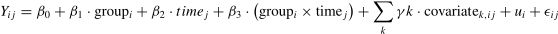Subthreshold depression (SD) affects a significant proportion of adolescent females, posing a risk of major depression in later life. This study examines the effects of open-skill exercise (OSE) and closed-skill exercise (CSE) on SD, executive function (EF), and emotional states in female adolescents.
MethodsA double-blind randomized controlled trial involved 95 female adolescents (mean age = 16.73 ± 0.42 years) with SD symptoms. Participants were assigned to OSE, CSE, or control (CON) groups and underwent an 8-week exercise program. Primary outcomes were assessed using the Beck Depression Inventory (BDI-13) and the Center for Epidemiological Studies Depression Scale (CES-D), with secondary outcomes including EF tasks and emotional assessments. Differences were examined using generalized linear mixed models with intention-to-treat and multiple imputation.
ResultsBoth OSE and CSE significantly reduced depressive symptoms, with CSE showing greater improvement. EF assessments showed enhanced cognitive flexibility and working memory in both exercise groups at 4 weeks, and superior inhibitory control and cognitive flexibility in the CSE group at 8 weeks. Emotional assessments indicated a notable reduction in negative emotions in the CSE group after 8 weeks.
ConclusionsBoth OSE and CSE reduce SD symptoms in female adolescents, with CSE providing more sustained benefits for EF and emotional states. Further research on exercise interventions for mental health is warranted.
Trial registration number: ChiCTR2400081139
Depression is the second most prevalent mental disorder among children and adolescents, affecting between 5 and 11 % of adolescents worldwide (Erskine et al., 2016; Noetel et al., 2024). The primary symptoms of depression include persistent sadness, loss of interest, difficulty concentrating, decreased self-esteem, and impaired functioning in academic and social activities (Austin et al., 2001; Gotlib & Joormann, 2010; McDermott & Ebmeier, 2009). Research suggests that depression can disrupt cognitive processes, leading to difficulties in decision-making, reduced attention, and diminished memory capacity (Friedman & Robbins, 2021; Snyder, 2013). Impairments in executive functions (EFs), particularly the core EFs—inhibitory control, working memory, and cognitive flexibility—may further hinder the ability to regulate emotions (Gabrys et al., 2018; Morris et al., 2015). These cognitive and emotional challenges not only impact adolescents' daily lives and academic performance but can also lead to more severe mental health issues, such as substance abuse and an increased risk of suicide (Balázs et al., 2013; Erskine et al., 2016; Sihvola et al., 2007; Wesselhoeft et al., 2013).
In recent years, as attention to adolescent mental health issues has increased, researchers have found that a significant proportion of adolescents exhibit subthreshold depressive (SD) symptoms, which fall short of clinical diagnostic criteria but pose a high risk of developing into major depression later in life (American Psychiatric Association, 2013; Lee et al., 2018). Approximately 29.2 % of adolescents are affected by SD, with the prevalence of this condition increasing steadily between the ages of 12 and 20 (Bertha & Balázs, 2013; Carrellas et al., 2017; Wesselhoeft et al., 2013). The physiological, psychological, and social factors associated with puberty result in a clear gender difference in SD prevalence, with females experiencing approximately double the rate of SD compared to males (Balázs et al., 2013; Crockett et al., 2020; Shankman et al., 2007). Adolescence represents a particularly vulnerable period in the life cycle of females, as well as being a crucial stage of brain maturation. Adolescent females are particularly susceptible to emotional health problems, which makes them the primary target population for interventions aimed at reducing mild depressive symptoms and prevent the onset of more severe forms of depression (Eaton et al., 1995; Stewart et al., 2016).
It has been demonstrated that physical exercise has a positive effect on mental health and cognitive function (Erickson et al., 2019; Kramer & Erickson, 2007; Schuch et al., 2018). One key mechanism is the enhancement of cerebral blood flow (CBF), which delivers oxygen and nutrients to the brain's executive centers, improving cognitive performance (Lucas et al., 2015). Exercise also promotes neuroplasticity, the brain's ability to reorganize itself by forming new neural connections, leading to improvements in attention, memory, and problem-solving skills (Cassilhas et al., 2015; Mattson et al., 2018). Additionally, the brain-derived neurotrophic factor (BDNF) model suggests that exercise stimulates the production of BDNF, a protein essential for the growth, maintenance, and survival of neurons, which plays a critical role in cognitive function and emotional regulation (Marosi & Mattson, 2014). Moreover, physical activity increases the secretion of serotonin and dopamine, neurotransmitters associated with improved mood and emotional resilience (Cooney, 2018; Mikkelsen et al., 2017). Given its non-pharmacological nature, physical activity has emerged as an effective intervention to address the emotional and cognitive challenges associated with SD in adolescents, offering benefits in areas such as attention, memory, and emotional resilience (Schuch et al., 2018).
In high school, the motor skills practiced in physical education classes are not only critical for demonstrating physical abilities but also have significant benefits for adolescents' physical health, emotional state, and cognitive functioning (Biddle & Asare, 2011; Darla, 2022). Motor skills can be divided into open skills and closed skills based on the predictability of the sport environment (Gu et al., 2019; Schmidt RA, 2008). Open-skill exercise (OSE), such as badminton, table tennis, and basketball, emphasizes adaptability and coordination in changing environments (Tsai et al., 2017; Yu et al., 2017). This not only improves physical health but also enhances cognitive flexibility and problem-solving through better coordination of the brain and nervous system. In contrast, closed-skill exercise (CSE) involves repetitive, self-paced activities (e.g., swimming, running) in a stable environment (Tsai et al., 2017; Yu et al., 2017). These exercise can improve focus and stress management through rhythmic and meditative movement (Saltzman, 2018). From the perspective of emotion regulation, moderate physical exercise has a significant effect on relieving anxiety and depression (Aldao et al., 2010; Salmon, 2001). This is because moderate exercise stimulates the release of endorphins, which enhance mood and create a sense of well-being (Aldao et al., 2010; Salmon, 2001). Furthermore, regular physical activity can reduce stress levels and improve overall mental health by providing a healthy outlet for emotional stress (Paluska & Schwenk, 2000). For adolescents with SD, these forms of exercise may provide effective emotion regulation strategies that help them better cope with challenges (Biddle et al., 2019; Noetel et al., 2024; Singh et al., 2023).
A primary goal of high school physical education programs is the development of both physical and mental health (Trudeau & Shephard, 2008). Consequently, it is of the utmost importance to ascertain which types of exercises, namely OSE or CSE, are most effective in addressing SD. The identification of the most beneficial forms of exercise can assist educators in the design of more effective PE curricula, which will in turn facilitate the delivery of targeted support for adolescents struggling with SD. This study aims to explore the therapeutic benefits of physical exercise for female adolescents with SD by implementing an 8-week physical exercise intervention program in schools. The research will examine whether different motor skills exercises can improve subthreshold depressive states in adolescents and have varying effects on specific EFs and positive emotional aspects. The objective is to provide more effective support and intervention strategies for adolescents with SD.
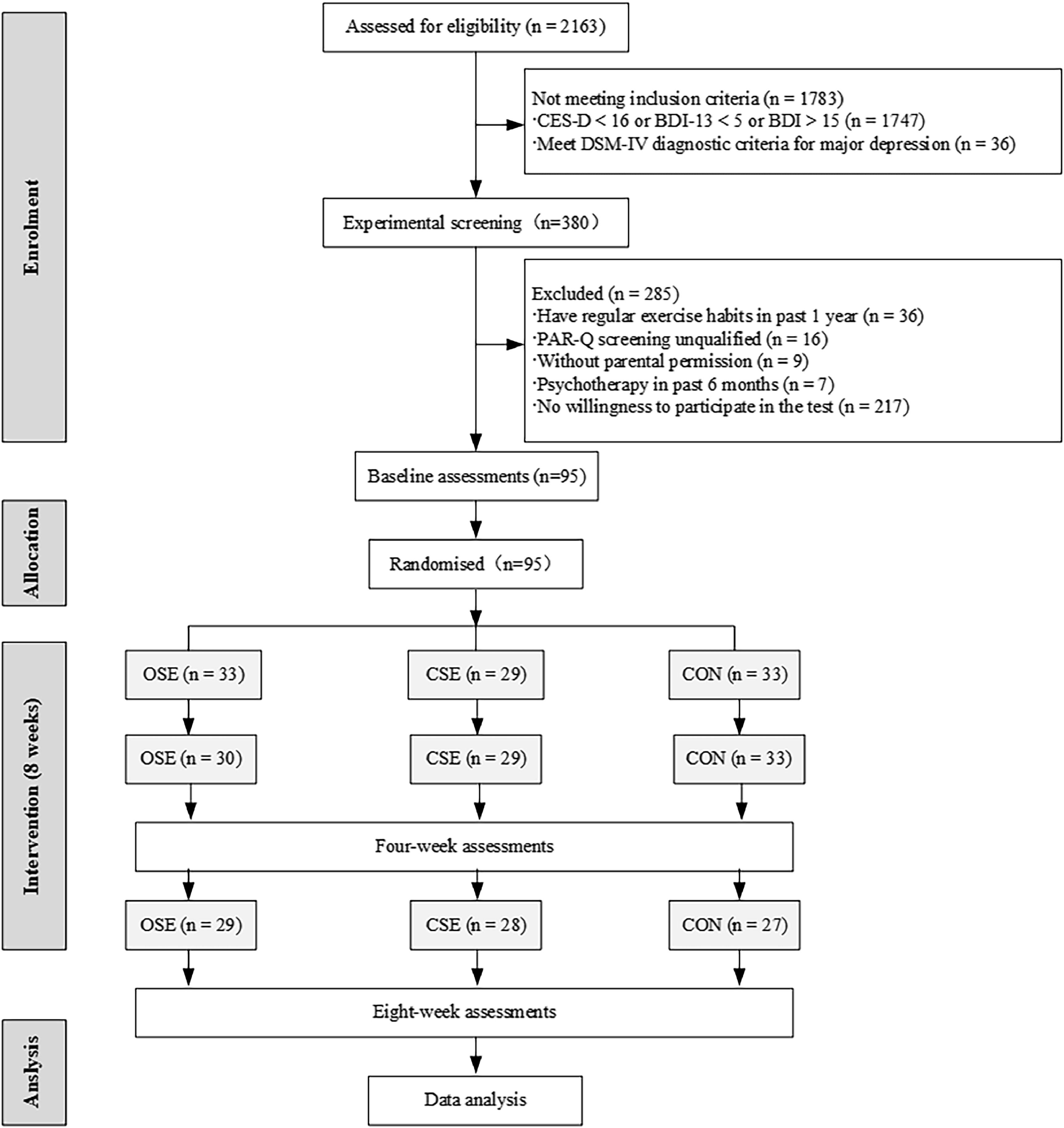
MethodsParticipantsThe participants for this study were recruited through screenings conducted in high schools using two validated instruments: the Center for Epidemiologic Studies Depression Scale (CES-D) and the Beck Depression Inventory (BDI). Adolescents exhibiting only SD symptoms were selected based on their scores on the CES-D (≥16) and BDI (5–14), thereby ensuring that no participants met the criteria for a full diagnosis of depression. Following an interview with the school's psychological counsellor, the selected adolescents were invited to participate in the study. In total, 95 female adolescents who met the eligibility criteria were included in the study. None of the participants had been diagnosed with a neurological or psychiatric disorder or informed of their SD status prior to the study (Fig. 1). All participants provided verbal consent, and their parents provided written informed consent. The study was conducted in accordance with the ethical guidelines set forth in the Declaration of Helsinki and received approval from the Ethics Committee of the Wuxi Higher Health Vocational Technical School (Ethics Approval No: 2,024,003). Furthermore, the study protocol was registered in the Chinese Clinical Trial Registry (Registration No: ChiCTR2400081139).
The sample size was determined based on a previous randomized controlled in which the authors utilised the CES-D to assess the variability in depression scores in conjunction with physical activity as the treatment (Philippot et al., 2022). In order to detect a difference of −2.4 at the 5 % significance level with 82 % efficacy and assuming a standard deviation of 2.4 (corresponding to an effect size of 1), the sample size should be at least 18 participants per group. In consideration of the possibility of subject attrition, 95 participants were recruited.
Study designThis research employed a double-blind, randomized, controlled trial design to investigate the impact of OSE and CES on subthreshold depressive states, cognitive functions, and emotional responses in high school students. Following their inclusion in the study, participants were randomly allocated to one of three groups: an OSE group, a CSE group, or an active control group (CON). Participants were unaware of the purpose of the experiment and were not informed of their group allocation. This was achieved through a random draw from envelopes, with a total of 95 participants. Prior to the intervention, designated teachers collected basic demographic information, including height, weight, the Mobile Phone Addiction Index (MPAI), and the Pittsburgh Sleep Quality Index (PSQI), from the participants. Within one week of the initial data collection, participants were required to complete three EF tests independently: the Flanker task, task switching test, and N-back task. Additionally, they were required to complete an emotional task. At the fourth and eighth weeks of the intervention, participants repeated the baseline data collection, SD questionnaires, and underwent identical cognitive and emotional assessments. Throughout the study period, participants adhered to the prescribed training protocols and demonstrated a high level of engagement (90 ± 2 %).
MeasuresPrimary outcome measure: clinical assessmentsBDI-13: Depressive symptoms were assessed using the 13-item Beck Depression Inventory short form (Beck & Beamesderfer, 1974). Emotional and behavioral states in the past week was assessed on a 4-point scale ranging from 0 to 3. Total scores are interpreted as follows: scores of 0 to 4 indicate no or slight depression, scores of 5 to 7 indicate mild depression, scores of 8 to 15 indicate moderate depression, and scores of 16 or more indicate severe depression. The internal consistency reliability of the BDI-13 was 0.9, while the test-retest reliability ranged from 0.73 to 0.96 (Proença Lopes et al., 2022).
CES-D: The Center for Epidemiological Studies Depression Scale (CES-D) a frequently employed instrument for the screening of depression in non-clinical populations (Radloff, 2016). The scale comprises 20 items that measure a range of symptoms, including sadness, feelings of worthlessness, poor concentration, appetite loss, and sleep disturbances. Participants are required to rate the frequency of these symptoms over the past week using a 4-point scale (0–3 points). The response options are as follows: 0 indicates none or rarely (less than one day), 1 sometimes or a little (1–2 days), 2 often or half the time (3–4 days), and 3 most or all the time (5–7 days). A score of 16 or above is generally indicative of depressive symptoms. In China, the CES-D demonstrates an internal consistency of 0.9 and a test-retest reliability of 0.49 (Wang et al., 1997).
Secondary outcome measures: executive function and emotional assessmentsEF assessments were conducted using a series of comprehensive tasks. Before the official testing for all EF measures, participants completed a practice module consisting of two block to ensure their comprehensive understanding of the tasks. Results were recorded using response time (RT) and accuracy (ACC), including the following components:
Eriksen Flanker task. A modified version of the task was employed to assess inhibitory control (Hillman et al., 2009). The participants were required to react to congruent and incongruent arrow stimuli by pressing the respective keys on a keyboard. Each stimulus was presented for a duration of 120 ms, with a response window of 1350 ms and intervals ranging from 1250 to 1550 ms. Two 150-trial blocks were conducted.
Task-switching test. A paradigm was employed to assess cognitive flexibility. Participants viewed numbers (1–9, excluding 5) on a black screen, encircled by solid or dashed squares. They determined if the solid square number was higher or lower than 5 and if the dashed square number was odd or even. The task was comprised of six blocks, each containing 64 trials. The initial two blocks were homogeneous tasks (i.e., all trials were either high or low task and odd or even task), whereas blocks 3–6 were heterogeneous tasks (i.e., a mix of high/low numbers and even/odd numbers). In this section, the task transitioned between two options every two times in a predictable optional run-transition paradigm (i.e., AABBAA) (Rogers & Monsell, 1995). The variable block included an equal number of switch and non-switch tasks (Dai et al., 2013). The stimulus duration was 200 ms, and the interval between stimuli was 1500 ms.
N-back task. Working memory was assessed using a modified N-back task with 1-back, 2-back, and 3-back variations (Kao et al., 2020). A total of 176 trials were distributed across 12 blocks, with 12 trials in the 1-back and 2-back blocks and 20 trials in the 3-back block. Each trial displayed a digit for 200 ms, followed by a 2300 ms inter-stimulus interval. Participants were afforded 1000 ms to respond, with task blocks, signalled by a 5-second cue, being separated by 15-second rest periods. The entire session, which placed a premium on the prompt and precise execution of responses, spanned a duration of ten minutes.
Self-Assessment Manikin (SAM), revised by Bradley and Lang (1994), is a non-verbal tool for assessing self-reported emotional states. It quantifies emotional states in terms of arousal and valence rapidly and non-verbally. For valence, a continuum of five stylized human figures (manikins) depicts feeling very happy (score of 1) to very sad (score of 9). In terms of arousal, the first continuum, also consisting of five stylised human figures, depicted a variety of emotions ranging from a state of complete calm (score of 1) to a state of extreme distress (score of 9). The SAM was validated using 60 images from the Chinese Affective Picture System (CAPS) (Gong & Wang, 2016). It has demonstrated high internal consistency in the dimensions of arousal and valence, with Cronbach's α coefficients of 0.63 and 0.98, respectively (Gong & Wang, 2016).
Intervention contentThe study involved an eight-week exercise intervention targeting adolescents exhibiting subthreshold depressive symptoms. The CON group only participated in regular physical education classes. In addition to regular physical education, the experimental group participated in three 40-minute OSE or CSE sessions per week (see Supplementary Table1 for details for details), which were led by physical education professionals and conducted at a moderate intensity. The intensity of the activity was quantified by monitoring each participant's heart rate (HR) using a Polar HR monitor (RX800CX, Finland). The target heart rate reserve (HRR) during exercise was set at 40 % to 59 % of HRR, which is an indicator of moderate intensity exercise (Kesaniemi et al., 2001). Each session commenced with a warm-up and concluded with a muscle stretch. To reduce bias, the assessors and trainers were unaware of the purpose of the intervention.
The OSE training program comprised the following elements: The training content encompassed a diverse array of techniques, including forehand serve, backhand serve, return of serve, over smash, serve, lob, lift, and footwork combination. Each session comprised 10 min of focused skill practice. The duration of each match was 30 min, regardless of whether it was played as a singles or doubles event. The structure of the training sessions was designed to become progressively more complex in order to facilitate the improvement of the participants' overall skills. The CSE training program comprised individual running exercises, with different exercises performed each week. These included interval runs, timed runs and long runs. The training program placed particular emphasis on the importance of rhythmic movement, proper running form and breathing control.
Statistical analysisThe statistical analysis was conducted using the R programming language (version 4.3.2, R Foundation for Statistical Computing, Vienna, Austria). A variance analysis was performed on the continuous variables representing the baseline characteristics of the participants. All data from subjects randomly grouped were analyzed in accordance with intention-to-treat (ITT) principles. To account for missing data, sensitivity analyses were conducted for clinical assessments, EF, and SAM outcomes under the assumption that the data were missing at random, using multiple imputation (MI) via chained equations with the mice package in R. A total of 200 imputations were performed. Baseline values and factors significantly associated with the outcomes were included in the imputation model. To perform the EF, the inverse efficiency score (average correct RT / ACC of responses) was calculated, which can be used to measure the relationship between reaction speed and accuracy. Consequently, a smaller IES value indicates superior task performance. All data pertaining to the reaction time and accuracy of each measure of EF can be found in the Supplementary Table 2. All statistical analyses were performed and reported as IES for all measures.
The between-group differences in clinical assessments, EF, and SAM outcomes over time were examined using generalized linear mixed models (GLMMs). The models included fixed effects for group (intervention vs. control), time (baseline, post-intervention), and their interaction (group × time), as well as covariates such as age, BMI, sleep score, and cell phone use. A random intercept was included to account for intra-subject variability. The GLMM model can be expressed as:
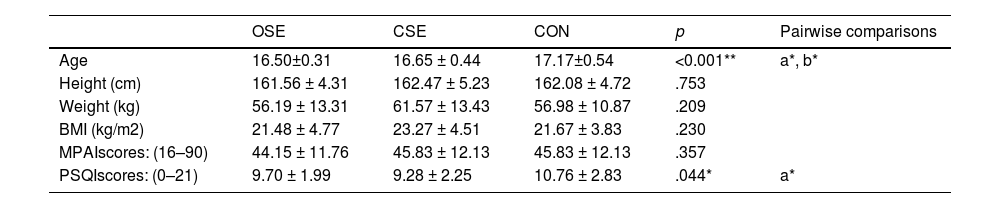
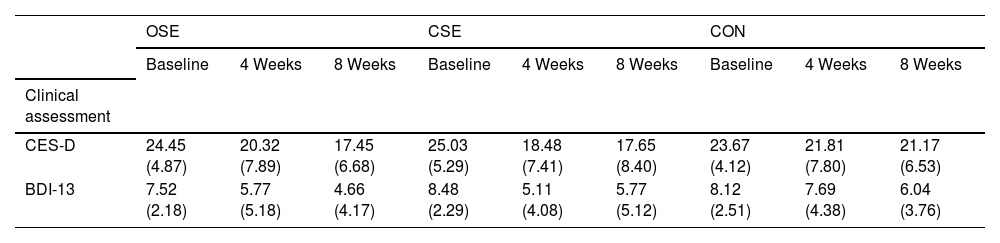
Where Yijis the outcome variable (clinical assessment, EF, or SAM) for participant i at time j, β0 is the overall intercept, β1 represents the effect of group, β2 represents the effect of time, and β3 is the interaction effect between group and time. γk represents the coefficients for the covariates (e.g., age, BMI, sleep score, and cell phone use), ui is the random intercept for participant i, accounting for intra-subject variability, and ϵij is the residual error term. Continuous outcomes were modeled using gamma distributions with log-links, which are appropriate for positively skewed data. Results were reported as percentage changes with 95 % confidence intervals (CIs), and the significance level (α) was set at 0.05.ResultsTable 1 presents a summary of the baseline characteristics of participants in the OSE, CSE, and CON. Significant differences were observed in age (p < 0.001) and sleep scores (p = 0.044) among the groups, while other variables such as height, weight, BMI, and MPAI scores demonstrated no significant differences. Table 2 presents the descriptive data for clinical assessments, EF, and SAM outcomes, categorized by study group and evaluation time points.
Participant characteristics at baseline.
| OSE | CSE | CON | p | Pairwise comparisons | |
|---|---|---|---|---|---|
| Age | 16.50±0.31 | 16.65 ± 0.44 | 17.17±0.54 | <0.001** | a*, b* |
| Height (cm) | 161.56 ± 4.31 | 162.47 ± 5.23 | 162.08 ± 4.72 | .753 | |
| Weight (kg) | 56.19 ± 13.31 | 61.57 ± 13.43 | 56.98 ± 10.87 | .209 | |
| BMI (kg/m2) | 21.48 ± 4.77 | 23.27 ± 4.51 | 21.67 ± 3.83 | .230 | |
| MPAIscores: (16–90) | 44.15 ± 11.76 | 45.83 ± 12.13 | 45.83 ± 12.13 | .357 | |
| PSQIscores: (0–21) | 9.70 ± 1.99 | 9.28 ± 2.25 | 10.76 ± 2.83 | .044* | a* |
Note: OSE: open-skill exercise; CSE: closed-skill exercise; CON: control group. MPAI: the Mobile Phone Addiction Index; PSQI: Pittsburgh Sleep Quality Index (PSQI). a = between OSE and CON, b = between OSE and CON.
Descriptive statistics for clinical assessment, executive function assessments, and emotional assessment by group and time.
| OSE | CSE | CON | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 4 Weeks | 8 Weeks | Baseline | 4 Weeks | 8 Weeks | Baseline | 4 Weeks | 8 Weeks | |
| Clinical assessment | |||||||||
| CES-D | 24.45 (4.87) | 20.32 (7.89) | 17.45 (6.68) | 25.03 (5.29) | 18.48 (7.41) | 17.65 (8.40) | 23.67 (4.12) | 21.81 (7.80) | 21.17 (6.53) |
| BDI-13 | 7.52 (2.18) | 5.77 (5.18) | 4.66 (4.17) | 8.48 (2.29) | 5.11 (4.08) | 5.77 (5.12) | 8.12 (2.51) | 7.69 (4.38) | 6.04 (3.76) |
| Executive function assessment | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Eriksen·flanker·task | |||||||||
| Congruent | 324.46 (67.49) | 638.22 (1819.30) | 288.66 (41.98) | 323.42 (61.01) | 313.81 (56.63) | 294.91 (49.65) | 327.00 (72.91) | 318.45 (46.36) | 364.45 (54.93) |
| Incongruent | 377.56 (82.42) | 720.35 (1997.95) | 333.97 (52.20) | 374.34 (63.96) | 363.92 (67.42) | 338.03 (57.78) | 377.98 (87.25) | 359.13 (51.89) | 343.99 (72.43) |
| Task–switching·task | |||||||||
| Non-switch | 401.08 (107.94) | 406.01 (124.17) | 390.58 (114.45) | 395.78 (171.76) | 395.68 (126.29) | 405.40 (141.78) | 386.87 (114.70) | 413.50 (131.55) | 408.93 (91.74) |
| Switch | 858.16 (217.03) | 703.37 (231.20) | 592.71 (198.24) | 870.19 (270.35) | 741.19 (251.01) | 627.78 (251.35) | 785.21 (188.49) | 706.33 (196.25) | 627.98 (130.86) |
| N-back task | |||||||||
| 1-back | 471.10 (80.63) | 449.97 (91.05) | 431.57 (97.90) | 521.73 (139.05) | 496.27 (122.46) | 461.61 (102.24) | 554.94 (99.78) | 542.75 (100.05) | 508.37 (97.77) |
| 2-back | 620.25 (202.08) | 556.77 (172.96) | 553.48 (179.70) | 691.99 (199.14) | 588.06 (172.58) | 563.96 (185.53) | 714.21 (161.71) | 655.66 (153.67) | 645.57 (130.34) |
| 3-back | 792.92 (245.27) | 829.40 (275.72) | 795.32 (299.07) | 845.37 (241.92) | 746.24 (245.75) | 765.25 (260.44) | 882.49 (233.86) | 853.27 (203.75) | 822.60 (237.05) |
| Emotional assessment | |||||||||
| Valence | |||||||||
| Neutral emotion | 4.02 (0.98) | 4.18 (1.02) | 3.93 (1.24) | 3.78 (0.99) | 4.01 (1.21) | 3.96 (1.16) | 3.52 (1.13) | 3.51 (1.11) | 3.60 (1.29) |
| Negative emotion | 4.01 (1.24) | 3.84 (1.01) | 3.69 (1.12) | 3.47 (1.08) | 3.73 (1.16) | 3.86 (1.21) | 3.73 (1.22) | 3.42 (1.23) | 3.52 (1.51) |
| Arousal | |||||||||
| Neutral emotion | 3.82 (1.05) | 3.99 (1.02) | 3.83 (1.15) | 3.51 (1.08) | 3.58 (1.26) | 3.83 (1.08) | 3.33 (1.11) | 3.39 (1.07) | 3.25 (1.08) |
| Negative emotion | 4.01 (1.11) | 4.22 (1.09) | 4.05 (1.03) | 3.84 (1.11) | 3.95 (1.14) | 4.24 (1.25) | 3.98 (1.26) | 4.08 (1.42) | 4.00 (1.44) |
å.
Note: CES-D: The Center for Epidemiological Studies Depression Scale; BDI: Beck Depression Inventory; OSE: open-skill exercise; CSE: closed-skill exercise; CON: control group; Data are expressed as Mean±SD.
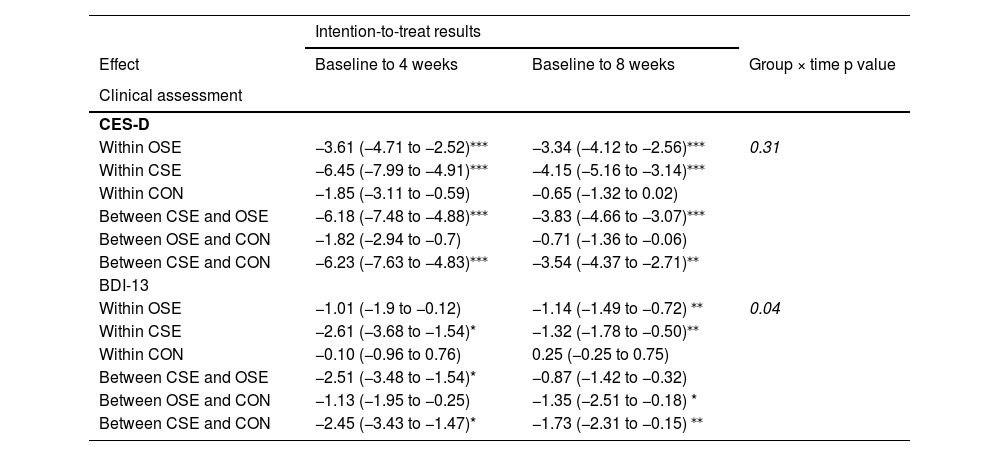
Table 3 presents the within-group and between-group changes in clinical assessments, EF, and SAM outcomes for the intention-to-treat (ITT) and sensitivity analyses. In comparison to the ITT analysis, the sensitivity analysis (see Supplementary Table 3) demonstrated similar overall patterns and magnitudes of results.
Results of the generalised linear mixed models for the outcome measures.
| Intention-to-treat results | |||
|---|---|---|---|
| Effect | Baseline to 4 weeks | Baseline to 8 weeks | Group × time p value |
| Clinical assessment | |||
| CES-D | |||
| Within OSE | −3.61 (−4.71 to −2.52)⁎⁎⁎ | −3.34 (−4.12 to −2.56)⁎⁎⁎ | 0.31 |
| Within CSE | −6.45 (−7.99 to −4.91)⁎⁎⁎ | −4.15 (−5.16 to −3.14)⁎⁎⁎ | |
| Within CON | −1.85 (−3.11 to −0.59) | −0.65 (−1.32 to 0.02) | |
| Between CSE and OSE | −6.18 (−7.48 to −4.88)⁎⁎⁎ | −3.83 (−4.66 to −3.07)⁎⁎⁎ | |
| Between OSE and CON | −1.82 (−2.94 to −0.7) | −0.71 (−1.36 to −0.06) | |
| Between CSE and CON | −6.23 (−7.63 to −4.83)⁎⁎⁎ | −3.54 (−4.37 to −2.71)⁎⁎ | |
| BDI-13 | |||
| Within OSE | −1.01 (−1.9 to −0.12) | −1.14 (−1.49 to −0.72) ⁎⁎ | 0.04 |
| Within CSE | −2.61 (−3.68 to −1.54)* | −1.32 (−1.78 to −0.50)⁎⁎ | |
| Within CON | −0.10 (−0.96 to 0.76) | 0.25 (−0.25 to 0.75) | |
| Between CSE and OSE | −2.51 (−3.48 to −1.54)* | −0.87 (−1.42 to −0.32) | |
| Between OSE and CON | −1.13 (−1.95 to −0.25) | −1.35 (−2.51 to −0.18) * | |
| Between CSE and CON | −2.45 (−3.43 to −1.47)* | −1.73 (−2.31 to −0.15) ⁎⁎ | |
| Executive function assessment (IES) | |||
|---|---|---|---|
| Eriksen·flanker·task | |||
| Congruent | |||
| Within OSE | −10.41 (−16.84 to 13.66) | −23.40 (−31.24 to −15.56)⁎⁎ | 0.37 |
| Within CSE | −12.31 (−20.55 to −4.07) | −18.23 (−23.57 to −12.89)⁎⁎ | |
| Within CON | −6.79 (−17.09 to 3.51) | −7.37 (−14.16 to −0.58) | |
| Between CSE and OSE | −68.41 (−303.43 to 166.63) | −17.14 (−23.32 to −10.96)⁎⁎ | |
| Between OSE and CON | −34.24 (−247.52 to 179.04) | −47.89 (−322.36 to 173.42) ⁎⁎ | |
| Between CSE and CON | −10.09 (−19.53 to −0.68) | −18.91 (−29.48 to 57.28)* | |
| Incongruent | |||
| Within OSE | −14.28 (−17.54 to 6.10) | −23.44 (−32.58 to −14.30)* | 0.40 |
| Within CSE | −10.45 (−20.52 to −0.38) | −21.13 (−27.11 to −15.09)⁎⁎⁎ | |
| Within CON | −16.32 (−29.02 to −3.62) | −15.79 (−27.12 to −4.46) | |
| Between CSE and OSE | −4.38 (−5.34 to 6.58) | −21.61 (−28.64 to −14.58)⁎⁎ | |
| Between OSE and CON | −6.69 (−23.5 to 9.12) | −13.61 (−4.25 to 12.97) * | |
| Between CSE and CON | −8.97 (−20.59 to 2.65) | −18.28 (−26.4 to −3.67)⁎⁎ | |
| Task-switching test | |||
| Non-switch | |||
| Within OSE | 15.63 (−4.97 to 36.23) | 17.24 (2.21 to 32.27) | 0.27 |
| Within CSE | 20.32 (1.01 to 39.63) | 2.59 (−19.13 to 24.31) | |
| Within CON | 31.15 (3.95 to 58.35) | 23.06 (10.55 to 35.57) | |
| Between CSE and OSE | 17.55 (−2.41 to 37.51) | 13.35 (−3.49 to 30.19) | |
| Between OSE and CON | 30.53 (6.8 to 54.26) | 26.72 (13.49 to 39.91)* | |
| Between CSE and CON | 19.83 (−4.18 to 43.84) | 18.33 (2.24 to 34.42) | |
| Switch | |||
| Within OSE | −123.07 (−160.09 to −86.05)⁎⁎ | −122.07 (−87.73 to −16.41)⁎⁎ | 0.31 |
| Within CSE | −114.33 (−163.45 to −65.21)* | −126.28 (−151.20 to −101.36) ⁎⁎⁎ | |
| Within CON | −76.35 (−109.16 to −43.54)* | −57.65 (−79.71 to 43.31) ⁎⁎⁎ | |
| Between CSE and OSE | −105.89 (−146.71 to −65.07)* | −114.53 (−143.2 to −85.8)⁎⁎⁎ | |
| Between OSE and CON | −78.46 (−111.84 to −45.08)* | −67.93 (−88.71 to −47.15)⁎⁎ | |
| Between CSE and CON | −102.33 (−141.43 to −63.23)* | −98.17 (−122.45 to −73.75)⁎⁎⁎ | |
| N-back task | |||
| 1-back | |||
| Within OSE | −16.66 (−37.27 to 3.95) | −3.07 (−14.75 to 8.61) | 0.72 |
| Within CSE | −26.86 (−50.56 to −3.16) | −38.32 (−52.98 to −23.66)* | |
| Within CON | −8.46 (−24.14 to 7.22) | −13.74 (−24.37 to −3.11) | |
| Between CSE and OSE | −22.98 (−44.68 to −1.28) | −28.41 (−40.28 to −16.54)* | |
| Between OSE and CON | −6.25 (−23.98 to 11.48) | −10.16 (−20.07 to −0.25) | |
| Between CSE and CON | −23.12 (−42.24 to −4) | −26.76 (−38.76 to −14.76)* | |
| 2-back | |||
| Within OSE | −71.07 (−97.42 to −44.72)⁎⁎ | −20.98 (−43.38 to 1.42) | 0.25 |
| Within CSE | −102.20 (−135.72 to −68.68) ⁎⁎ | −66.43 (−88.14 to −44.72)⁎⁎ | |
| Within CON | −35.29 (−57.48 to −13.1) | 5.56 (−12.88 to 23.88) | |
| Between CSE and OSE | −104.64 (−133.28 to −76)⁎⁎⁎ | −64.33 (−83.66 to −45)⁎⁎ | |
| Between OSE and CON | −46.45 (−69.33 to −23.57)* | −2.14 (−20.25 to 15.97) | |
| Between CSE and CON | −86.22 (−113.55 to −58.89)⁎⁎ | −44.06 (−63.2 to −24.92)* | |
| 3-back | |||
| Within OSE | 6.88 (−34.93 to 48.69) | −8.76 (−39.44 to 21.92) | 0.70 |
| Within CSE | −93.33 (−145.48 to −41.18) | −46.43 (−72.79 to −20.07)⁎⁎⁎ | |
| Within CON | −12.69 (−54.97 to 29.59) | −13.95 (−37.72 to 9.82) | |
| Between CSE and OSE | −109.72 (−155.48 to −63.92)* | −45.26 (−70.75 to −19.77) | |
| Between OSE and CON | −28.64 (−69.21 to 11.93) | −16.13 (−40.19 to 7.93) | |
| Between CSE and CON | −86.68 (−134.38 to −38.98) | −31.63 (−54.58 to −8.68) | |
| Emotional assessment | |||
|---|---|---|---|
| Valence | |||
| Neutral emotion | |||
| Within OSE | 0.02 (−0.17 to 0.21) | −0.19 (−0.35 to −0.03) | 0.12 |
| Within CSE | 0.20 (−0.02 to 0.42) | 0.09 (−0.06 to 0.24) | |
| Within CON | 0.03 (−0.18 to 0.24) | −0.05 (−0.18 to 0.08) | |
| Between CSE and OSE | 0.14 (−0.06 to 0.34) | 0.03 (−0.11 to 0.17) | |
| Between OSE and CON | −0.01 (−0.2 to 0.18) | −0.06 (−0.19 to 0.07) | |
| Between CSE and CON | 0.22 (0.03 to 0.44) | 0.04 (−0.09 to 0.17) | |
| Negative emotion | |||
| Within OSE | −0.26 (−0.44 to −0.08) | −0.23 (−0.37 to −0.09) | 0.53 |
| Within CSE | 0.25 (0.04 to 0.46) | 0.23 (0.10 to 0.36) | |
| Within CON | −0.16 (−0.31 to −0.01) | −0.02 (−0.13 to 0.09) | |
| Between CSE and OSE | 0.15 (−0.04 to 0.34) | 0.16 (0.04 to 0.28) | |
| Between OSE and CON | −0.26 (−0.42 to −0.1) | −0.07 (−0.18 to 0.04) | |
| Between CSE and CON | 0.28 (0.10 to 0.46) | 0.22 (0.11 to 0.33) | |
| Arousal | |||
| Neutral emotion | |||
| Within OSE | 0.04 (−0.19 to 0.19) | −0.07 (−0.21 to 0.07) | 0.09 |
| Within CSE | 0.09 (−0.21 to 0.21) | 0.10 (−0.04 to 0.24) | |
| Within CON | 0.06 (−0.14 to 0.26) | −0.04 (−0.15 to 0.07) | |
| Between CSE and OSE | −0.02 (−0.21 to 0.17) | 0.09 (−0.05 to 0.23) | |
| Between OSE and CON | 0.04 (−0.18 to 0.18) | −0.09 (−0.21 to 0.03) | |
| Between CSE and CON | 0.08 (−0.12 to 0.28) | 0.13 (0.01 to 0.25) | |
| Negative emotion | |||
| Within OSE | 0.10 (−0.11 to 0.31) | 0.14 (−0.04 to 0.32) | 0.23 |
| Within CSE | 0.10 (−0.13 to 0.33) | 0.42 (0.23 to 0.61)* | |
| Within CON | 0.23 (0.06 to 0.4) | 0.11 (−0.04 to 0.26) | |
| Between CSE and OSE | 0.06 (−0.16 to 0.28) | 0.37 (0.24 to 0.54)* | |
| Between OSE and CON | 0.14 (−0.05 to 0.33) | −0.06 (−0.22 to 0.15) | |
| Between CSE and CON | 0.14 (−0.06 to 0.34) | 0.48 (0.31 to 0.65)⁎⁎ |
Note: OSE: open-skill exercise; CSE: closed-skill exercise; CON: control group.
In the clinical assessment results, the CES-D scores indicated that both the OSE and CSE group exhibited significant time effects following the intervention (all p < 0.001), suggesting improvements in CES-D scores. There were no significant differences between the OSE and CON groups. However, the CSE group demonstrated significantly greater improvements in CES-D scores than both the OSE and CON group (all p < 0.001). The BDI-13 results demonstrated significant interaction effects between the three groups. Further analysis revealed that the CSE group exhibited significant time effects following four weeks of intervention (all p < 0.05), with BDI-13scores demonstrating a decline over the course of the intervention. The OSE group demonstrated significant time effects only after eight weeks of intervention (p = 0.035). After four weeks of intervention, the CSE group demonstrated significantly greater improvements in BDI-13 compared to the OSE group (p = 0.012) and the CON group (p = 0.015). After eight weeks of intervention, both the OSE group (p = 0.042) and the CSE group (p = 0.032) demonstrated significant differences compared to the CON group. However, no significant differences were observed between the OSE and CSE group.
In regard to the EF results, the Flanker task results indicated no significant differences between the groups following four weeks of intervention. However, following eight weeks of intervention, both the OSE and CSE groups exhibited improvements in both congruent and incongruent IES, exhibiting significant time effects (all p < 0.001), regardless of the type of intervention. Additionally, the CSE group demonstrated significantly greater improvements in inhibition compared to the OSE group (p < 0.01). In the task-switching test, all three groups exhibited significant time effects after eight weeks of intervention (all p < 0.05), with both exercise interventions leading to significant improvements in cognitive flexibility (all p < 0.01). Furthermore, the CSE group demonstrated significantly greater improvements than the OSE group (p < 0.001). The N-back task results indicated that, under the 1-back condition, the CSE group exhibited significant time effects after eight weeks of intervention, with performance significantly better than that of the OSE and CON groups (all p < 0.001). In the 2-back condition, both the OSE and CSE groups demonstrated significant time effects after four weeks of intervention (p < 0.01). The CSE group exhibited superior performance to the OSE group (p < 0.01) and the CON group (p = 0.024). However, this improvement was observed only in the CSE group after eight weeks of intervention. In the 3-back condition, only the CSE group demonstrated significant time effects after eight weeks of intervention (p < 0.001).
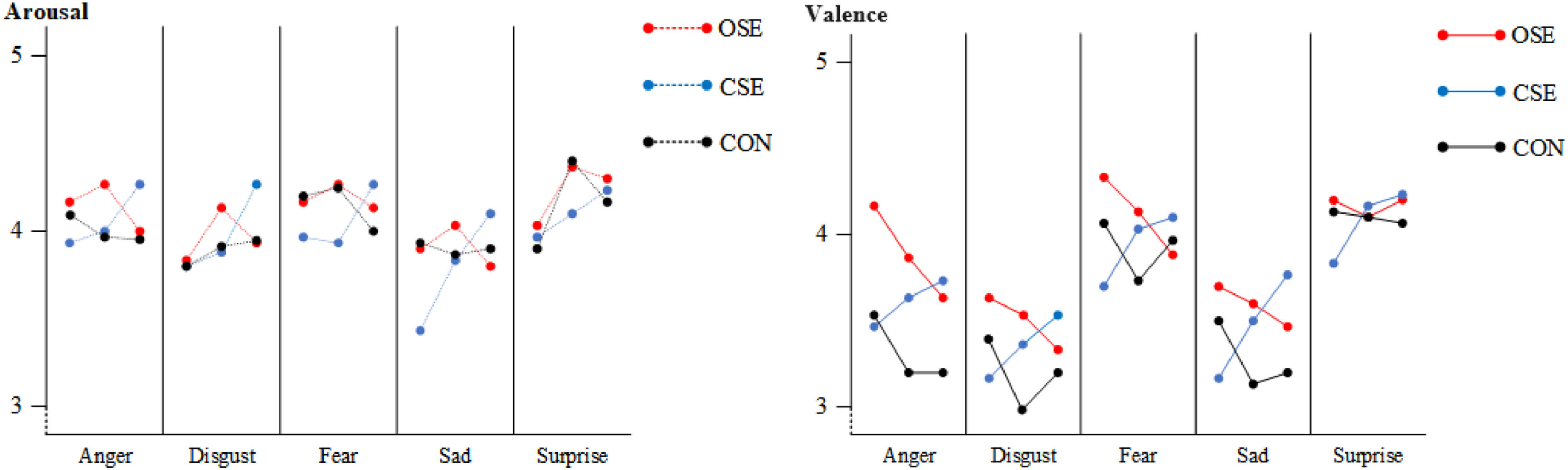
The results of the SAM indicated that the CSE group exhibited a significant improvement in arousal related to negative emotions following an eight-week intervention (p = 0.048). Furthermore, a notable difference was evident between this group and the OSE group (p = 0.039) and the CON group (p = 0.007). Fig. 2 further examined the alterations in valence and arousal related to negative emotions among the three groups. In comparison to the pre-intervention period, the CSE group exhibited enhanced arousal levels for anger (p = 0.041), disgust (p = 0.040), fear (p = 0.050), and sadness (p = 0.032) following the eight-week intervention.
DiscussionThis study aimed to investigate the effects of 8-week extracurricular physical education programs, focusing on OSE and CSE, on female adolescents with SD symptoms. Additionally, it evaluated the impact of different types of exercise on their EF and emotions. The findings revealed that both OSE and CSE interventions significantly improved participants' depressive symptoms, with the CSE group showing more pronounced improvements compared to the OSE group and the CON. In terms of EF, both the OSE and CSE groups showed improvements in various tasks. Notably, the CSE group exhibited more significant progress in cognitive flexibility and working memory, indicating stronger effects. Furthermore, the CSE intervention was superior in improving arousal related to negative emotions. Overall, exercise had a positive impact on subthreshold depressive symptoms in adolescents. From the perspective of improving EF and negative emotions, CSE might be a more suitable exercise intervention for female adolescents with subthreshold depression.
This study relied on extracurricular physical education interventions conducted outside of regular school sports classes, demonstrating high adherence rates. This suggests that female adolescents with SD can be systematically and intensively encouraged to participate in various forms of exercise to improve their condition (Biddle et al., 2019; Dishman et al., 2012). The results align with previous research indicating the efficacy of exercise in reducing depressive symptoms. Physiologically, exercise stimulates the release of endorphins, serotonin, and dopamine, increases the expression of BDNF, and reduces inflammation, thereby improving mood and cognitive function (Dinoff et al., 2018; Wegner et al., 2014). Psychologically, exercise enhances self-efficacy, alleviates stress, increases social interaction, breaks negative behavior patterns, and enhances well-being (Lubans et al., 2011; Mammen & Faulkner, 2013). Therefore, regular exercise, as a non-pharmacological treatment, can effectively improve the emotional and psychological health of individuals with SD (Lubans et al., 2011).
Previous studies suggested that OSE might be a better exercise method compared to CSE (Feng et al., 2023; Gu et al., 2019). However, in this study, we found that CSE had a more pronounced effect on improving both EFs and emotional regulation in female adolescents with SD. The structured, repetitive, and predictable nature of CSE may offer greater stability and emotional security, which can be especially beneficial for adolescents with subthreshold depression. By reducing uncertainty and anxiety caused by environmental changes, CSE provides a sense of security that may be particularly valuable for females, who tend to be more sensitive to environmental fluctuations and social pressures (Diamond & Ling, 2016; Lubans et al., 2011). Compared to OSE, CSE's lower levels of confrontation and competition may reduce the anxiety and stress related to social comparison, which tends to be more pronounced in female adolescents (Balázs et al., 2013; Feng et al., 2023) . These factors suggest that CSE may offer a more suitable exercise intervention for females with SD, promoting emotional well-being and enhancing EFs more effectively than OSE.
Different types of exercise skills have varying effects on brain tissues and neural activation, leading to different expressions of executive control (Feng et al., 2023; Gu et al., 2019; Hillman et al., 2008). Our study found that OSE and CSE improved EF at different times. This discrepancy is due to the different brain regions and neural mechanisms involved in these functions, as well as the varying speed and manner in which exercise impacts these regions and mechanisms (Chaddock et al., 2011). Cognitive flexibility and working memory, which require high levels of environmental adaptability and information processing, can show significant improvements early on (Hillman et al., 2020; Khan & Hillman, 2014). In contrast, the improvement of inhibitory control and negative emotions requires longer sustained training, related to the enhanced function of the prefrontal cortex and long-term regulation of neurotransmitters (Hillman et al., 2020; Khan & Hillman, 2014). Meanwhile, the improvement of negative emotions typically requires a long-term process of neurotransmitter regulation and psychological adaptation (Dishman et al., 2012). The repetitiveness and low confrontational nature of CSE provide a stable and safe environment, contributing to long-term emotional stability and mental health.
While this study provided valuable insights into the psychological effects of OSE and CSE, there are several limitations that we need to acknowledge. Firstly, this study focused specifically on female adolescents with SD. The generalisability of the results to males and other age groups is limited. Future research should therefore explore the effects in male adolescents and across different age groups in order to enhance the applicability of the findings. Secondly, the intervention period was only eight weeks, and future research could explore the effects of the intervention over a longer period of time to observe sustained changes and long-term effects. Furthermore, this study only focused on two exercise types. Future investigate should focus on the effects of other types of exercise (such as team or individual sports) on adolescents with SD and examine the combined effects of different exercise combinations on mental health and cognitive function. With this said, the strengths of our study included a comprehensive investigation of the psychological and cognitive effects of two distinct types of exercise, OSE and CSE, on female adolescents with SD. By evaluating at two time points, mid- and post-intervention, the study was able to observe changes in short- and medium-term intervention effects, revealing differences in the effects of different exercise types at different time periods. Finally, as the intervention uses existing sport and exercises that are already familiar to students, implementation of these interventions are likely to be highly feasible and easy for schools, families and communities to assimilate into existing curriculum and education programs.
ConclusionThe study investigated the impact of an 8-week intervention using OSE and CSE on SD symptoms in female adolescents. The findings revealed that both OSE and CSE interventions significantly improved participants' depressive symptoms, with the CSE group showing more pronounced improvements compared to the OSE group and the CON. In terms of EF, both intervention groups showed enhancements in cognitive flexibility and working memory, but CSE led to more significant gains in inhibitory control, cognitive flexibility, and working memory. Regarding emotional improvement, only CSE showed a notable reduction in negative emotions after eight weeks. Overall, CSE demonstrated more sustained effects in long-term interventions, suggesting it may be more suitable for addressing SD in adolescents. Future research should verify the consistent effectiveness of CSE and investigate the mechanisms behind these changes. It is also important to identify specific components of exercise interventions that lead to positive mental health outcomes to design more effective programs for adolescent depression.
Footnotes
Competing interestsThe authors declare that they have no competing interests.
Data availabilityData are available upon reasonable request. Anonymous data are available on reasonable request to the corresponding author.
Ethics statementsPatient consent for publication. Not applicable.
The authors sincerely thank the expert panel and students who participated in this study.