Childhood trauma is a leading risk factor for adolescents developing major depressive disorder (MDD); however, the underlying neuroimaging mechanisms remain unclear. This study aimed to investigate the association among childhood trauma, MDD and brain dysfunctions by combining static and dynamic brain network models. We recruited 46 first-episode drug-naïve adolescent MDD patients with childhood trauma (MDD-CT), 53 MDD patients without childhood trauma (MDD-nCT), and 90 healthy controls (HCs) for resting-state functional magnetic resonance imaging (fMRI) scans; all participants were aged 13–18 years. Compared to the HCs and MDD-nCT groups, the MDD-CT group exhibited significantly higher global and local efficiency in static brain networks and significantly higher temporal correlation coefficients in dynamic brain network models at the whole-brain level, and altered the local efficiency of default mode network (DMN) and temporal correlation coefficients of DMN, salience (SAN), and attention (ATN) networks at the local perspective. Correlation analysis indicated that altered brain network features and clinical symptoms, childhood trauma, and particularly emotional neglect were highly correlated in adolescents with MDD. This study may provide new evidence for the dysconnectivity hypothesis regarding the associations between childhood trauma and MDD in adolescents from the perspectives of both static and dynamic brain topology.
Major depressive disorder (MDD) is a common illness and a significant public health problem; moreover, it is the leading cause of illness and disability, especially among young people (Liu et al., 2022). The prevalence of adolescents reporting MDD symptoms has nearly doubled in the last decade, increasing from 8.3% in 2008 to 14.4% in 2018 (Sekhar et al., 2021). Childhood trauma has been associated with the appearance of various psychopathological disorders, such as mental disorders, posttraumatic stress disorder, and substance use disorders (Aas et al., 2017; Gidzgier et al., 2019; Kim & Lee, 2016). In particular, MDD is commonly observed among individuals reporting exposure to childhood traumatic events (Baldwin et al., 2019; Green et al., 2010), and childhood trauma has been established as a major risk factor for depression in adolescents (Chen et al., 2021;De Bellis et al., 2019). Moreover, meta-analyses show that childhood traumatic events are associated with a 2- to 3-fold increase in the risk of MDD and other mental disorders (Trotta et al., 2015; van Dam et al., 2012; Varese et al., 2012).
Past neuroimaging studies have demonstrated that childhood trauma is associated with MDD-related changes in brain structures and functions, e.g., reductions in total gray matter volume and the volumes of the hippocampus, amygdala, anterior cingulate cortex (ACC), and ventromedial prefrontal cortices (PFCs) (Frissen et al., 2018; Teicher et al., 2016; Vythilingam et al., 2002). Studies have also shown that childhood traumatic experiences and dimensional symptoms are associated with abnormal functional network architecture in individuals with MDD (Yu et al., 2019). Furthermore, MDD patients with childhood trauma have increased amplitudes of low-frequency fluctuations (ALFFs) in the bilateral amygdala and left orbit/cerebellum and decreased fractional amplitudes of low-frequency fluctuation (fALFFs) in the left inferior temporal gyrus and right middle frontal gyrus compared to those of healthy controls (Du et al., 2016; Heim et al., 2008), demonstrating that childhood trauma may contribute to brain dysfunction and increase the risk of MDD. Moreover, in a multimodal study, childhood trauma was found to have long-term effects on the function and structure of the brain (Duncan et al., 2015). In adolescents with MDD, recent evidence has suggested that childhood trauma is indeed associated with the dynamic function among the hub brain regions of the bilateral intracalcarine cortex (ICC), right supracalcarine cortex (SCC), right cuneal cortex, right lingual gyrus, bilateral superior division of the lateral occipital cortex and right paracingulate gyrus (Zhang et al., 2022).
Despite accumulating knowledge, recent studies on the relationships between childhood trauma and MDD are still limited in several ways. First, many of the prior studies were limited in that they only focused on functional connectivity (FC) patterns in several predefined regions of interest (ROIs) (Fan et al., 2021; He et al., 2022). Although there have been some attempts (Yu et al., 2019), knowledge about how childhood trauma affects the configuration of large-scale brain networks in MDD patients is relatively limited. This question is important since functional dysconnectivity in MDD patients is not constrained in a circumscribed area but is usually associated with alterations of the entire brain (Yang et al., 2021; Zhang et al., 2011). In particular, it has been suggested that graph-theoretical-based topological measures (e.g., global and local efficiency) (Yang et al., 2021) can provide a powerful framework for understanding brain pathology, but their possible relationships with childhood trauma in MDD patients have seldom been reported. Second, most conventional neuroimaging studies were performed under the assumption that FC strengths between various cortical and subcortical regions of the brain are static over the entire duration of an fMRI experiment (Biswal et al., 2010; Chen et al., 2017;Smith et al., 2009). However, it has been suggested that brain FC fluctuates dynamically, and much important information is missed by conventional static analysis methods (Bassett et al., 2011; Hutchison et al., 2013; Siebenhuhner et al., 2013). Third, most neuroimaging studies are focused on the relationship between adult MDD and childhood trauma, while research on alterations in the brain function of adolescent MDD patients with childhood trauma is still scarce. Hence, there is a need to combine static and dynamic brain network measures to fully characterize the possible relationships between childhood trauma and MDD-related brain dysfunctions, especially in adolescents with MDD.
To overcome the above limitations, the aim of the present study was to investigate possible associations between childhood trauma and brain functions in first-episode, drug-naïve patients with MDD by combining both static and dynamic brain network models. To our knowledge, our work is one of the first to incorporate both static and dynamic brain topological organizations in an investigation of the effects of childhood trauma on brain function.
Materials and methodsParticipantsA total of 99 drug-naïve adolescents with first-episode MDD and 90 demographically matched healthy controls (HCs) were recruited. Adolescents with MDD were recruited from the First Affiliated Hospital of Chongqing Medical University, and HCs were enrolled through local media advertising in Chongqing, China. The Ethics Committee of the First Affiliated Hospital of Chongqing Medical University approved this research protocol (approval ID: 2020–864), and all patients provided written informed consent.
We measured adverse childhood events using the 28-item Childhood Trauma Questionnaire (CTQ), a retrospective questionnaire that assessed childhood traumatic experiences (Bernstein et al., 1994). The CTQ contains five subscales to assess different forms of trauma: emotional abuse, physical abuse, sexual abuse, emotional neglect, and physical neglect. Standardized cutoffs recommended by the CTQ manual (Bernstein et al., 1997) and used in previous studies (Huang et al., 2021; Wu et al., 2022) were used to assign patients who exceeded the cutoff on one or more CTQ subscales to the MDD with childhood trauma (MDD-CT) group (cutoff scores: emotional abuse, 13; physical abuse, 10; sexual abuse, 8; emotional neglect, 15; and physical neglect, 10). The MDD without childhood trauma (MDD-nCT) group included patients without severe abuse or neglect and were defined as having scores below the cutoff point on all five subscales.
The inclusion and exclusion criteria for those three groups are shown in Table 1.
The inclusion and exclusion criteria.
Inclusion and exclusion criteria. MDD-CT, MDD with childhood trauma; MDD-nCT, MDD without childhood trauma; HCs, healthy controls; DSM-5, Diagnostic and Statistical Manual of Mental Disorders, 5th edition; HAMD-17, 17-item Hamilton Depression Scale; CTQ, Childhood Trauma Questionnaire; MRI, magnetic resonance imaging.
MRI scans were acquired on a Siemens Magnetom Skyra 3T scanner with a 32-channel head coil. High-resolution transaxial T1-weighted structural images were obtained using 3D-MPRAGE with the following parameters: repetition time (TR) = 2000 ms; echo time (TE) = 2.56 ms; inversion time (TI) = 900 ms; flip angle = 9°; matrix size, 256 × 256; field of view (FOV) = 256 × 256 mm2; slice thickness = 1 mm; slices per slab = 192; and voxel size, 1 × 1 × 1 mm3. A total of 240 resting-state blood oxygen level-dependent (BOLD) volumes were obtained. The parameters for BOLD scans were as follows: TR = 2000 ms; TE = 30 ms; flip angle = 90°; FOV = 220 × 220 mm2; number of slices = 36; reconstructed voxel size = 3.4 × 3.4 × 3 mm3; and layer thickness = 3.0 mm.
Data processingAfter data acquisition, the images were preprocessed using the standard pipeline of the DPARSF toolbox (http://rfmri.org/DPARSF) (Chao-Gan & Yu-Feng, 2010; Yan et al., 2016). Briefly, the pipeline includes removing the first 10 time points, slice-timing correction, motion realignment, spatial normalization, temporal filtering, and nuisance regression. Global signal regression was not performed, as this process is controversial (Murphy & Fox, 2017). Specifically, for those participants who were under 18 years of age, an adolescent brain template obtained from 316 Chinese adolescents (https://github.com/zuoxinian/CCS/tree/master/H3/GrowthCharts/Templates/IPCAS/BrainTemplate) (Dong et al., 2020) was used for normalization instead of the default brain template. More details of the preprocessing procedures can be found in the Supplementary Materials and some previous publications (Huang et al., 2021; Long et al., 2020; Zhang et al., 2021). To ensure data quality, the following steps, from which 10 subjects were excluded, were performed: 1) all images were visually inspected before analysis to ensure the absence of apparent or aberrant artifacts, and all scans were inspected visually for any gross structural abnormality by an expert neuroradiologist; 2) data were excluded from the analyses when excessive head motion occurred during scanning, as defined by a mean framewise-displacement (FD) > 0.2 mm (Long et al., 2020; Yan et al., 2019); and 3) in all the following analyses, the mean FD values were further used as a covariate to minimize possible effects of head motion.
Static and dynamic brain network constructionsThe Power functional atlas was used to define the nodes in both static and dynamic brain networks, resulting in a total of 264 ROIs distributed across the brain (Power et al., 2011) (Supplementary Figure S1). Referring to previous work (MW Cole et al., 2013; Mohr et al., 2016; Niu et al., 2022), these ROIs were further parcellated into 9 well-established, large-scale networks: the default mode network (DMN), fronto-parietal network (FPN), sensorimotor network (SMN), visual network (VIS), subcortical network (SUB), cingulo-opercular network (CON), salience network (SAN), attention network (ATN), and auditory network (AUD).
The rs-fMRI signals of all voxels within each of the 264 nodes were first averaged. The static FC strength was computed as the Fisher's r-to-z transformed Pearson's correlation coefficients of the averaged rs-fMRI signals between each pair of nodes, yielding a 264×264 FC matrix that represents the network organization of the static brain. The extracted mean rs-fMRI signals were further subdivided into a number of time windows using a common sliding-window approach (Long et al., 2020; Zhao et al., 2022) to construct dynamic brain networks. In the primary analyses, based on previous recommendations (Long et al., 2020; Sun et al., 2019; Tang et al., 2022), a window width of 100 s and a step length of 6 s were used, resulting in a total of 61 time windows. The effects of using different window widths or step lengths were investigated in the latter part of this study. Similar to the static FC matrices, a 264×264 dynamic FC matrix was then generated for each time window based on the Fisher's r-to-z transformed FC strengths between pairs of nodes within that window. These time-ordered matrices then formed a dynamic brain network G = (Gt)t = 1, 2, 3, …, 61, where the tth matrix (Gt) represents the “snapshot” of dynamic brain FC patterns within the tth time window (Huang et al., 2021; Sun et al., 2019).
Topological brain network metricsAfter brain network construction, several commonly used static and dynamic topological network metrics were calculated for each participant. All metrics were computed at the whole-brain level and network level separately. For static brain networks, we calculated the global efficiency (Eglob) and local efficiency (Eloc), which are both intuitive and widely used metrics to measure the topological features of a static brain network (Meijer et al., 2020; Rubinov & Sporns, 2010; Yang et al., 2021). For dynamic brain networks, a validated metric called the temporal correlation coefficient was computed to quantify the temporal stability of brain networks, with a higher value indicating higher stability (fewer fluctuations) of the dynamic FC patterns over time (Long et al., 2020, 2023; Sizemore & Bassett, 2018). The static and dynamic brain function metrics used in this study have been shown to have high test-retest reliability in previous studies (Braun et al., 2012; Cao et al., 2014; Long et al., 2023). All these metrics were calculated based on thresholded, undirected graphs in a wide range of densities from 0.10 to 0.34 with an interval of 0.01. Such a range was chosen to ensure that all metrics are estimable while spurious connections can be minimized (Achard & Bullmore, 2007; Zhang et al., 2011). The area under the curve (AUC) for each metric across this range (0.10–0.34) was calculated and evaluated by statistical analyses to avoid possible bias caused by a single density level (Yang et al., 2021; Zhang et al., 2011). More details are provided in the Supplementary Materials.
The static brain network metrics were computed using the Brain Connectivity Toolbox (BCT) (Rubinov & Sporns, 2010). The codes for computing the temporal correlation coefficient for dynamic brain networks are available at https://github.com/Yicheng-Long/dynamic_graph_metrics.
StatisticsGroup differences in demographics and clinical characteristics were compared between groups using the chi-square test or analysis of variance. Differences were considered significant at p < 0.05.
All network metrics for both the whole-brain and each of the nine networks were compared among the MDD-CT, MDD-nCT and HCs groups by analysis of covariance (ANCOVA) covarying for sex, age and mean FD. The ANCOVA models were first used to detect significant main effects, with FDR corrections performed across the multiple metrics and multiple networks examined. When main effects were significant (FDR-corrected p < 0.05), Bonferroni post hoc pairwise comparisons were further adopted between all pairs of groups (MDD-CT vs. MDD-nCT, MDD-CT vs. HCs, and MDD-nCT vs. HCs), and differences were considered significant at Bonferroni-corrected p < 0.05. Statistical analyses were performed using SPSS 23.0 (IBM Corporation, Armonk, NY, USA), with significance set at p < 0.05 after FDR corrections.
Validation analysesSeveral supplementary analyses were further performed to validate the results. Firstly, when constructing dynamic brain networks using the sliding-window approach, there is still no consensus on the optimal window width and step length. Therefore, we repeated the analyses on the temporal correlation coefficients using a range of different window widths (80/100/120 s) and step lengths (6/8/10 s) to validate that the results were not affected by different sliding-window parameters.
Secondly, a split-half validation analysis was performed by randomly spliting the whole sample into two subsets. In both subsets, the age, sex, education, and clinical symptoms were all still matched between groups (Supplementary Tables S1-S2). The analyses were then repeated independently in two subsets. See Supplementray Materials for more details.
ResultsA schematic diagram of the entire process is shown in Fig. 1.
The demographic characteristics of all patients are recorded in Table 2. Among the MDD patients, 46 and 53 patients were assigned to the MDD-CT and MDD-nCT groups, respectively. There were no significant differences among the three groups in terms of age, sex, body mass index (BMI), and years of education (p > 0.05). Both the MDD-CT and MDD-nCT groups showed significantly higher HAMD-17 and CTQ total scores than the HCs group (all p < 0.05, Bonferroni post hoc comparisons).
Demographic characteristics.
Demographic characteristics. MDD-CT, MDD with childhood trauma; MDD-nCT, MDD without childhood trauma; HCs, healthy controls; BMI, body mass index; HAMD-17, 17-item Hamilton Depression Scale; HAMA, Hamilton Anxiety Scale; CTQ, Childhood Trauma Questionnaire; CTQ-PN, CTQ Physical Neglect subscale; CTQ-EN, CTQ Emotional Neglect subscale; CTQ-SA, CTQ Sexual Abuse subscale; CTQ-PA, CTQ Physical Abuse subscale; CTQ-EA, CTQ Emotional Abuse subscale. Unless otherwise indicated, data are presented as the mean ± standard deviation.
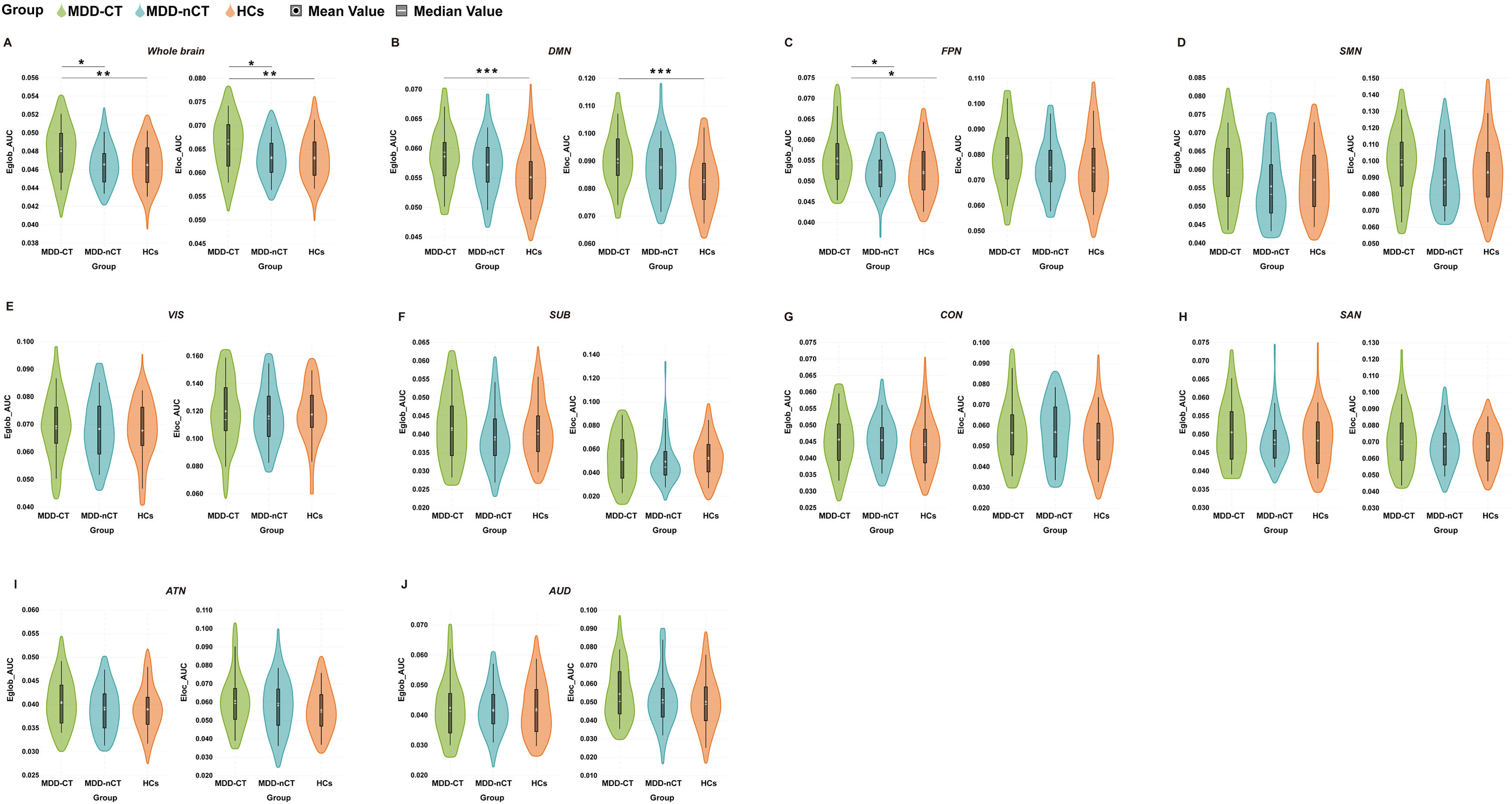
Compared to the HCs and MDD-nCT groups, the MDD-CT group showed significant increases in both the Eglob (observed power = 0.858, partial η2 =0.058, Bonferroni-corrected p = 0.014, 95% CI of difference = 0.000–0.003/Bonferroni-corrected p = 0.006, 95% CI of difference = 0.000–0.002 when compared to MDD-nCT/HCs groups) and Eloc (observed power = 0.838, partial η2 =0.056, Bonferroni-corrected p = 0.017, 95% CI of difference = 0.000–0.005/Bonferroni-corrected p = 0.008, 95% CI of difference = 0.001–0.005 when compared to MDD-nCT/HCs groups) for the whole-brain (Fig. 2A). Moreover, at the local network level, the MDD-CT group had significantly higher Eglob in the DMN (observed power = 0.950, partial η2 =0.079, Bonferroni-corrected p < 0.001, 95% CI of difference = 0.001–0.006 when compared to the HCs group), higher Eglob in the FPN (observed power = 0.810, partial η2 =0.052, Bonferroni-corrected p = 0.021, 95% CI of difference = 0.000–0.006/Bonferroni-corrected p = 0.012, 95% CI of difference = 0.001–0.006 when compared to the MDD-nCT/HCs groups) and higher Eloc in the DMN (observed power = 0.970, partial η2 =0.088, Bonferroni-corrected p < 0.001, 95% CI of difference = 0.003–0.012 when compared to the HCs group) (Fig. 2B, C). However, no significant differences in the Eglob and Eloc were found between MDD-nCT and HC groups (all Bonferroni-corrected p>0.05).
Group differences in network topological properties among major depressive disorder (MDD) patients with childhood trauma, MDD patients without childhood trauma patients and HCs. Violin plots illustrating the area under the curve (AUC) parameters of global efficiency (Eglob) and local efficiency (Eloc) for the three groups. A: whole-brain; B: default mode network (DMN); C: fronto-parietal network (FPN); D: sensorimotor network (SMN); E: visual network (VIS); F: subcortical network (SUB); G: cingulo-opercular network (CON); H: salience network (SAN); I: attention network (ATN) and J: auditory network (AUD). MDD-CT, MDD with childhood trauma; MDD-nCT, MDD without childhood trauma; HCs, healthy controls. Means and standard deviations are depicted. *: p < 0.05. **: p < 0.01, ***: p < 0.001.
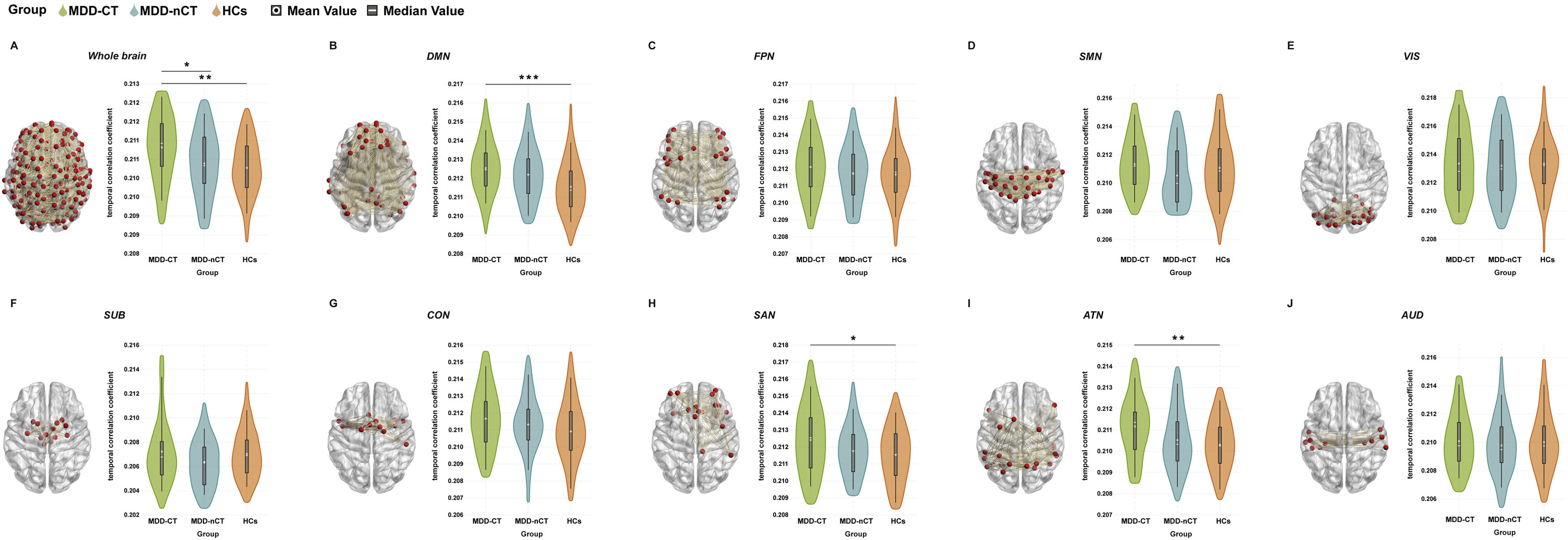
Comparisons of the temporal correlation coefficients among the three groups are shown in Fig. 3. We observed significantly increased temporal correlation coefficients for the whole-brain (observed power = 0.915, partial η2 = 0.069, Bonferroni-corrected p = 0.001, 95% CI of difference = 0.000–0.001), DMN (observed power = 0.952, partial η2 =0.080, Bonferroni-corrected p < 0.001, 95% CI of difference = 0.000–0.002), SAN (observed power = 0.764, partial η2 =0.047, Bonferroni-corrected p = 0.010, 95% CI of difference = 0.000–0.002) and ATN (observed power = 0.870, partial η2 =0.060, Bonferroni-corrected p = 0.002, 95% CI of difference = 0.000–0.001) in the MDD-CT group compared to the HCs group (Fig. 3A, B, H, I). In addition, the temporal correlation coefficient at the whole-brain level (observed power = 0.915, partial η2 =0.069, Bonferroni-corrected p = 0.025, 95% CI of difference = 0.000–0.001) was significantly higher in the MDD-CT group than in the MDD-nCT group (Fig. 3A). There were no significant differences in the temporal correlation coefficients between the MDD-nCT and HC groups (all Bonferroni-corrected p > 0.05).
Group differences in the temporal correlation coefficients of network topological properties and schematic of brain network connectivity. A: whole-brain; B: default mode network (DMN); C: cognitive control network (FPN); D: sensorimotor network (SMN); E: visual network (VIS); F: subcortical network (SUB); G: cingulo-opercular network (CON); H: salience network (SAN); I: attention network (ATN) and J: auditory network (AUD). MDD-CT, MDD with childhood trauma; MDD-nCT, MDD without childhood trauma; HCs, healthy controls. Means and standard deviations are depicted. *: p < 0.05. **: p < 0.01, ***: p < 0.001.
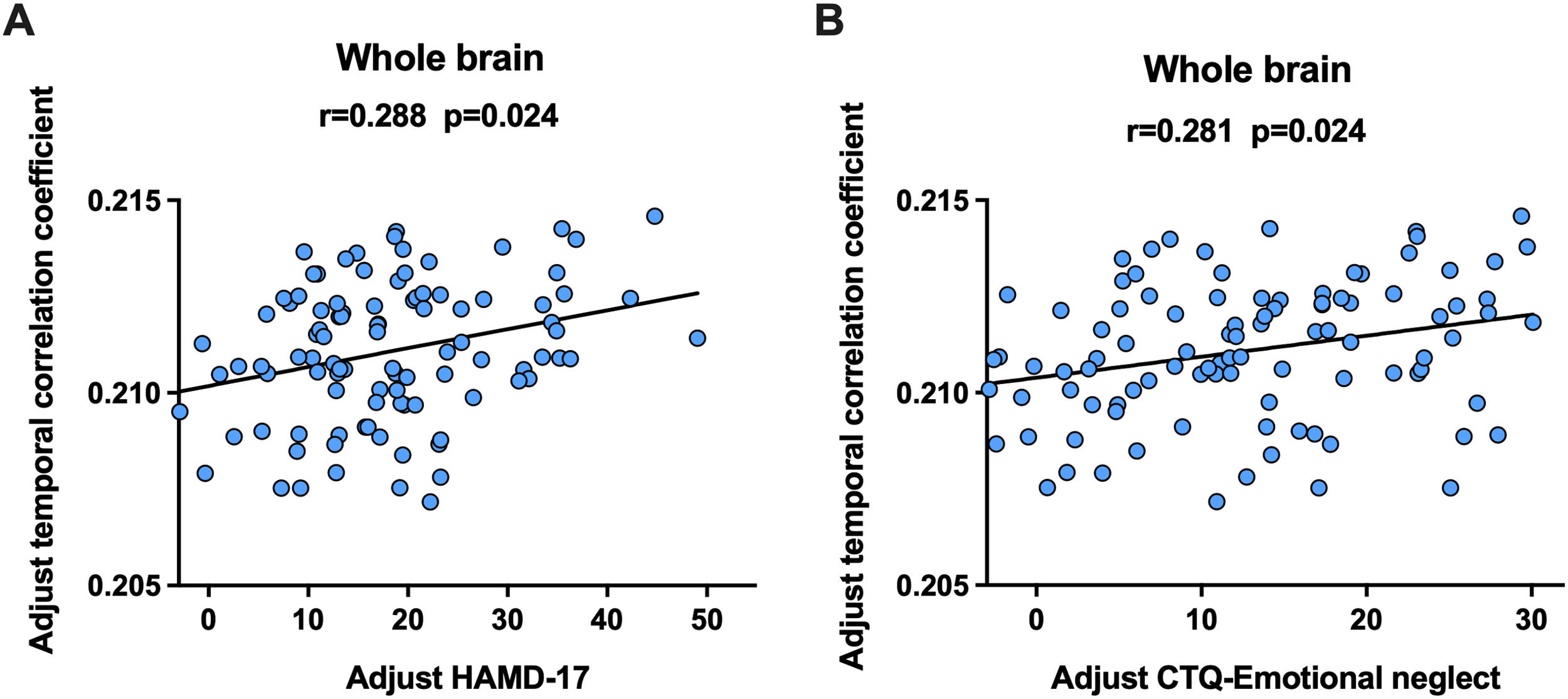
Partial correlation analyses were performed to examine the relationship between alterations in brain measures and clinical symptoms in patients with MDD, controlling for sex, age, and mean FD. Acquired p values were adjusted using the Benjamini and Hochberg FDR method. A significant positive correlation was observed between the temporal correlation coefficients of the whole-brain and HAMD-17 scores (r = 0.288, FDR p = 0.024, Fig. 4A), as well as between the temporal correlation coefficients of the whole-brain and CTQ Emotional Neglect scores (r = 0.281, FDR p = 0.024, Fig. 4B).
The significant correlations detected in adolescents with MDD. A: correlation between adolescents with MDD and the HAMD-17 scores, B: correlation between adolescents with MDD and CTQ Emotional Neglect scores. The partial correlation coefficients (r) and p values are presented in the figure. MDD-CT, MDD with childhood trauma; MDD-nCT, MDD without childhood trauma; HCs, healthy controls.
The results were generally unchanged when re-calculating the temporal correlation coefficients using a range of different window widths and step lengths: we consistently observed significantly increased temporal correlation coefficients for the whole brain, DMN, SAN, and ATN in the MDD-CT group compared to the HC group; in addition, the temporal correlation coefficient was significantly higher in the MDD-CT group than in the MDD-nCT group at the whole-brain level (Supplementary Table S3).
As shown in Supplementary Figures S2-S3, for most brain network metrics, similar trends as found in the whole sample were consistently observed in both of the two split-half subset, which could suggest that our findings are not likely to be coincidental. Nevertheless, differences in statistical significance were only observed in one of the subsets (Supplementary Figures S2-S3); such lack of significant differences in a smaller subset may underscore the importance of using a larger sample to detect group effects.
DiscussionIn the current study, using both static and dynamic brain network measures, we identified significant differences between adolescent MDD patients with and without childhood trauma, and these differences were related to changes in symptoms. The main finding was that MDD adolescents with childhood trauma had higher Eglob and Eloc values and higher temporal correlation coefficients of the whole brain than those without childhood trauma and HCs. Furthermore, at the local level, we found increased Eglob and Eloc in the DMN, increased Eglob in the FPN, and increased temporal correlation coefficients in the DMN, SAN and ATN functional networks in MDD adolescents with childhood trauma. These findings may provide new evidence for the dysconnectivity hypothesis regarding the associations between childhood trauma and MDD in adolescents from the perspectives of both static and dynamic brain topology.
Trauma-associated alterations at the whole-brain levelIn this study, we combined both static (global and local efficiency) and dynamic (temporal correlation coefficient) network measures to better understand the possible trauma-associated alterations in functional brain network topologies in MDD adolescents. We found significantly increased Eglob and Eloc in the MDD adolescents with childhood trauma than HCs and those patients without childhood trauma. While global efficiency quantifies the communication efficiency between long-range connections and promotes functional integration, local efficiency is an indicator of the regional network's fault tolerance and reflects functional segregation (Sporns, 2013). The higher Eglob and Eloc values indicated stronger small-worldization (Suo et al., 2018), which means that the network may transform from an optimal organization to an excessively stronger small-world network. Using the dynamic brain network model, our results showed that the adolescents with MDD and childhood trauma showed a higher temporal correlation coefficient, which indicates higher consistency of FC patterns between consecutive time points (higher temporal stability) (Sizemore & Bassett, 2018). In summary, compared to HCs and those patients without childhood trauma experiences, the MDD adolescents with childhood trauma showed excessively stronger small-worldization and excessively increased temporal stability in their brain network topologies. It may thus be speculated that these changes may play important roles in the associations between childhood trauma and adolescent MDD. Of note, prior studies have reported significant changes in these network metrics in patients with MDD (Borchardt et al., 2015, 2016; Luo et al., 2015; Ye et al., 2015), but their possible relationships with childhood trauma in MDD patients were seldom reported. Here, our findings may partly fill such gaps, which will help future studies to identify the existence of neuroimaging distinguishable subtypes of MDD associated with childhood trauma and provide precise diagnosis and individualized treatment.
Another noteworthy point is that the observed MDD-related alterations in brain network topology in the current study are different from many of the previous ones conducted in adult MDD patients, or patients with several other trauma-associated disorders. For example, multiple previous studies have shown a shift toward a weaker small-world network with decreased segregation and integration in the FC network of adults with MDD (Li et al., 2015; Luo et al., 2015; Wang et al., 2016). Moreover, in adults with posttraumatic stress disorder, it was found that segregation (increased Eloc) and integration (increased Eglob) increased, showing a shift toward a stronger small-world network (Lei et al., 2015), whereas another study reported increased segregation (increased Eloc) and decreased integration in children with posttraumatic stress disorder, indicating a shift toward regularization (Suo et al., 2015). Inconsistent with our study, a significantly lower temporal correlation coefficient in adult MDD patients than in HCs was also observed in a multicenter study (Long et al., 2020). In summary, previous and our current studies seem to reveal some differences in the MDD-related changes in the brain network topology between adolescents and adults. It is unclear why the transformation direction of the brain network differs between adolescents and adults. One possibility is that the neurobiological effects of depressive symptoms and stress vary at different developmental periods. These hypotheses clearly require further testing in future studies, including longitudinal examination of children with childhood trauma as they continue into adulthood.
Trauma-associated alterations in local network organizationsThe brain is organized into networks, and alterations in network architecture may underlie many forms of psychopathology (van den Heuvel & Hulshoff Pol, 2010). At the local level, we identified significant abnormalities within the DMN in both static and dynamic network metrics in the MDD-CT group than HCs group (Figs. 2-3). Abnormal FC patterns within the DMN have been frequently reported in patients with MDD in previous neuroimaging studies (Hamilton et al., 2011; Sheline et al., 2010; Yan et al., 2019; Yu et al., 2019) and large meta-analyses (Kaiser et al., 2015). The DMN is known to be involved in the processing of self-related awareness and emotions (Sheline et al., 2009). Therefore, it has been widely suggested that dysfunctions within the DMN may be associated with the common repetitive and passive focus on one's distress (rumination) in patients with MDD (Hamilton et al., 2015; Tozzi et al., 2021). Notably, multiple previous studies have documented the mediating role of rumination in the relationship between childhood trauma and depression (Kim et al., 2017; Raes & Hermans, 2008). Considering these findings of prior studies and our results, it may thus be hypothesized that dysfunction within the DMN (and possible consequences of increased self-focused rumination) might moderate the relationship between childhood trauma and adolescent MDD. Here, our work may be one of the first to support such hypothesis from the perspectives of static and dynamic brain topographical organizations to our knowledge. However, since rumination was not assessed in the current study, this hypothesis can be further tested in future studies.
This study also showed that the global efficiency of the FPN increased in the MDD-CT group compared to that in both the MDD-nCT and HCs groups. The FPN has been referred to by various terms, including the cognitive control network or system (MW Cole et al., 2013), the superordinate cognitive control network (Niendam et al., 2012), the multiple-demand system (Duncan, 2010) and the task-positive network (Fox et al., 2005). Task-positive networks are mainly involved in executive control and external attention. Our findings indicated that abnormal connectivity patterns of this network are associated with dysfunction in executive control (Cole et al., 2014; Dosenbach et al., 2008). Notably, in contrast to our findings, the global efficiency of the FPN was reported to be reduced in adult MDD patients (Tan et al., 2021; Yu et al., 2019), which may again suggest the potential differences between adolescent and adult MDDs.
Furthermore, the temporal correlation coefficients of the SAN and ATN increased in the dynamic brain network analysis. While the SAN was originally defined as a set of regions encoding emotional and cognitive processes associated with individuals, the prominent role of the SAN in salient events and emotional regulation of feelings explains how the abnormal increase in intranetwork connectivity (segregation) in the SAN contributes to ruminative responses to negative emotional states and life events in MDD patients (Seeley et al., 2007). Moreover, another study found that childhood trauma was associated with abnormal SAN connectivity (van der Werff et al., 2013). The attention network (including the VAN and DAN) has been implicated in stimulus-driven attention (Corbetta et al., 2008) and is involved in recognizing the emotional significance of stimuli, generation of affective states, and automatic regulation of emotional responses (Phillips et al., 2003). Thus, it is likely that childhood trauma is associated with altered development of the DMN, FPN, SAN and ATN in ways that may have important clinical implications. Overall, our current study not only confirms the significant relationship between childhood trauma and MDD but also links patients’ childhood traumatic experiences to specific functional brain network abnormalities, indicating that environmental factors may contribute to the neurobiological clinical symptom profile.
Associations between network measures and clinical symptomsWe established the associations between clinical symptoms (including depression level measured by the HAMD-17 and childhood trauma measured by the CTQ) and network metrics. We found that higher network metrics were significantly associated with higher HAMD-17 and CTQ Emotional Neglect scores in adolescents with MDD. As described in the above sections, the temporal correlation coefficients indicate the consistency of FC patterns between consecutive time points (Long et al., 2020). Our results suggest that increased consistency of FC is associated with more severe depressive symptoms. The fact that the adolescents with MDD had the highest correlation for the emotional neglect subscale of the CTQ suggests that emotional neglect indeed seems to affect dynamic brain FC development more profoundly compared to other types of childhood trauma. Our results revealed positive correlation between HAMD-17, emotional neglect, and brain networks in adolescents with MDD, and clinicians must pay more attention to childhood trauma among adolescent patients with MDD.
Limitations and future directionsSeveral limitations of the current study must be noted. First, we used a cross-sectional approach, which does not allow cause and effect to be examined. Second, those with childhood trauma were excluded from the HCs group, so no comparisons were made between healthy controls for the presence or absence of concomitant childhood trauma. In addition, childhood trauma subtype analyses were not conducted due to the small sample size of MDD patients. Future studies may focus on the unique impact of a particular childhood trauma subtype, such as neglect or abuse. Third, childhood trauma was assessed retrospectively through self-report; although the CTQ is reliable and widely used, childhood trauma history may be over- or underreported due to recall bias. Future studies are encouraged to investigate the associations of childhood trauma with other aspects of static and dynamic brain networks. Fourth, while this study focused on several validated topological metrics for brain networks, other measures such as the brain network flexibility (Bassett et al., 2011) may be also relevant to the association between childhood trauma and MDD, which can be explored in further studies. Sixth, the test-retest reliability of a measure of brain functioning is a fundamental factor to detect meaningful interindividual differences (Noble et al., 2019; Zuo et al., 2019). It is necessary to note that our participants were adolescents, while previous studies on the test-retest reliability of the brain network metrics have been done mainly in adults (Braun et al., 2012; Cao et al., 2014; Long et al., 2023); whether there are differences in the reliability of brain network metrics between adolescents and adults remains to be further investigated in the future. Lastly, although the sample size in this study is larger than most prior studies on the possible neuroimaging effects of childhood trauma in MDD, it may still be relatively small for getting robust results based on recent opinions (Marek et al., 2022). It is necessary for future research to further expand the sample size to verify the repeatability of the results.
In summary, we provide evidence that brain network abnormalities in patients with MDD compared to HCs, as well as static and dynamic brain network associations of patients with clinical symptoms, are most obviously driven by childhood traumatic experiences. The exploration of neurobiological markers of individuals with MDD and childhood trauma will be essential to advance our knowledge of this disabling condition, which will lead to the development of new diagnostic classifications, treatment options, and prevention methods. Future neuroimaging studies also need to shift the focus toward examining adolescents with MDD and childhood trauma to better understand brain markers of high-risk disease subtypes.