologically, this table tennis group demonstrated strong neural network regulation under pre-SMA-M1 subthreshold conditions and stable inhibitory regulation under DLPFC-M1 suprathreshold conditions.
Behavioral advantagesThis study investigated whether different types of exercise yield distinct effects in the elderly population. Specifically, we recruited elderly individuals with experience in two distinct activities: table tennis (an open-skill sport) and fit aerobics (a closed-skill sport). The reason for selecting these two exercise types was because table tennis is an open-skill sport with a smaller activity area and environmental factors. On the other hand, fit aerobics takes place in a stable environment where individuals only need to focus on their own actions according to the requirements, without significant interaction with the surroundings. Both exercise types are common in elderly populations.
Based on previous studies, moderate-intensity exercise, defined as 3–4 sessions per week, lasting 40–60 min per session, and performed at 60 %−70 % of maximum heart rate, has been shown to have positive effects on both the physiological and psychological well-being of older adults(Lambourne & Tomporowski, 2010; Lautenschlager et al., 2008; McMorris & Hale, 2012). Exercise lasting for more than 6 months has been associated with significant improvements in cognitive function, while exercise lasting for 12 months or more has been shown to benefit overall functional status(Baker et al., 2010; Langoni et al., 2019; Lazarou et al., 2017; Sink et al., 2015; Suzuki et al., 2012). The duration of over 2 years was chosen to ensure stability in exercise habits, minimizing any potential interruptions or changes in routine that could affect the comparability between groups(Sink et al., 2015). Therefore, we required the exercise group to perform exercise at least three times a week, with each session lasting for at least 40–60 min, for a duration of over two years.
Our study revealed noteworthy distinctions in reactive control abilities among the groups. The elderly individuals in the table tennis group exhibited significantly shorter SSRT compared to both the aerobic and control groups (see Fig. 3A), as well as shorter SSD (see Fig. 3C). Regarding reaction speed, Stop RT, Maybe go RT, and Never RT were all significantly shorter in the table tennis group compared to the other groups. In terms of reaction accuracy, Maybe go ACC and Never ACC were also significantly improved with table tennis, compared to the control condition. In older adults, playing table tennis resulted in a swifter and more accurate response pattern, highlighting the positive impact of table tennis on reactive control in this demographic.
This advantage was not observed in the fit aerobics group. We propose that this difference may arise due to the dynamic and interactive nature of table tennis, in contrast to the relatively monotonous activities associated with fit aerobics. The latter typically involves individuals performing steadily within a predictable environment(Di Russo et al., 2010). In the control group, participants did not have a regular exercise practice, but their daily physical activity reached moderate levels (e.g., housework, cycling, walking), which may explain the maintenance of motor control behavior in the control group.
No discernible differences in proactive control were identified among groups (see Fig. 3B), perhaps due to a general decline in proactive control associated with aging, encompassing facets such as fluid intelligence(Braver & Barch, 2002; Braver & West, 2008).
Characteristics of pre-SMA-M1 connectivityPrevious studies have indicated that the neural conduction pathway of pre-SMA-M1 varies with different stimulus intervals. Specifically, at shorter intervals, pre-SMA-M1 functions as a direct pathway(Rae et al., 2015). In our investigation, we observed that at short intervals (6 ms, 8 ms), pre-SMA exhibits a facilitatory effect on M1. At short time intervals, this facilitation effect is significantly negatively correlated with SSRT, as depicted in Fig. 4. Additionally, we noted that the facilitation effects vary based on exercise type. In the table tennis group, a significant facilitation was observed under subthreshold conditions at 8 ms, as illustrated in Fig. 4A. Moreover, under this condition, a correlation with SSRT was identified, as shown in Fig. 4D. Conversely, the aerobic and control groups demonstrated significant facilitation under suprathreshold conditions at 6 ms and 8 ms, as illustrated in Fig. 4B. Similarly, correlations with SSRT were observed, as depicted in Fig. 4E and F. Overall, the table tennis group achieved facilitation effects at lower stimulus conditions compared to the other two groups at higher stimulus conditions. Table tennis experience may confer an advantage in facilitating these effects. The facilitation observed in the fit aerobics and control groups may reflect heightened neuronal excitability due to an increase in stimulus intensity(Gordon et al., 2018), rather than the nature of the exercise itself. Consequently, facilitation of the direct pathway of pre-SMA-M1 may represent a key pathway affecting the control of responsiveness.
In this study, we found that table tennis exercise can enhance the facilitation ability of the pre-SMA-M1 direct pathway. Previous studies have shown that, under longer stimulus intervals, pre-SMA-M1 involves an indirect pathway mediated by the subthalamic nucleus (STN)(Rae et al., 2015). Different pathways of pre-SMA-M1 conduction exert distinct regulatory effects on M1, typically facilitation with short-interval stimulation and inhibition with long-interval stimulation(Rae et al., 2015). From a neural network perspective, these distinct conduction pathways likely constitute a pre-SMA-M1 neural network. We identified a correlation between slope and SSRT in the table tennis group under the subthreshold condition. In the other two groups, we did not find any significant correlation. Our findings suggest that table tennis confers a significant advantage in enhancing the neural network regulatory capacity of pre-SMA-M1, potentially influencing reactive control abilities. The activation of pre-SMA in combination with M1 during preparation for a movement(Ball et al., 1999) may simultaneously enhance the conduction ability of excitatory and inhibitory neurons in different pre-SMA-M1 pathways, thereby enhancing its regulatory ability(Fiori et al., 2016; Tokuno & Nambu, 2000). We therefore posit that one of the direct factors influencing reactive control abilities may be the neural network regulatory capacity of pre-SMA-M1.
Characteristics of DLPFC-M1 connectivitySimilarly, research indicates that DLPFC-M1 conduction pathways differ across stimulus intervals, where short intervals may represent the direct pathway, and long intervals the indirect pathway(Cao et al., 2022; Xia et al., 2022). Under subthreshold conditions, the table tennis group exhibited short-term facilitation and long-term inhibition, as illustrated in Fig. 5A. This phenomenon may be associated with sustained attention during table tennis activities. The DLPFC remains consistently activated during movement, collaboratively coordinating with M1 to execute actions,(Aron et al., 2003; Aupperle et al., 2015) thereby enhancing its connectivity with M1. Heightened attention may have been maintained less effectively in the fit aerobics and control groups.
As nerve signals are transmitted from the DLPFC to M1, with an extended stimulus interval, the indirect pathway likely involves subcortical circuits.(Ni et al., 2009) This may encompass transcallosal projections from the right DLPFC to the contralateral homologous region, subsequently reaching left M1. Alternatively, the circuit could involve projections from the right DLPFC to the ipsilateral M1, eventually affecting left M1(Cao et al., 2022; Xia et al., 2022). Our investigation revealed that, during prolonged stimulation (40 ms, 50 ms) of DLPFC-M1, inhibitory effects on M1 were observed in both the table tennis and fit aerobics (Fig. 5A and B). Studies have pointed out that no matter what kind of exercise, there will be functional and structural changes in the elderly brain(Chen et al., 2020). Therefore, this change may bring about changes in neural connections between different cortical areas, which in turn affect the connectivity of multiple subcortical DLPFC-M1 pathways.
Only the table tennis group showed global inhibition of the direct and indirect pathway under subthreshold conditions. Inhibition of the indirect pathway (40 ms, 50 ms) was significantly correlated with SSRT, as illustrated in Fig. 5C and D. Prior research has emphasized the pivotal role of the right DLPFC in co-regulating cognitive processes with M1(Cao et al., 2022; Ceceli et al., 2023), and such regulatory dynamics may be altered by training(Ni et al., 2009). Playing table tennis requires constant movement adjustments to adapt to unpredictable environmental stimuli, which in turn require activation of attention, sensory, motor, and other relevant cortical areas(Carius et al., 2022; Faber et al., 2014). With repeated activation, the subcortical pathway establishes stable inhibitory connections. Conversely, no analogous correlations were observed in the fit aerobics group. Therefore, we speculate that another direct factor contributing to the enhancement of table tennis reactivity control is the subcortical multipathway inhibitory connectivity of DLPFC-M1.
Previous studies have primarily focused on exploring the positive effects of exercise on cognitive aging, with a concentration on changes in physical function, cognitive ability, and brain structure. Few studies have focused on exploring the brain networks related to motor control in older adults by measuring the neural connectivity between the sensorimotor-related cortex and M1, and further examining the relationship among these networks, motor experience, and motor control ability. Therefore, our study is the first to use double-pulse TMS at two sites to directly assess the sensorimotor cortical neural connectivity among older adults with different exercise backgrounds. This innovative method can serve as a parametric reference for Paired Associated Stimulation (PAS) interventions, potentially offering an alternative approach to enhancing motor control and neural connectivity among older adults.
LimitationsOne limitation of our study is the relatively narrow range of exercise experience among our participants. While this allowed us to focus on the effects of different exercises on cortical connectivity, it may have limited our ability to detect correlations between neural connectivity and years of exercise experience or exercise intensity. Future studies could consider including a broader range of exercise experience to explore these relationships further. Another limitation is the cross-sectional design of our study, which Limits the causal inferences about the relationship between exercise and neural connectivity. Longitudinal studies are needed to establish causality and track changes in neural connectivity over time in response to exercise.
ConclusionThe preliminary findings of this study suggest that table tennis exercise may enhance the motor system regulated by neural networks and stabilize inhibitory regulation of DLPFC-M1, thereby affecting motor control in older adults.
CRediT authorship contribution statementJia-Ning Wei: Data curation, Software, Writing – original draft, Validation. Ming-Kai Zhang: Investigation, Project administration, Resources. Zhen Wang: Methodology, Formal analysis. Yu Liu: Funding acquisition, Conceptualization. Jian Zhang: Supervision, Visualization, Writing – review & editing.
The study was supported by a grant from the National Natural Science Foundation of China (11932013, 31971024) . For the purpose of open access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission.
Table Tennis Experience Enhances Motor Control in Older Adults: Insights into Sensorimotor-related Cortical Connectivity