Attention Deficit/Hyperactivity Disorder (ADHD) negatively affects social functioning; however, its neurological underpinnings remain unclear. Altered Default Mode Network (DMN) connectivity may contribute to social dysfunction in ADHD. We investigated whether DMN's dynamic functional connectivity (dFC) alterations were associated with social dysfunction in individuals with ADHD.
MethodsResting-state fMRI was used to examine DMN subsystems (dorsal medial prefrontal cortex (dMPFC), medial temporal lobe (MTL)) and the midline core in 40 male ADHD patients (7-10 years) and 45 healthy controls (HCs). Connectivity correlations with symptoms and demographic data were assessed. Group-based analyses compared rsFC between groups with two-sample t-tests and post-hoc analyses.
ResultsSocial dysfunction in ADHD patients was related to reduced DMN connectivity, specifically in the MTL subsystem and the midline core. ADHD patients showed decreased dFC between parahippocampal cortex (PHC) and left superior frontal gyrus, and between ventral medial prefrontal cortex (vMPFC) and right middle frontal gyrus compared to HCs (MTL subsystem). Additionally, decreased dFC between posterior cingulate cortex (PCC), anterior medial prefrontal cortex (aMPFC), and right angular gyrus (midline core) was observed in ADHD patients relative to HCs. No abnormal connectivity was found within the dMPFC.
ConclusionPreliminary findings suggest that DMN connectional abnormalities may contribute to social dysfunction in ADHD, providing insights into the disorder's neurobiology and pathophysiology.
Social functioning is an important aspect of an individual's overall well-being and daily life, encompassing the ability to form and maintain relationships, adapt to social situations, and effectively communicate with others (Blakely & Dziadosz, 2007; Eisenberger & Cole, 2012). Although social functioning is not considered a psychiatric disorder/symptom itself, neuropsychiatric disorders such as ADHD can have a significant impact on social conduct (Fateh et al., 2022). ADHD is one of the most prevalent mental disorders in childhood and often persists into adolescence and adulthood (Willcutt, 2012). It is a debilitating neurodevelopmental condition characterized by age-inappropriate inattention, extreme restlessness, and lack of self-control (Posner et al., 2020; Willcutt, 2012). ADHD symptoms, including inattention, hyperactivity, and impulsivity, can contribute to difficulties in social interactions and relationships (Harpin et al., 2016). These symptoms affect interpersonal relationships, self-esteem, and self-regulation (Danielson et al., 2018; Katzman et al., 2017). In this context, social functioning can be considered a secondary outcome of ADHD, influenced by the primary symptoms of the disorder. While there is literature addressing the impact of ADHD on social functioning (Harpin et al., 2016), there are very few studies, particularly fMRI-based ones, that specifically investigate the neurobiological underpinnings of social functioning impairments in ADHD. In comparison to disorders such as autism, which have been more extensively addressed by fMRI studies (Kim et al., 2015; Sato & Uono, 2019), research on the relationship between ADHD and social dysfunction using neuroimaging techniques is relatively scarce. While our study specifically investigates the default mode network (DMN) in relation to social dysfunction in ADHD, it is essential to acknowledge the complex and variable symptoms associated with psychiatric disorders, including ADHD. Furthermore, autism-spectrum disorders (ASD) involve impairment in social communication, affecting both social perception and expression. By appreciating the breadth of psychiatric symptomatology, our aim is to gain unique insights into the underlying pathophysiological mechanisms of social dysfunction in ADHD and inform improvements in therapeutic management strategies (Posner et al., 2014).
The brain's DMN, which has been demonstrated to play a crucial role in several facets of human social behavior, is a neurobiological system that may be related to both social (dys)functioning and ADHD pathology (Li et al., 2014; Mars et al., 2012a; Padmanabhan et al., 2017). Resting-state networks (RSNs) are specific areas within the brain that demonstrate synchronous activity during resting-state functional magnetic resonance imaging (rs-fMRI), characterized by low-frequency signal fluctuations, typically below 0.1 Hz (Seitzman et al., 2019). One of the most extensively studied RSNs is the DMN, associated with internal mental processes such as memory retrieval and self-referential processing. The DMN can be distinguished from other RSNs by its characteristic decrease in activity during goal-directed behavior, referred to as "deactivation", particularly during cognitive tasks. DMN activity has been shown to be involved in stimulus-independent memory retrieval processes and self-referential processing, which are closely connected to depressive symptoms (Hamilton et al., 2015). Several studies have used rs-fMRI and identified prominent FC differences of DMN in ADHD individuals compared to healthy controls (HCs). For instance, previous studies by using graph theory methods detected disrupted integration of DMN regions in ADHD patients (Kucyi et al., 2015). One study performed independent component analysis on rs-fMRI data and showed that resting state connectivity pattern of DMN had higher dispersion in ADHD children (Kumar et al., 2021). Furthermore, mounting evidence points to the involvement of DMN in cognitive processes such as contemplation of the future, reviewing memories (Raichle & Snyder, 2007; Whitfield-Gabrieli & Ford, 2012) and personal introspection (Dudukovic et al., 2011; Gusnard & Raichle, 2001; Whitfield-Gabrieli et al., 2011). Although both ADHD and DMN have been the subject of substantial research, yet, no studies have been conducted on the likely involvement of DMN in the social dysfunction exhibited by ADHD patients (Fassbender et al., 2009; Garrity et al., 2007; Posner et al., 2014; Uddin et al., 2008; Whitfield-Gabrieli et al., 2009).
The DMN is a complex network composed of two subsystems and a midline core, each with distinct contributions to cognitive processes (R. Buckner et al., 2008). The two subsystems, namely the dorsal medial prefrontal cortex (dMPFC) and the medial temporal lobe (MTL), along with the midline core, have been identified as critical components in constructing personal meaning, metacognitive processes, mentalizing, and episodic memory, respectively (Andrews-Hanna et al., 2010, 2014a; Poerio et al., 2017). These components of the DMN interact in intricate ways, and each contributes to various aspects of social cognition and self-referential thinking (Mars et al., 2012b).
Disruptions in one region or subsystem of the DMN have been observed to affect the entire network, which can potentially lead to changes in its functionality (Menon, 2011). The complex interactions and dependencies within the DMN support various social behaviors, and alterations to individual subsystems can result in significant consequences, potentially giving rise to altered social functioning. In our study, we opted to focus on specific DMN subsystems using a region-of-interest (ROI) analysis, rather than a whole-network approach. This decision was based on the rationale that certain subsystems might be more relevant to social behavior in patients with ADHD, and by investigating these subsystems in a targeted, hypothesis-driven manner, we can potentially gain a deeper understanding of the neural mechanisms underlying social dysfunction in ADHD. However, it is important to briefly acknowledge the limitations and trade-offs associated with this approach, which are discussed in the limitations section of our study. Future research can expand upon our findings by examining other DMN regions and conducting whole-network analyses to complement the insights gathered from the ROI-based investigation.
There exist no relevant work examining the alterations in DMN connectivity or connectional integrity with regard to social dysfunctioning in ADHD so far. To the best of our knowledge, this is the first work addressing DMN's subsystems FC abnormalities and its implications to social disabilities in children with ADHD. However, the significance of DMN to both normal and impaired social functioning is further supported by the observation of DMN disruptions in several neuropsychiatric illnesses with severe social dysfunctioning. These disorders include autism, schizophrenia, social phobia, and major depressive disorder (Arnold Anteraper et al., 2014; Di Martino et al., 2009; Mazza et al., 2013; Saris et al., 2020). In their review paper, Kaiser et al. (Kaiser et al., 2015) posited that certain patterns of network disruption could underlie fundamental deficiencies in social, cognitive, and emotional functioning that might set off clinical symptoms in neuropsychiatric illnesses like ADHD. Thus, a DMN-based FC approach to social functioning in ADHD allows for the investigation of the interplay between many systems, thereby facilitating the development of adaptive social behaviors.
Exploring the possible categorical and dimensional relationship between social dysfunction and DMN's FC in ADHD may, however, help us learn more about the underlying neurobiology and, maybe, guide future treatment strategies. The idea that social dysfunction may have a distinct neurobiological signature, be transdiagnostic in nature, and bear clinical/therapeutic value is gaining traction in the field and is supported, in particular, by the Pan-European PRISM research (Kas et al., 2019). However, social functioning is a multifaceted and ever-evolving process that might be hard to pin down in a single area. Here, we examine the cumulative connection between DMN FC and three significant indicators of social dysfunction in individuals with ADHD, providing a more comprehensive picture of patients' social functioning. In addition to being present in variable degrees in male patients with ADHD, these indices are also related with detrimental neurobiological alterations. These indices include loneliness, perceived social impairment, and limited social network. by using the cumulative social dysfunction score, we tested our hypotheses about the relationship between social dysfunction and FC in the DMN subsystems of ADHD patients. So, we hypothesized that: Boys with ADHD will exhibit reduced FC within the DMN, as compared to typically developing boys, across multiple subsystems.
We predict that this reduced connectivity will be related to deficits in social functioning, as indexed by a composite score of measures including loneliness, perceived social impairment, and limited social network, as well as measures of social, thought, and delinquent behavior problems. As part of the hypothesis, FIQ scores, working memory scores, behavioral memory scores, learning problems, and anxiety scores are also taken into account.
Materials and methodsParticipants and measuresThis study recruited 40 children with ADHD aged between 7 and 10 years (mean age: 10.6 ± 0.85) from Shenzhen Children's Hospital and 45 age-matched HCs (mean age: 9.1 ± 0.52). Prior to enrollment, all participants and their parents were interviewed to confirm or exclude a diagnosis of ADHD or any other psychiatric disorder using a clinical interview and the Schedule for Affective Disorders and Schizophrenia for School-Age Children - present and lifetime version (K-SADS-PL) (Joan et al., 1997), based on the DSM-V criteria (American Psychiatric Association, 2013). Children with ADHD were required to meet the following inclusion criteria: (1) 7-10 years old, (2) educated in private or public schools, and (3) diagnosed with ADHD. HCs subjects had the same age and education requirements as ADHD subjects. The exclusion criteria for both groups included a history of head injury with loss of consciousness, severe physical disease or neurological abnormalities, drug or substance misuse, full-scale IQ measured by Wechsler Intelligence Scale for Chinese Children-IV (WISC-IV-Chinese) below 70, prescription medications for ADHD or other medical conditions used over the long term, and comorbid conduct disorder or Oppositional Defiant Disorder (ODD). The MRI scans were only performed on participants who were right-handed dominant and who did not have any visible abnormalities on their MRI images or a history of claustrophobia. ADHD participants presented with six or more inattentive symptoms as well as six or more hyperactive/impulsive symptoms, and in subsequent statistical analysis, the summing severity scores of each symptom were used as indicators of symptom severity. Parents provided informed consent, and children gave their assent to participate in the study, which was approved by the Ethics Committee of Shenzhen Children's Hospital.
Assessments of social and cognitive function and clinical outcomesADHD patients and HCs underwent a comprehensive array of social and cognitive assessments, along with clinical and semi-structured interviews. Parents of the children filled out the Child Behavior Checklist (CBCL) (Achenbach & Ruffle, 2000). This 113-item questionnaire assesses everyday behavior and captures eight distinct factors: withdrawal, somatic complaints, anxiety/depression, social problems, thought problems, attention problems, and delinquent behavior.
Cognitive function, particularly behavioral memory and executive functions, was evaluated using selected tests from the Cambridge Neuropsychological Test Automated Battery (CANTAB).1 The term “behavioral memory” refers to the memory processes that underpin the learning and recall of behaviors, which were assessed using specific tasks within the CANTAB.
The Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IVWISC-IV-Chinese), a widely accepted intelligence test for children aged 6 to 16 years, was also administered by trained professionals. The WISC-IV yields a Full-Scale Intelligence Quotient (FIQ) as well as other indices, including the Verbal Comprehension Index (VCI), the Perceptual Reasoning Index (PRI), the Working Memory Index (WMI), and the Processing Speed Index (PSI).
In the context of clinical and scientific settings, the CBCL is extensively utilized, with well-established reliability and validity (Leung et al., 2006). Due to the unavailability of standardized t-scores in mainland China, we employed the original summary score for each factor in this study.
Social composite scoreIt is important to note that, to date, there is no specific standard validated assessment protocol for measuring social dysfunction in children with ADHD, primarily because it is considered a secondary symptom in comparison to core ADHD symptoms like inattention and impulsivity. This presents challenges in evaluating social functioning in this population compared to other disorders like ASD and Social Anxiety Disorder (SAD), which have more established assessment scales and tools (Chan et al., 2019). Therefore, our study aims to contribute to the existing literature by utilizing a composite score approach that encompasses specific aspects of social dysfunction relevant to children with ADHD while acknowledging the challenges posed by the lack of a specific standard validated assessment tool.
Following (Saris et al., 2020), a social composite score was computed to assess specific aspects of social dysfunction, using data coming from three validated (subscales of) questionnaires that looked at loneliness (social isolation), perceived social disability, and a limited social network size. The subjective experience of loneliness was assessed with the help of a questionnaire comprised of 11 questions, each of which was evaluated on a 3-point Likert scale (de Jong-Gierveld & Kamphuls, 1985). The World Health Organization Disability Assessment Schedule (WHO-DAS) features a 5-item social interaction subscale domain, which we used to assess the degree of difficulty participants experienced in forming and maintaining social relationships (Üstün et al., 2010). It is important to note that our study primarily focused on social dysfunction in children with ADHD, an area that may not be completely captured by conventional ADHD severity scales, such as the Conners or the Special Needs Assessment Profile (SNAP) scales. While these instruments are invaluable for evaluating overall ADHD symptom severity, the WHO-DAS, specifically its social interaction subscale, offers a more nuanced view of social isolation and social disability. This comprehensive approach allows for a targeted investigation of social dysfunction, which is often a secondary but significant aspect of the ADHD experience. The size of children's social networks was calculated using the close contacts inventory (Stansfeld & Marmot, 1992). To make a more intuitive and accurate composite score, the results for social network size were inverted so that, in accordance with the other two questionnaires, greater scores would signify more social dysfunction. The individual questionnaire scores were log-transformed and standardized before being summed, and then the resulting sum was divided by three to get the composite score. Therefore, more social dysfunction is reflected by a higher total score (more loneliness, higher perceived social disability, smaller social network) (Gaspersz et al., 2017). By incorporating these selected measures of social dysfunction into a single composite score, we aim to provide insight into how these specific aspects of social dysfunction affects DMN connectivity as a whole without conducting repeated tests on each individual measure. Social composite score is calculated using the following formula:
Here is an expanded explanation of each component:
- 1.
Loneliness: This component represents the total score from the loneliness questionnaire, measuring the child's subjective experience of social isolation. To account for possible zero scores, we add 1 before taking the logarithm. This standard statistical practice accommodates zero values in log transformations, ensuring all data points are appropriately considered in the composite score.
- 2.
Social Disability: Derived from the social interaction subscale domain of the WHO-DAS, this score reflects the difficulty respondents have in forming and sustaining social relationships. The distribution of these scores in our sample was already normal, so we did not apply a log transformation. Log transformations are generally used to reduce skewness and bring the data closer to normal distribution, but in our case, this step was unnecessary (Feng et al., 2019).
- 3.
Social Network Size: This value represents the size of the child's social network as assessed by the close contacts inventory. We invert this value (using 1/“Social Network Size”) so that greater scores contribute to signifying heightened social dysfunction, in accordance with the other two components. Then, we apply a log transformation to better align this value with the other components of the composite score.
We sum the three components and divide by three to create an average, producing the “Social Composite Score”. This score provides a holistic view of selected aspects of the child's social functioning, with higher scores indicating more significant dysfunction, including increased loneliness, heightened perceived social disability, and a smaller social network. Concerning the translation of these results into current medical knowledge, we acknowledge that capturing and compressing social processing and functioning into numerical values is challenging, as stated in the limitations section of our study. Although no specific works for ADHD have utilized these measures, our intention is to contribute to the understanding of social dysfunction in ADHD by offering a broader perspective on overall social functioning. We are aware that additional research and validation, alongside in-depth examination of components like social networks, loneliness, and social disability, are necessary for a better integration of these findings into current medical practice and knowledge.
Multiple linear regression analysis of DMN functional connectivity and covariatesIn this section, a multiple linear regression analysis was conducted to investigate the associations between DMN functional connectivity (FC) and various covariates, including age, FIQ, and measures of social, thought, and delinquent behavior problems. The social composite score, which was computed from three validated questionnaires assessing social dysfunction, was also included as a covariate. The beta coefficients, standard errors, t-values, and p-values for each variable were reported in Table 3. This analysis aimed to provide insight into the factors that influence DMN FC and shed light on the role of social dysfunction in this relationship.
Resting-state fMRI data acquisitionThe rs-fMRI data for all participants were acquired using a 3.0-T Siemens Magnetom Skyra system scanner at the Radiology Department of Shenzhen Children's Hospital. Echo-planar imaging (EPI) sequence was employed with the following parameters: repetition time (TR) = 2000ms; echo time = 30ms; flip angle = 90°; matrix size = 64 × 64; 32 axial slices; field of view (FOV)= 24 × 24cm2; slice thickness = 3mm with no gap. A 3D magnetization-prepared rapid gradient-echo (MPRAGE) T1-weighted sequence was also acquired with the following parameters: Repetition Time [TR, ms] = 2300, Echo Time [TE, ms] = 2.26; Number of Averages = 1.0, Slice Thickness =1.0mm, and FOV=256mm.
Data Pre-processingData pre-processing was carried out using the DPARSF toolkit (Yan et al., 2016) based on SPM12 (https://www.fil.ion.ucl.ac.uk/spm/software/spm12). The first 10 volumes were discarded to account for the initial magnetic resonance imaging signal instability and participant adaptation to experimental conditions. The remaining 230 volumes were realigned for head motion correction and slice timing adjustment. All ADHD patients met the criterion of head motion translation < 3mm or rotation < 3° in any direction. Following normalization and resampling into a voxel size of 3 x 3 x 3mm³, nuisance covariates (global signal, white matter signal, cerebrospinal fluid signal, and Friston-24 parameters of head motion) were regressed out from each voxel's time course.
The data were linearly detrended and filtered within the 0.01-0.08 Hz range to minimize the low-frequency drift and high-frequency physiological noise influences. A 6mm full-width-at-half-maximum Gaussian kernel was applied for smoothing. Frame-wise displacement (FD) was calculated for each subject to assess head motion at each time point. Using the scrubbing methods (Power et al., 2012) with an FD threshold of 0.5mm, the bad time points and their 1 back and 2 forward volumes were estimated through cubic spline interpolation.
Head of motionThe mean frame-wise displacement (FD) created during the scanning process was removed using Jenkinson's relative root-mean-square technique (Jenkinson et al., 2002). To evaluate the voxel-wise motion differences between the two groups, the mean FD (Jenkinson) was determined. The mean FD did not change substantially between the ADHD and HCs groups (p ˃ 0.6).
Statistical analysisIn this study, we employed two sample t-test (model A) to examine the differences in dFC of the DMN subsystems between HCs and patients with ADHD. We regressed out the mean FD, age, FIQ, and years of education to account for potential confounding factors. Group effects were assessed by converting F-statistic images to z-statistic images and applying a threshold of z > 2.3, along with a cluster-level threshold p value < 0.05, corrected for whole-brain multiple comparisons using Gaussian random field theory. We selected the surviving brain clusters as regions of interest (ROIs) for subsequent post-hoc analyses. These areas were selected due to their significant differences in DMN subsystems connectivity and relevance for understanding the FC within the DMN as documented in previous research (Li et al., 2014; Wen et al., 2020). Two-tailed, two-sample t-tests were conducted on these ROIs to determine differences between ADHD and HCs groups while controlling for mean FD, age, gender, and years of education. A statistical significance level of (p < 0.05/11) (Bonferroni corrected) was considered significant.
FC (seed-to-voxel) of DMN subsystems analysesIn neuroimaging research, a subsystem within the DMN refers to a smaller, specific collection of brain regions within the larger DMN that tend to co-activate, or “communicate,” with each other during resting-state fMRI scans. These subsystems are typically defined based on their functional connectivity profiles, meaning that regions within the same subsystem show more significant synchronized activity over time compared to regions in different subsystems.
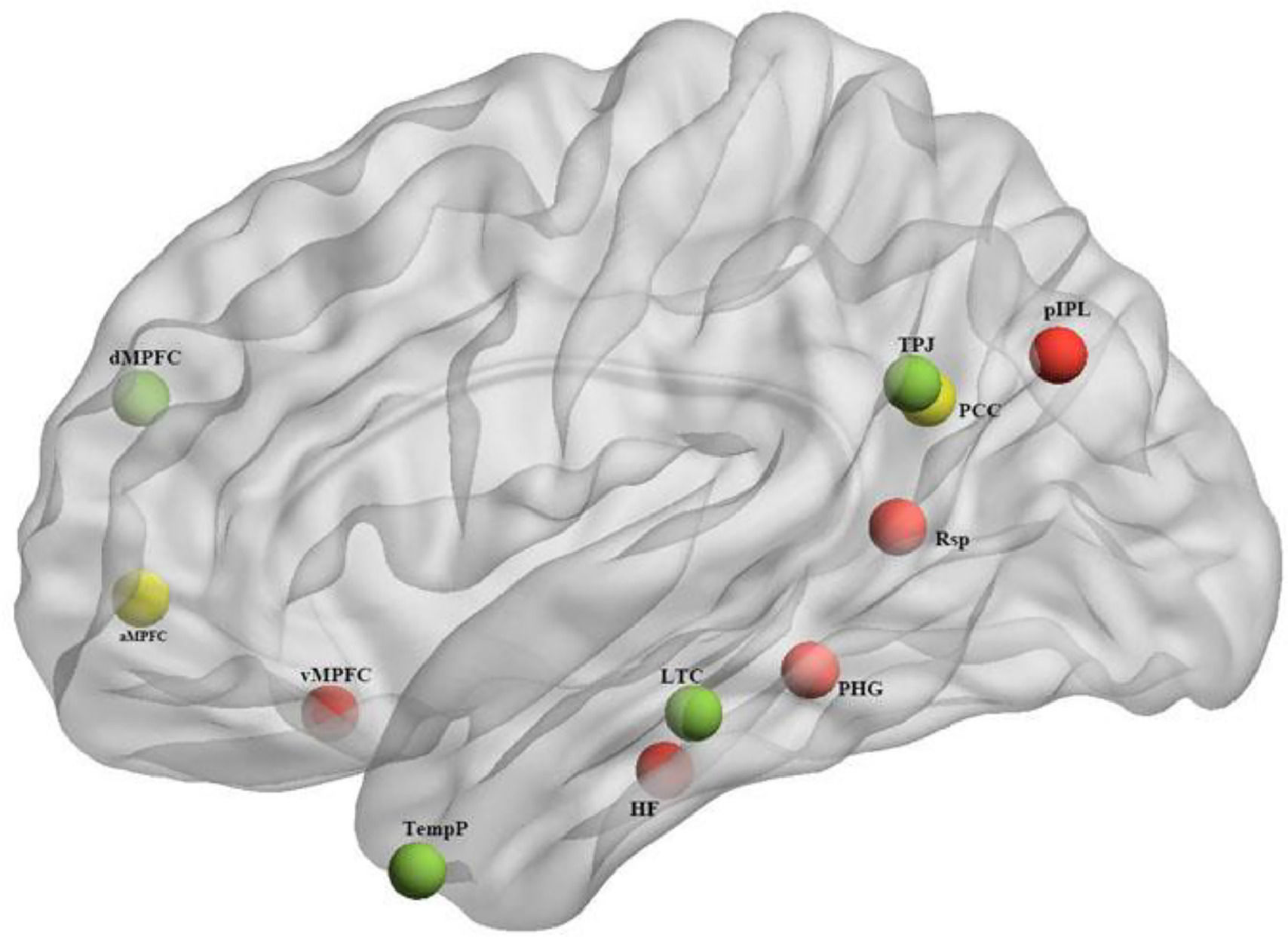
While the full DMN is implicated in many internal mental processes like daydreaming or mind-wandering, the subsystems of the DMN have been found to correlate with more specific cognitive functions. The division into subsystems is largely based on patterns of correlation and covariation in neural activity, as well as overlaps with other well-established functional brain networks. The exact definition and boundaries of these subsystems may vary slightly depending on the research, but there is consensus on two primary subsystems and one midline core (Andrews-Hanna, 2012; Du et al., 2016), as depicted in Fig. 1 and Table 1:
- 1.
The Midline core: generally including the Posterior Cingulate Cortex (PCC) and Anterior Medial Prefrontal Cortex (aMPFC), is thought to be involved in self-referential mental activity.
- 2.
The Dorsomedial Prefrontal Cortex (DMPFC) subsystem: including regions like the DMPFC, Temporo-parietal Junction (TPJ), Lateral Temporal Cortex (LTC), and Temporal Pole (TempP), has been linked with tasks involving thinking about other people's perspectives or mental states (a process known as mentalizing).
- 3.
The Medial Temporal Lobe (MTL) subsystem: encompassing the Ventral Medial Prefrontal Cortex (vMPFC), Posterior Inferior Parietal Lobule (pIPL), Retrosplenial Cortex (Rsp), Parahippocampal Cortex (PHC), and Hippocampal Formation (HF), is primarily associated with memory processing and scene construction.
The 11 ROIs of the DMN subsystem (depicted with BrianNet viewer (Xia et al., 2013)), Red: Medial temporal lobe; Green: dorsal medial prefrontal cortex; Yellow: Midline core.
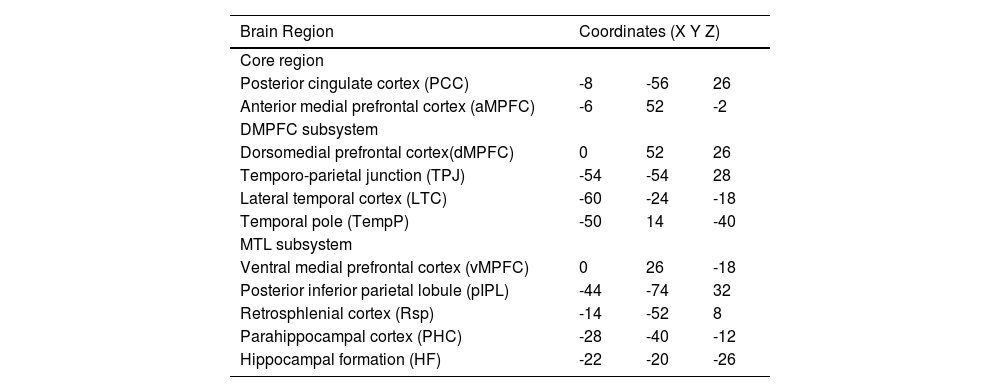
Montreal Neurological Institute (MNI) coordinates of 11 DMN regions of interest (ROIs).
These subsystems are not rigid, and some regions may participate in multiple subsystems. They are best thought of as operational units within the DMN, each with its own characteristic pattern of activity and cognitive function (Menon, 2011). In the FC analysis, correlation maps were generated for each of the 11 DMN seeds, defined as spheres with an 8 mm radius. To create these spheres, multiple adjacent voxels were incorporated within each seed region despite the voxel size being normalized and resampled to 3 × 3 × 3 mm. This method accounts for the variations in the functional interactions within the seed regions and adheres to standard practices in FC analysis (Uddin et al., 2011). The regional time series of each seed, computed by averaging the time series of the voxels encompassed within the 8 mm radius sphere, were then correlated with each individual voxel in the gray matter mask derived from the Automated Anatomic Labeling-116 (AAL) atlas (Tzourio-Mazoyer et al., 2002). The AAL atlas comprises 116 anatomical regions, enabling a comprehensive assessment of FC between the DMN seeds and other regions across the brain.
Following the generation of the correlation maps, they were transformed into Fisher z-maps to facilitate subsequent statistical analyses. This procedure ensures that the data adhere to the normal distribution assumptions underlying many statistical tests and minimizes the impact of potential biases present in raw correlation coefficients. More specifically, separate Fisher z-maps were generated for each group – HCs and ADHD patients – representing the group-specific FC patterns. The group FC maps were derived by averaging the Fisher z-maps within each group. Following this, a two-sample t-test was conducted to compare the FC patterns between the HCs and ADHD patient groups.
We first performed a two-sample-t-test first to examine the differences in DMN subsystems maps. The specific z-maps were entered into random effects analyses using age, grade, number of scrubbing cut time points, and mean FD as covariates of interest to compare connectivity maps of each seed between the HCs and ADHD groups (two-sample t-test). The gray matter mask, which included 116 automated anatomic labelling atlas regions (Tzourio-Mazoyer et al., 2002), was used in statistical analyses. Resulting t-maps indexing differences between the ADHD and HCs groups were corrected using a two-step approach, initially applying Gaussian random field theory and followed by Bonferroni correction for multiple comparisons as implemented in the SnPM (Nichols & Holmes, 2002), with a statistical threshold of voxel-level P < 0.005 and cluster-level P < 0.001 (representing a Bonferroni corrected P value adjusted for 11 comparisons after cluster-level correction). This combination of correction methods was chosen to maintain a balance between reducing Type I errors and not being overly stringent, which could increase Type II errors (missing true positives). To create histograms, we extracted the mean z-scores of clusters that revealed differences between ADHD and HCs.
Correlation analysisThe zFC values that significantly deviated from the baseline were extracted, and Pearson's correlation analysis was used to examine the relationships between these altered zFC values and various psychopathology domains of the CBCL, such as anxiety, thought problems, aggressive behavior, and others. However, significant correlations were only found in specific areas. These included the relationship between the altered zFC values and FIQ score with the mean value of the right angular gyrus, the correlation between behavioral memory and the right middle frontal gyrus, and the correlation between the social composite score and the left superior frontal gyrus.
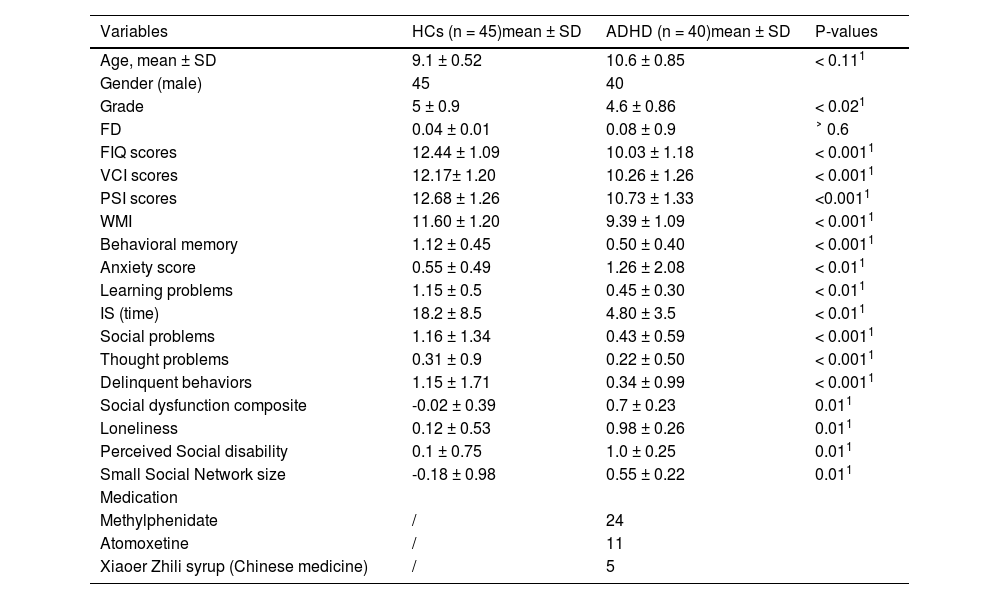
ResultsThe sample's demographic and clinical characteristicsThe demographic and clinical characteristics of the HCs and ADHD groups can be found in Table 2.
Demographic and clinical information.
HCs: healthy control.
ADHD: Attention deficit hyperactivity.
FD: frame-wise displacement.
SD: Standard deviation.
1: Two-tailed two-sample t-test.
IS: interference score.
FIQ: full scale intelligence.
VCI: verbal comprehension Index.
PSI: Processing Speed Index.
WMI: Working Memory Index.
There were no statistically significant differences in age or frame-wise displacement (FD) between the two groups. However, significant differences were observed in cognitive and behavioral assessments. The ADHD group had significantly lower scores in full scale intelligence (FIQ), verbal comprehension index (VCI), processing speed index (PSI), working memory index (WMI), and behavioral memory (p < 0.0011 for all). Conversely, the ADHD group exhibited higher scores in social composite score, loneliness, perceived social disability, and small social network size (p < 0.011 for all).
The report of medication intake was limited to the participants diagnosed with ADHD, wherein 24 were found to be taking methylphenidate, 11 were taking atomoxetine, and 5 were using a type of Chinese medicine called Xiaoer Zhili syrup. We would like to clarify that while our exclusion criteria included the long-term use of ADHD medications, participants who were on short-term prescription (defined as less than six months of continuous use) were not excluded. This decision was based on our aim to reduce potential confounding effects from long-term medication use, while still allowing us to include a representative sample of children with ADHD, many of whom receive medication as part of their management plan. In our study, 35 participants were receiving short-term ADHD medication.
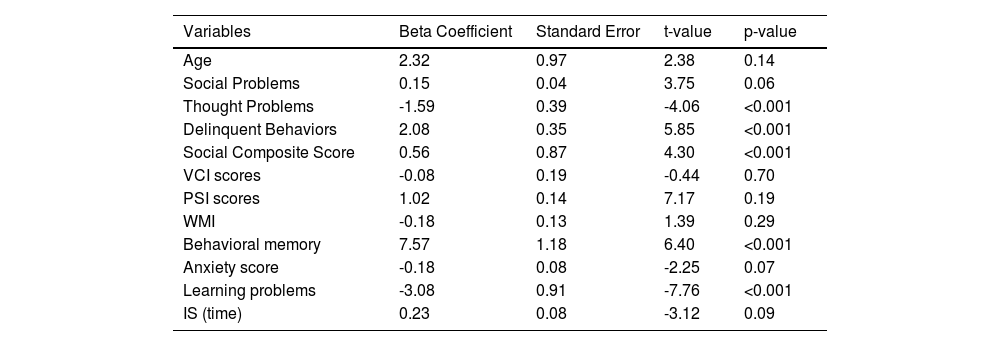
Results of multiple linear regression analysis examining the associations between DMN FC and different covariatesTable 3 presents the results of a multiple linear regression analysis designed to investigate the relationship between DMN dFC and various covariates, including age, VCI scores, PSI scores, WMI, behavioral memory, anxiety score, social problems, thought problems, delinquent behaviors, and the social composite score. We have omitted the use of FIQ as a covariate, in line with established recommendations in neurodevelopmental disorder studies (Dennis et al., 2009).
Results of multiple linear regression analysis examining the associations between DMN FC and various covariates.
After adjusting for age and other behavioral measures, the regression analysis shows that both social problems (beta = 0.15, p = 0.06) and social composite score (beta = 0.56, p < 0.001) exhibit significant and positive associations with DMN dFC. These findings suggest that higher values of social problems or social composite scores are associated with increased DMN dFC.
In addition, delinquent behaviors (beta = 2.08, p < 0.001) and behavioral memory (beta = 7.57, p < 0.001) demonstrate significant relationships with DMN dFC. These results indicate that higher scores in delinquent behaviors and better memory functioning are linked with altered DMN dFC.
Conversely, thought problems (beta = -1.59, p < 0.001) have a significantly negative association with DMN dFC, which implies that an increase in thought problems corresponds to a decrease in DMN dFC.
It is important to note that age, VCI scores, PSI scores, WMI, anxiety score, and IS (time) do not exhibit significant associations with DMN dFC in this analysis. This suggests that these variables do not substantially impact DMN dFC in our study.
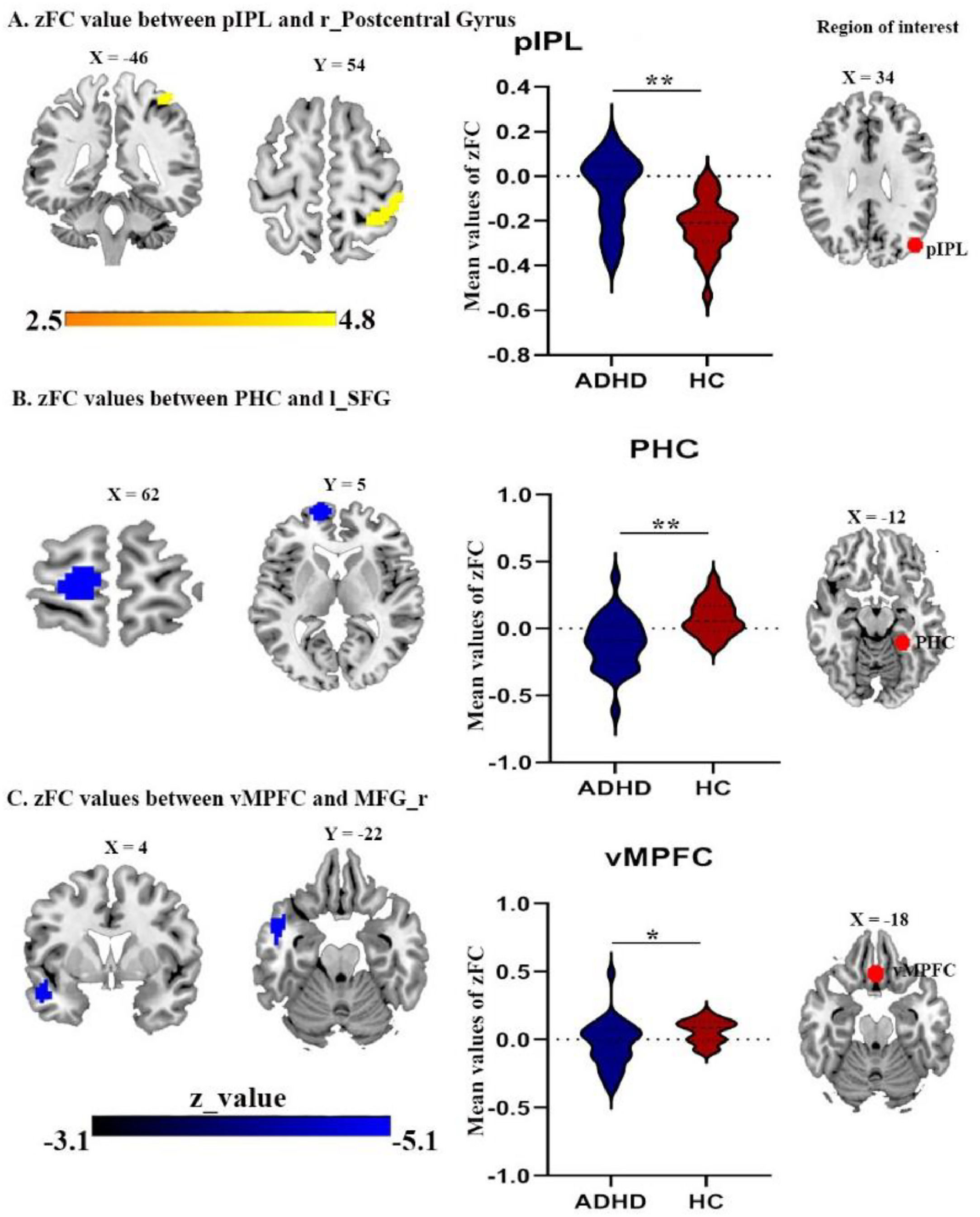
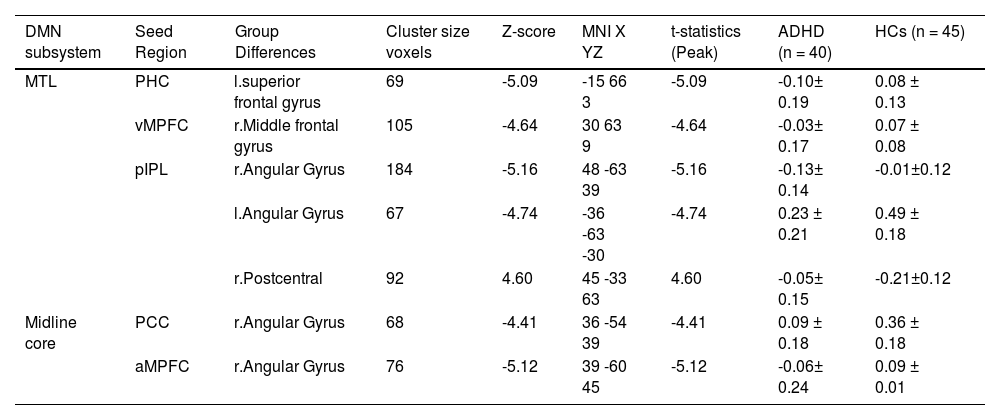
Difference in FC of DMN subsystemsWe detected FC disruptions in only two subsystems, namely MTL and midline core. ADHD patients demonstrated a decreased FC between the parahippocampal cortex (PHC) and the left superior frontal gyrus (Fig. 3B, Table 4), as well as between the ventral medial prefrontal cortex (vMPFC) and the right middle frontal gyrus (Fig. 3C, Table 4), within the MTL, when compared to HCs group.
Brain clusters showing significant effects in the DMN subsystems.
SD: Standard Deviation;
M: Mean value;
PHC: Parahippocampal cortex;
MTL: Medial temporal lobe;
pIPL: Posterior inferior parietal lobule;
aMPFC: Anterior medial prefrontal cortex;
vMPFC: Ventral medial prefrontal cortex;
PCC: Posterior cingulate cortex;
r: Right;
l: Left.
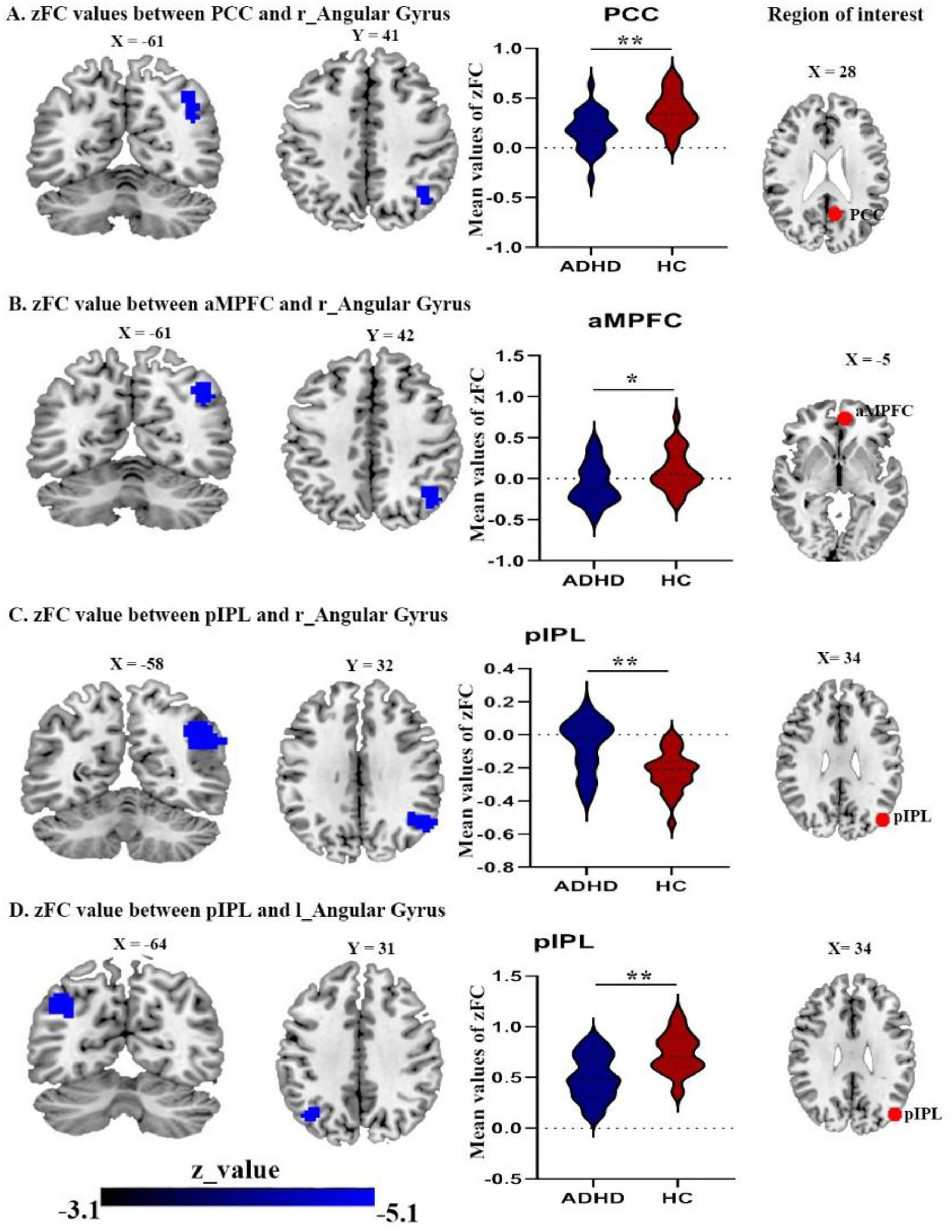
In addition, while ADHD patients exhibited a decreased FC between posterior inferior parietal lobule (pIPL) and both right and left angular gyrus (Fig. 2C and 2D) Table 4. While, the FC between pIPL and right postcentral gyrus was increased (Fig. 3A).
Brain areas showing significantly reduced connectivity with default mode network seeds in the ADHD group compared with the control group(A) right angular gyrus with PCC seed; (B) right angular gyrus with aMPFC seed; (C) right angular gyrus with pIPL seed; (D) the left angular gyrus with pIPL seed. Abbreviations: PCC, posterior cingulate cortex; pIPL, posterior inferior parietal lobule, aMPFC, anterior medial prefrontal cortex; r, right; l, left.
(A) the right postcentral gyrus with pIPL seed (B) the superior frontal gyrus with PHC seed; (C) the right middle prefrontal cortex with vMPFC seed. Abbreviations: PHC, parahippocampal cortex; vMPFC, ventral medial prefrontal cortex; MFG, middle frontal gyrus; SFG, superior frontal gyrus; r, right; l, left.
ADHD patients displayed a decreased FC between the posterior cingulate cortex (PCC) and the right angular gyrus (Fig. 2A), also decreased FC between anterior medial prefrontal cortex (aMPFC) and the right angular gyrus (Fig. 2B), in comparison to HCs. The dMPFC did not show in significant connectivity. Finally, the brain clusters showing a significant effect in the zFC with the DMN in (Table 4).
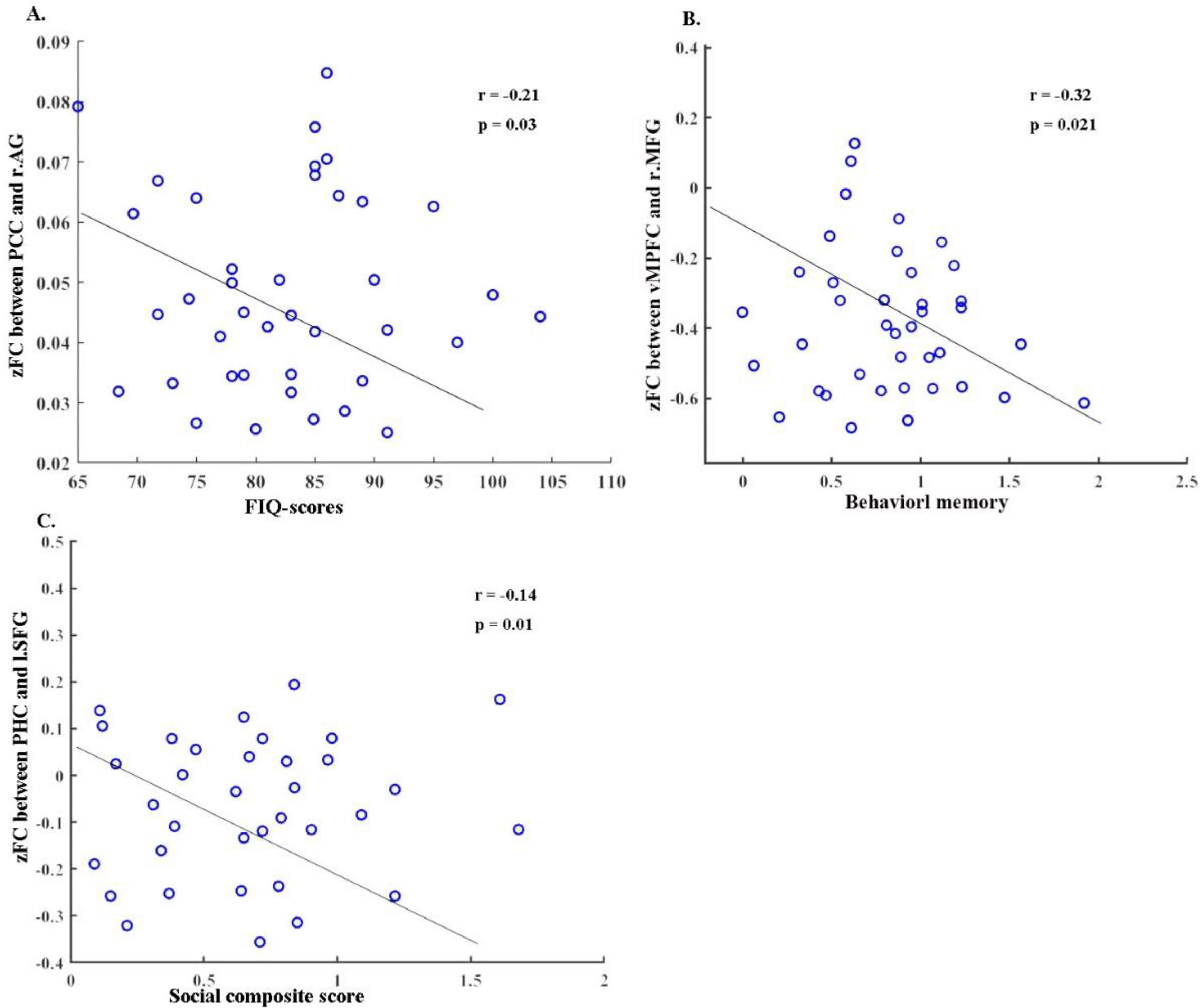
Correlation resultsOur correlation analysis uncovered a number of significant associations. First, we found a negative relationship between the altered zFC of the right angular gyrus and the PCC and the FIQ-score (r = -0.21, p = 0.03), as illustrated in Fig. 4A. Next, a significant negative correlation was observed between the disrupted zFC of the vMPFC and the rMFG and behavioral memory (r = -0.32, p = 0.021), as depicted in Fig. 4B. Finally, we detected a negative association between the zFC of the PHC and the left SFG and the social composite score (r = -0.14, p = 0.01), as seen in Fig. 4C.
Significant correlation (with p value < 0.05) between the total FIQ, Behavioral memory and social dysfunction. Abbreviations: PHC, parahippocampal cortex; SFG, superior frontal gyrus; vMPFC, ventral medial prefrontal cortex; MFG, middle fontal gyrus; PCC, posterior cingulate cortex; AG, angular gyrus; l, left; r, right.
It is important to emphasize that these correlations represent relationships among the variables rather than causative effects. In other words, although the results indicate that certain variables are related, we cannot conclude that one variable directly causes changes in the other based on this analysis alone. More in-depth studies are required to further explore these relationships to potentially determine any causal connections and to further refine and validate the measures of social functioning used in this analysis.
Composite score vs. individual social dysfunction indicesIn order to investigate the relationship between DMN subsystems FC and ADHD, we opted to utilize a cumulative measure of social dysfunctioning. This cumulative score was derived by integrating together the results of the three questionnaires that showed the greatest impairment in patients with ADHD who showed social dysfunction. Furthermore, there is substantial evidence supporting the influence of these three questionnaires on neurobiological markers (Mars et al., 2012a). Two other research, including the Pan-European PRISM project on the neuroscience of social dysfunction, also make use of these markers (Kas et al., 2019; Saris et al., 2020). The respective questionnaires measure loneliness, perceived social disability, and having a small social circle are all aspects of social dysfunction that may be measured by the respective questionnaires. This process led to the development of a social dysfunction composite index, which does the following: (1) captures social dysfunction across multiple domains simultaneously and more fully than each individual measure separately; (2) eliminates the need to conduct multiple tests of brain-behavior relations for each individual measure; (3) provides insight into the cumulative association of social dysfunction on DMN subsystems connectivity. Further supporting the usage of the composite score rather than the individual questionnaires is the fact that they were significantly correlated (r = -0.14, P=0.011, Fig. 4C). No significant DMN effects emerged when we reran our connectivity analyses with the total sum scores of each social dysfunction questionnaire (both individually and in one model), and no total sum score was predictive of DMN connectivity strength. These results suggest, however tentatively, that the cumulative social dysfunction score is presumably better able to pick up subtle brain-behavior links, at least in this specific dataset.
DiscussionOur study reveals potential associations between DMN subsystems and social dysfunction in children with ADHD. Specifically, we identified disrupted dFC within the MTL and midline core subsystems. ADHD patients displayed decreased dFC between the PHC and the left superior frontal gyrus, and between the vMPFC and the right middle frontal gyrus compared to HCs. Similarly, differences were found between pIPL and both right and left angular gyrus. These findings support the notion that the DMN may be closely linked with social impairment in ADHD individuals.
One of the key findings of this study is the decreased FC within the MTL subsystem and the midline core, with the exception of an increase in FC between pIPL and right postcentral gyrus within the MTL subsystem. Moreover, ADHD patients exhibited more loneliness, higher perceived social disability, and smaller social network than HCs, consistent with previous studies in other neuropsychiatric illnesses characterized by significant social impairments. This finding is further supported by the results of our regression analysis, which revealed a significant relationship between DMN dFC and social problems, as well as social composite scores. These results contribute to the growing body of evidence that dFC of the DMN subsystems is critical not just for adaptive human social functioning (Andrews-Hanna et al., 2014b; Mars et al., 2012a) but also for constructive social engagement (Che et al., 2014). This underscores the potential relevance of DMN alterations in understanding social dysfunction in ADHD and, by extension, various neuropsychiatric conditions such as schizophrenia, autism, and major depressive disorder (Fox et al., 2017; Padmanabhan et al., 2017; Saris et al., 2020). Particularly, our findings of negative correlation between within-MTL subsystem's FC (i.e., between PHC and lSFG) and the social composite score lend credence to the view that the MTL subsystem contributes to the ADHD social dysfunction.
The MTL subsystem, comprised of the hippocampus and the parahippocampal cortex (PHC), is involved in mnemonic processes and is active when retrieving previously learned knowledge. The DMN is known to play a role in the construction of self-relevant mental simulations using memories and associations from previous experiences as its building blocks. These simulations are then used by a variety of social cognitive processes, such as reflecting on the past, planning for the future, and interpreting others’ points of view. Accordingly, this finding of altered dFC within PHC-hippocampus may help explain the reductions we observed in ADHD patients' working memory index (mean ± SD = 9.39 ± 1.09) and behavioral memory (mean ± SD = 0.50 ± 0.40).
Moreover, the findings obtained in our study suggest that the ADHD group had significantly lower scores in several cognitive and behavioral domains compared to the HCs group, including FIQ, VCI, PSI, WMI, behavioral memory, learning problems, and social problems. Memory and cognitive deficits are common symptoms of ADHD, often attributed to impairment in executive functions, such as working memory, attention, and inhibition (Brown, 2013). However, recent research suggests that deficits in episodic memory and cognitive control may also play a role in ADHD (Skowronek et al., 2008). These deficits could be related to the inability to connect behavior with past consequences and difficulty in mentally simulating future events.
Studies have demonstrated that the brain regions involved in episodic memory and cognitive control, such as the hippocampus, prefrontal cortex, and parietal cortex, are also involved in social cognition and theory of mind (Zalla & Korman, 2018). Social cognition refers to the ability to comprehend and interpret social cues, while theory of mind involves attributing mental states to oneself and others. Therefore, it is plausible that deficits in social functioning among individuals with ADHD are linked to deficits in episodic memory and cognitive control. These deficits may hinder the ability to understand social cues and make accurate predictions about social outcomes, resulting in difficulties during social interactions.
Furthermore, our results from the multiple linear regression analysis suggest a possible association between DMN's dFC and thought problems, delinquent behaviors, social composite score, learning problems, and behavioral memory. This indicates that abnormalities in the DMN's dFC in children and adolescents with ADHD may contribute to the cognitive and social deficits observed in this population. In other words, while the study's primary goal may not have been to investigate the relationship between memory/cognitive deficits and social functioning in ADHD, there is evidence from our results suggesting that these deficits may be interconnected. Further research is needed to better understand the neural mechanisms underlying these deficits and to develop effective interventions that improve social functioning in individuals with ADHD.
In line with these implications, PHC has been found to be potentially related to the pathology underlying social dysfunction symptoms of subtle cognitive and behavioral disorders such as myotonic dystrophy (Morin et al., 2022). More precisely, these findings are also supported by the negative correlation found between the altered FC between vMPFC and rMFG and the behavioral memory. As a component of the MTL, the vMPFC is engaged, for example, when drawing on previous experiences to help shape present and future emotional states while planning social interactions (Andrews-Hanna et al., 2010). Research into theory of mind (Premack & Woodruff, 1978) and morality have both uncovered altered connectivity between the vMPFC and other DMN areas (such as the TPJ). Atique and collegues (Atique et al., 2011) observed that when mentalizing emotions, the vMPFC exhibited stronger FC. Decety et al. (Decety et al., 2012) found that adults exhibit increased FC in the vMPFC while observing moral deeds compared to adolescents. This may help explain and predict the neurodevelopmental nature and severity of ADHD-related social dysfunctioning symptoms in children as they may persist into adulthood (Breda et al., 2021).
In contrast to our hypothesis, the only increased dFC we found was between the pIPL and right postcentral gyrus within the MTL. This finding illustrates that the pIPL integrates some of the most fundamental and complex cognitive processes necessary for human communication and interaction. These results are congruent with existing literature demonstrating that patients' posterior IPL is actively stimulated when they adopt the mental perspectives of others (Numssen et al., 2021). Increased pIPL FC has also been associated with deficits in praxis and social skills that are often observed in school-age children with autism (Wymbs et al., 2021). Further research investigating the IPL's roles and connections with other brain regions beyond the MTL subsystem is required to provide replications and a better understanding of such an unpredictable increase in FC.
Another key finding is the midline core's reduced dFC (between PCC and right angular gyrus), which was also negatively correlated with the FIQ-score. The PCC, a key component of DMN, is closely linked with an introspective attentional orientation related to mentalizing and emotional processing during rest (R. Buckner et al., 2008) and contextual processing (Baeuchl et al., 2015; Szpunar et al., 2009). Our results are consistent with other evidence from anatomical and imaging studies suggesting the role of the midline core in emotional self-referential cognition and making self-relevant, affective decisions (Andrews-Hanna et al., 2010). Our correlation results were supported by the sample's characteristics of poor learning problems and increased VCI and PSI scores in ADHD patients compared to HCs.
Previous works have also reported cognitive deficits in ADHD individuals, particularly in the areas of working memory, attention, and executive functions (Barkley, 1997; Castellanos & Tannock, 2002; Willcutt et al., 2005). The diminished dFC within the midline core may play a role in these cognitive deficits, as this network is known to be involved in a range of cognitive processes, including self-referential processing, memory retrieval, and attentional control (Andrews-Hanna et al., 2010; R. L. Buckner et al., 2008).
Moreover, studies have shown that ADHD individuals often have difficulty with future-oriented thinking and are less able to consider the consequences of their actions (Barkley, 1997; Sonuga-Barke, 2003). This could be related to the altered dFC within the midline core, as this network is also involved in processing information related to the self, including autobiographical memory. Therefore, it is possible that the altered dFC within the midline core contributes to the cognitive deficits and behavioral problems commonly observed in ADHD individuals. It is important to note that the findings of this study are correlational and do not establish causation. Additional research, such as longitudinal studies or experimental manipulations, is needed to better understand the relationship between altered FC within the midline core and cognitive/behavioral deficits in ADHD individuals.
Surprisingly, we observed no significant connectivity strength of the dMPFC subsystem. Although this subsystem is suggested to be linked to theory of mind, mental simulations, and present-focused thoughts (Andrews-Hanna et al., 2014b), but had a lesser role in reflective thinking about the past or the future (Christoff et al., 2016). Conflicting with our findings, other imaging studies reported altered dMPFC FC when investigating the DMN in other mental disorders with common social dysfunction symptoms. For instance, reduced dMPFC FC was found in schizophrenia (Fan et al., 2020), major depressive disorder (Chen et al., 2020; Zhu et al., 2017) and in introspection of mental states (Wen et al., 2020). Possible explanations for the observed discrepancy include diverse sample sizes or analyses of the corresponding imaging data. It is important to consider that variations in methodology, preprocessing, and statistical approaches may contribute to the differences in findings related to connectivity within the DMN subsystems. Further research is needed to elucidate the precise nature of dMPFC connectivity in both healthy individuals and those with mental disorders, as well as the role of different methodological choices in producing diverse results.
Of note, the absence of significant dMPFC connectivity alterations in our study supports the concept that the DMN is made up of smaller and functionally distinct subsystems. This aligns with the idea that while the DMN is often considered as a single cohesive network, it might be more accurately characterized as a collection of multiple, interconnected subsystems with diverse functional roles. Understanding the unique roles and interactions of these subsystems, as well as how they are affected in different mental disorders, may lead to a more comprehensive understanding of the DMN's role in cognition and behavior.
LimitationsThis study's exploratory and cross-sectional design precludes drawing any definitive causal conclusions; hence, future research and more longitudinal studies should focus on accumulating larger data samples. Although the composite index of social dysfunction used in the present study has certain advantages, as mentioned before, it is still mostly an unreliable stand-in for a subjective proxy for social impairments among children with ADHD. It is difficult, if not impossible, to capture and compress the multiple and complex processes that constitute social processing and functioning to numerical values. Prospective future studies might benefit from an in-depth examination of the composition of social networks, loneliness or the social disability. Deciphering a complicated phenotypic like social dysfunction necessitates more evaluation of all potential causes. A more objective approach to social dysfunctioning might be helpful in balancing subjective self-assessments, even if the utilized questionnaires are validated and particularly intended to research various facets of social dysfunctioning. In this context, it is worth noting that there is a lack of information on the severity of social dysfunction. This study used a composite index of social dysfunction, but did not provide information on the severity of social dysfunction in the participating children. A more detailed assessment of social dysfunction could provide a better understanding of the relationship between brain connectivity and social functioning in ADHD patients.
Furthermore, the adopted subsystem approach to studying DMN in socially dysfunctional ADHD patients may oversimplify DMN as a complex network system. This results in a limited ability to capture the interaction between different subsystems and how they contribute to the overall function of the network. For future research, it may be possible to combine a whole-network analysis with a subsystem analysis in order to gain a better understanding of how the various subsystems interact with each other and how they contribute to the overall function of socially dysfunctional ADHD patients. An alternative approach would be to investigate the role of resting-state networks other than the DMN, such as the salience network and the executive control network.
Additionally, this research utilizes a within-patient design, with all participating children with hyperactivity or attention deficiencies presumably experiencing some level of social disabilities, as this is a disease-related trait. However, this lack of social confidence should not be considered as an extra source of bias in their self-reported social (dys)functioning. Thus, the present study and its conclusions should ideally serve as a starting point or a source of hypothesis establishment for further studies that investigate social dysfunction and its bio-behavioral foundations. On the other hand, the age range of the sample has been limited as we included children and adolescents between the ages of 8 and 16 years. It is unclear whether the findings can be generalized to younger or older individuals with ADHD. Future studies could examine the relationship between brain connectivity and social dysfunction in individuals across the lifespan. More importantly, limiting the study to only male participants is a potential limitation that should be considered. It is well-established that ADHD is more commonly diagnosed in males than females (Biederman & Faraone, 2005) and as such, the results of this study may not be generalizable to female populations with ADHD. Additionally, previous research has suggested that there may be differences in brain structure and function between males and females with ADHD (Quinn & Madhoo, 2014), which could impact the findings of this study if it were conducted solely with male participants. Therefore, future studies should aim to include both male and female participants to ensure a more comprehensive understanding of the neural mechanisms underlying social dysfunction in ADHD.
ConclusionIn summary, our exploratory results suggest a tentative association between increased social dysfunction and reduced DMN connectivity in children with ADHD, particularly in the midline core and the MTL subsystems. These results seem to reveal pertinent, yet preliminary, insights on the neurobiology underpinning social dysfunction in ADHD highlighting DMN connectional abnormalities as a potentially crucial element. A more fine-grained description of DMN and its network dynamics requires additional investigation and validation of these preliminary exploratory results, ideally through multimodal exploration of DMN connectivity and complex network studies (e.g., graph theory). Future studies may reasonably build on these existing findings as a starting point or as a basis for formulating hypotheses, potentially leading to a better understanding of the specific connectivity patterns and their contributions to social dysfunction in ADHD.
Data availableThe data sets generated and/or analysed during the current study are not publicly available due to the Chinese ethics committee regulations but are available from the corresponding author (Hongwu Zeng: homerzeng@126.com) on reasonable request.
The authors extend their sincere appreciation to all the participants who took part in this research. They also extend their gratitude to Dr. Abla Smahi and Dr. Cristina Cañete-Masséd for their valuable contributions and support in guiding and proofreading the manuscript. This work was supported by Natural Science Foundation of Guangdong Province (No. 2022A1515011427), Sanming Project of Medicine in Shenzhen (No.SZSM202011005) from Shenzhen Medical and Health Project.