As two typical types of social rewards, both value affirmation and emotional support could alleviate acute stress response, but it is not clear whether they can impact stress circuit function and regulation through different neural pathways.
MethodSixty-two participants were randomly assigned to the value affirmation, emotional support, and non-reward conditions, then administered an adapted version of the ScanSTRESS paradigm. Participants’ subjective reports of uncontrollability and social evaluative threat were measured to explore the mitigation of stress by social rewards at the behavioral level. Meanwhile, their acute salivary cortisol response to stress was compared among different social reward conditions. Furthermore, we computed linear contrasts for performance (vs relaxation) and reward (vs non-reward) and used psychophysiological interaction (PPI) analysis to explore the impact of social reward on stress circuit function and regulation.
ResultsBoth value affirmation and emotional support conditions reduced subjective reports of uncontrollability and social evaluation threat, but not cortisol response to stress. Furthermore, value affirmation reduced uncontrollability by enhancing putamen activation, whereas emotional support reduced social evaluation threat by enhancing putamen activation. More importantly, during stress, value affirmation enhanced the functional connectivity of the putamen-hippocampus and putamen-angular gyrus (AG), whereas emotional support enhanced the functional connectivity of the putamen-ventrolateral prefrontal cortex (vlPFC) and putamen-temporal pole mid, compared to the non-reward condition.
ConclusionValue affirmation and emotional support alleviated acute stress response in different neural pathways. These findings suggested a precise categorization of social reward in intervention of a range of adverse psychological and physiological responses caused by stress.
People are facing more and more stressful events in their daily lives, such as important exams and interviews, overloaded work, and poor interpersonal relationships. Prolonged exposure to these stressful scenarios can trigger negative emotions such as anxiety and depression, which can further affect people's mental health (Hammen, 2005; Morgan et al., 2001) and may even increase the risk of physical illnesses (Maria et al., 2004; Richter & Hoffmann, 2019; Steptoe & Kivimäki, 2012). Given the negative consequences of stress on people's mental and physical health, how to buffer the stress response has become one of the most important issues (O'Connor et al., 2021).
Social rewards are positive experiences gained during social interactions such as praise, approval, caring, hugs, and smiles (Bhanji & Delgado, 2014), which can buffer a range of adverse psychological and physiological responses caused by stress (Creswell et al., 2005; Phumdoung & Good, 2003; Ren et al., 2019; Sherman et al., 2009; Speer & Mauricio, 2017; Younger et al., 2010). The theory of fundamental dimensions of social judgment suggests that there are two basic content dimensions underlying social interactions, namely agency versus communion (Abele & Wojciszke, 2007). Agency is about showing skills and competitiveness to gain opportunities, whereas communion is related to making social connections with others, both of which are essential for survival (Ybarra et al., 2008). According to this theory, social rewards can be categorized into two typical types including value affirmation and emotional support. Value affirmation is the process of reflecting on important personal values (Cohen & Sherman, 2014) and belongs to the agency dimension, such as praise, approval, etc. Emotional support is emotionally cared for by others (Reblin & Uchino, 2008) and belongs to the communion dimension, such as caring, hugs, smiles, etc.
Previous research found that both value affirmation (Creswell et al., 2005, 2013; Dutcher et al., 2020; Sherman et al., 2009; Spicer et al., 2016) and emotional support (Ditzen et al., 2007; Edens et al., 1992; Heinrichs et al., 2003; Hofer et al., 2018; Jakubiak & Feeney, 2016; Kamarck et al., 1990; Kirschbaum et al., 1995; Lepore et al., 1993; Thorsteinsson et al., 1998) had a buffering effect of the stress response, which was reflected in both the physiological (cortisol, blood pressure, heart rate etc.) and psychological (perceived stress, emotions etc.) dimensions. However, there have been no studies comparing whether there is a difference between value affirmation and emotional support on stress-buffering effects. Further to the study of neural mechanisms, for value affirmation, only one study showed that value affirmation led to greater ventromedial prefrontal cortex (VMPFC) activity, and subsequently less left anterior insula (AI) activity during stress. Furthermore, PPI analyses revealed greater connectivity between the VMPFC and AI during self-affirmation compared to control (Dutcher et al., 2020). For emotional support, it has been shown that emotional support triggered by presenting participants with photographs of their intimate relationships alleviated pain by enhanced functional connectivity of VMPFC with AI and dorsal anterior cingulate cortex (dACC) (Eisenberger et al., 2011; Younger et al., 2010). Unfortunately, few studies have explored the difference between the two types of social rewards in modulating stress neural circuits under the same stress experiment paradigm. Solving this problem can articulate the neural circuits involved in social reward effects during exposure to stressors, and more broadly specify neural reward-based responses to stressful situations.
Stress can influence a wide range of brain regions including the frontal, limbic systems, and basal nucleus, which elicited a series of stressful physiological and psychological responses (Ulrich-Lai & Herman, 2009). The biphasic-reciprocal model (Hermans et al., 2014) suggested that stress increased salience network activation, including the limbic system, which enhanced the body's ability to be alert to and recognize the stressor, and induced a relevant stress response. However, it also suppressed prefrontal-centered executive control network activity, which impaired cognitive function in stressful situations. Arnsten (2009) further illustrated the function of these brain regions in the stress response. For example, dorsolateral PFC (DLPFC) is responsible for attention regulation and action, and ventromedial PFC (VMPFC) has extensive connections with the amygdala and the hypothalamus that generate emotional responses.
Across human and animal studies, reward system activation can reduce stress response, including changes in behavior and physiology (for a review: Dutcher & Creswell, 2018). For example, it has been found that activating the striatum to reward stimuli led to corresponding functional decreases in stress neural responses in human neuroimaging studies (Eisenberger et al., 2011; Younger et al., 2010). Different regions of the striatum (e.g., caudate and putamen) may correspond to different types of rewards. A neuroimaging meta-analysis confirmed that the money reward activity was located in the right caudate while the social reward outcome was more related to the dorsal regions of the striatum, i.e., the bilateral putamen (Bore et al., 2024). It has been shown that either value affirmation (Dutcher et al., 2016; Izuma, Saito & Sadato, 2008) or emotional support (Kraus et al., 2020) can lead to stronger activation of putamen. Furthermore, a previous study indicated that the strength of activation of putamen during stress was not only closely related to reward but also positively correlated with the rate of recovery from the stress response (Hu et al., 2022). Therefore, we hypothesized that both value affirmation and emotional support could further reduce the stress response by activating reward brain regions, especially putamen.
Besides the similarity, value affirmation and emotional support belong to two basic content dimensions underlying social interactions. According to previous research, value affirmation is a behavior that affirms self-worth and can be a way for people to positively process their selves and enhance perceptions of self-integrity or self-competence (Cohen & Sherman, 2014). Related studies have shown that value affirmation was strongly associated with brain regions related to self-processing, including mPFC, PCC, and AG (Cascio et al., 2016; Dutcher et al., 2016). Emotional support usually created a feeling of being closely connected to others and boosted positive emotions, which had strong brain connections to social information processing and emotion regulation, mainly including the temporal pole (Qin & Northoff, 2011; Yi et al., 2018) and vlPFC (Onoda et al., 2009). Importantly, a review of stress brain responses suggested that stress simultaneously affected relevant brain regions responsible for self-processing, social information processing, and emotion regulation (van Oort et al., 2017). Previous studies have found stronger putamen -vlPFC connectivity was correlated with emotion regulation (Tseng et al., 2021), which can further increase active stress coping (Zahniser & Conley, 2018). In addition, during the stressful process of presenting negative picture stimuli to subjects, the functional connectivity between the hippocampus and putamen was associated with depression severity. These findings suggested that the functional connectivity between putamen and stress-related brain regions may facilitate the alleviation of stress by reward. Therefore, we hypothesized that different social rewards would have a modulatory effect on the relevant brain regions affected during stress by increasing the activation of reward brain regions. Specifically, during stress, value affirmation enhances the functional connectivity of putamen to brain regions related to self-processing (e.g., mPFC, PCC, AG), while emotional support enhances the functional connectivity of putamen to brain regions related to social information processing and emotion regulation (e.g., temporal pole, vlPFC).
In the current study, all participants were manipulated to obtain the social reward by the writing task before the stress. Then, an adapted version of the ScanSTRESS paradigm was used to induce an acute stress response during functional magnetic resonance imaging (fMRI) scanning. Overall, the current study would like to hypothesize both value affirmation and emotional support could further reduce the stress response by activating reward brain regions, especially putamen. However, value affirmation enhances the functional connectivity of putamen to brain regions related to self-processing (e.g., mPFC, PCC, AG), while emotional support enhances the functional connectivity of putamen to brain regions related to social information processing and emotion regulation (e.g., temporal pole, vlPFC).
Materials and methodsParticipantsSixty-two participants from a local university in China were recruited via online advertisements (23 male, Mage = 21.16, SD = 1.66). All participants were free of psychological disorders, serious physical illnesses, head injuries, or alcohol and drug abuse. Meanwhile, female participants need to be in the luteal phase and not recently taking hormonal contraceptives as these factors could affect the cortisol response during stress (Allen et al., 2014). They were randomly assigned to one of three conditions, 20 (8 males, Mage = 21.45, SD = 1.43) in the value affirmation condition, 22 (8 males, Mage = 21.17, SD = 1.99) in the emotional support condition, and 20 (7 males, Mage = 20.84, SD = 1.46) in the non-reward condition. This study was approved by the Research Ethical Committee of the corresponding author (IRB NO.H23009).
Experimental procedureTo avoid the effects of biological rhythms on cortisol, all participants were required to participate in the experiment between 1:30pm and 6:30pm and were not allowed to eat, exercise vigorously, drink alcohol, or coffee, or brush their teeth for one hour before the experiment. Participants completed the experiment, one participant at a time, in the above two-hour-long session. The whole experiment was divided into three parts: 1) Reward manipulation part. Participants were required to familiarize themselves with the experimental environment and were required to complete a recall writing task in the reward manipulation part; 2) Stress induction part. Participants finished an adapted version of the ScanSTRESS paradigm in the Scanner, including ScanSTRESS ‘relaxation phase’, Resting state 1, ScanSTRESS ‘performance phase’, Resting state 2, and T1 image; 3) Stress recovery part. Participants took a 30-minute break in the rest room. Subjective reports of uncontrollability and social evaluation threat and saliva were collected several times throughout the experiment, as shown in Fig. 1. To avoid the influence of confounding factors on the stress process, participants were asked to complete the questionnaires for all demographic and independent variables online the day before they came to the laboratory. Each participant received 70 yuan (approximately US$ 10) after completing the whole experiment.
Social reward manipulationParticipants in the value affirmation condition were required to write a word that best reflected their ability or talent (e.g., capable, confident, motivated, efficient, intelligent, hardworking). Then, they need to spend 3–5 min carefully recalling the experience of getting achievements due to this value. Finally, participants were required to write down the recalled experiences on a piece of paper requiring at least 200 words for 15 min. Similarly, participants in the emotional support condition were required to write the name of a person (including family, friends, classmates, teachers, etc.) who regularly helps or supports them in their lives, and completed recall and writing tasks. Participants in the non-reward condition were required to describe a neutral item (e.g., pencils, computers, glue, scissors, notepads, backpacks, etc.) and wrote down its appearance features and use scenarios. After completing the reward task, all participants were required to complete the reward manipulation check.
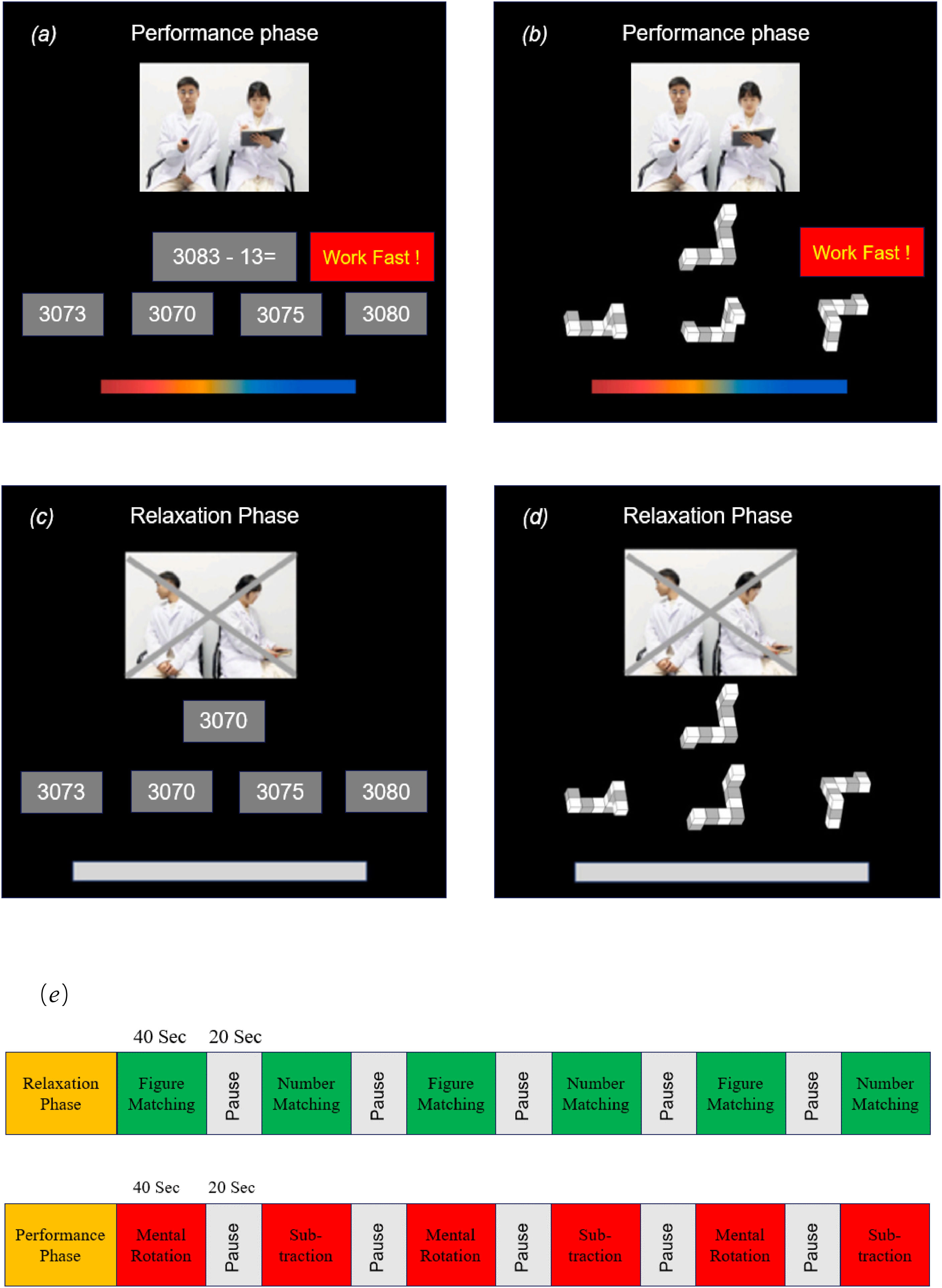
The compact ScanSTRESS paradigmThe ScanSTRESS-compact (ScanSTRESS-C) paradigm adopted in this study proved to be valid in evoking a subjective, endocrine, physiological, and neural stress response (Sandner et al., 2020). The paradigm included a stressful ‘performance phase’ with social evaluation and a control ‘relaxation phase’. The performance phase consisted of two cognitively challenging tasks (mental rotation and subtraction, see Fig. 2a and 2b). During mental rotation task, a three-dimensional geometric figure was presented at the top of the screen. Participants had to correctly select a matching but rotated figure from three alternatives. During the subtraction task, a subtraction task in which 13 consecutively were subtracted needs to be completed. Every item had a time limit, which was adapted to the individual's performance resulting in frequent failure. If the participants could not answer the question in time or correctly, there would be negative feedback shown on the screen as ‘work faster’ or ‘error’ insistently. Furthermore, the performance of the participants throughout the task period was observed by a panel of “experts” consisting of one male and one female. They dressed in a lab coat and kept their facial expressions neutral all the time. A live video of the “experts” was visible on the screen inside the scanner alongside the task. The relaxation phase consisted of two similar but much easier tasks (figure matching and number matching, see Fig. 2c and 2d). Participants were only required to select the same number or figure from the options as the given item with abundant time. The negative feedback from the screen and the “experts” were removed. Furthermore, the “experts” were instructed to look away to remove the social evaluative threat. As Fig. 2e shown, the ScanSTRESS-C consisted of an induction performance phase and relaxation phase, and both phases contained six blocks. All participants completed the relaxation phase first, followed by the performance phase.
ScanSTRESS-C paradigm. (a) Subtraction task during the performance phase (b) Mental rotation task during the performance phase (c) Number matching task during the relaxation phase (d) Figure matching task during the relaxation phase (e) Both the performance phase and relaxation phase consisted of six 40-second block tasks with a 20-second rest period between each block.
To check value affirmation, the 3-item self-value evaluation measure asked participants to rate how much they agreed with the statements on a 5-point scale (1 = not at all, 5 = very much), for example, “I feel confident about my abilities.” (Johnson & Stapel, 2007). Meanwhile, to check emotional support, they were asked to assess the extent to which they feel emotionally cared for on a 5-point differential emotion scale (Burson et al., 2012), including feeling loving, compassionate, and connected.
Salivary cortisol data acquisition and analysesSaliva samples were collected using a sampling device (Salivette, Sarstedt, Germany), and all saliva samples were stored at −20 °C until analysis. We used ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) to detect cortisol in saliva samples. Specifically, 300 ul each of saliva and methanol were mixed 1:1 in a test tube and were mixed for 10 s on a multivortex mixer first. Second, the mixed solution was extracted into a syringe and filtered through a filter membrane into a liquid phase bottle, which needs to be checked for clarity and absence of impurities. Third, the solution was transferred to a numbered location on a 96-well plate and injected into the UPLC–MS/MS system for analysis.
Subjective reports of uncontrollability and social evaluation threatParticipants were asked to indicate their sense of uncontrollability and social evaluation threat (Dickerson & Kemeny, 2004) level with one item each, i.e. 'Please rate the level of uncontrollability/social evaluation threat you feel at this moment', during the stress task on a 7-point Likert scale: 1 (not at all) to 7 (totally).
Confounding variablesTo verify the successful randomization of participants and to rule out the influence of some confounding variables on the social reward and stress manipulations, participants filled out several questionnaires online the day before they came to the laboratory, including Perceived Social Support Scale (PSSS, α = 0.90, Yan & Zheng, 2006), Core Self- Evaluations Scale (CSES, α = 0.76, Judge et al., 2003), Perceived Stress Scale (PSS, α = 0.85, Cohen et al. 1994), The Center for Epidemiological Studies Depression Scale, (CES-D, α = 0.95, Zhang et al., 2011), Trait Anxiety Inventory (T-AI, α = 0.91, Spielberger, 1971). All scales had good reliability and validity.
fMRI data acquisition and preprocessingAll fMRI images were acquired using a 3T Siemens Trio MRI scanner (Munich, Germany). A total of 420 vol functional images were acquired from each participant using a T2-weighted gradient echo-planar imaging sequence during the task (repetition time (TR) = 2 s; echo time (TE) = 30 ms; flip angle (FA) = 90°; slices number = 72; slice thickness = 2 mm; voxel size = 2 × 2 × 2 mm3; field of view (FOV), 224 × 224 mm2). High-resolution T1-weighted three-dimensional (3D) fast-field echo sequences were obtained for anatomical reference (TR = 2.53 s; TE = 2.98 ms; FA = 7°; slices number =192; slice thickness = 1 mm; voxel size = 0.5 × 0.5 × 1 mm3; field of view (FOV), 224 × 224 mm2).
Functional MRI data were processed with MATLAB (Natick, MA) using the DPABI toolbox (Yan et al., 2016). This process included: (1) the 3D DICOM image data of each participant was converted to a 4D NIFTI format; (2) the image data of the first five time points in the scanning sequence were deleted to reduce the effect of the magnetic field instability at the beginning of the experiment; (3) the remaining images were subjected to slice timing (62 are intermediate reference slice) and head motion correction (six parameters); (4) the functional images after head motion correction were aligned to the participant's own structural image; (5) the aligned brain images were then segmented; (6) the functional images were normalized to Montreal standard space (MNI space); (7) spatially smoothed (smoothing) using a 4 mm Gaussian smoothing kernel to improve the signal-to-noise ratio of the image data.
Functional MRI data analysisThe preprocessed data were analyzed using SPM12 software (Statistical Parametric Mapping Software, SPM). A first-level general linear model incorporating the two conditions (performance and relaxation) was built and convolved with the canonical hemodynamic response function and six movement parameters as covariates of non-interest. Then for the Second-level analyses, we first used random effects models to assess any stress effects (performance versus relaxation). The contrast image of the performance condition versus the relaxation condition was obtained using a one-sample t-test, and all activated brain regions were identified using masks from the automated anatomical labeling (AAL) template. Second, to assess social reward effects during the performance phase, a 2 (performance versus relaxation) × 2 (value affirmation or emotional support versus non-reward) analysis of variance (ANOVA) was conducted with the performance and relaxation block as within-subject variables. Last, psychophysiological interaction (PPI) analysis was performed using the CONN toolbox based on SPM12 (http://www.nitrc.org/projects/conn) to explore the impact of social reward on stress circuit function and regulation. Specifically, ROIs (two putamen seeds) were defined in terms of the coordinates of the peak points of the two reward-activated brain regions, and beta values were extracted using the REX toolbox in the space of a 6 mm radius sphere. Then an ROI-ROI functional connectivity analysis was conducted with the stress-activated brain regions.
Statistical analysisAll statistical analyses were performed using SPSS (version 26). A natural logarithm (ln) procedure was applied to correct for skewed raw score distributions in the cortisol data. First, to analyze whether there were differences in stress responses across reward conditions, we conducted a repeated measures ANOVA with conditions as a between-subjects variable and uncontrollability/social evaluative threat/cortisol at different time points as within-subjects variables. Additionally, we used the area under the curve with respect to increases from baseline (AUCI) to analyze overall stress responses, including uncontrollability, social evaluative threat, and salivary cortisol response in the whole experiment, because it considers both time-related changes and overall intensity of the stress response (Fekedulegn et al., 2007). Second, we used a mediation model using social reward (value affirmation vs non-reward or emotional support vs non-reward) as the independent variable, activation of the putamen during the performance phase as the mediation variable, and subjective reports of uncontrollability and social evaluation threat as the dependent variable. Sex and age were included as covariates in the analysis process.
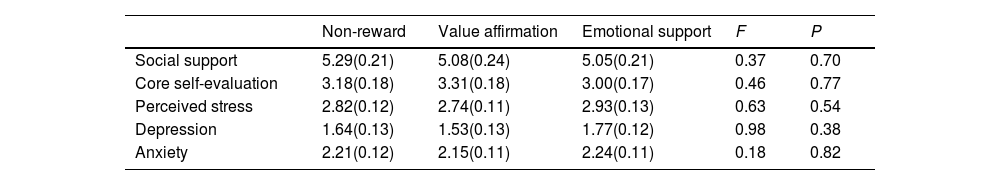
ResultsConfounding variables analysesWe compared reward and non-reward conditions on all confounding variables to verify the successful randomization of participants. As shown in Table 1, there were no significant differences in all confounding variables among the three conditions.
Tests of confounding variables by conditions.
A one-way ANOVA with the condition as the independent variable and each of the reward manipulation check variables as the dependent variable showed that the condition's main effects were significant on self-value evaluation (F (2,59) = 3.58, p < 0.05, ηp2 = 0.11) and differential emotion scores (F (2,59) = 13.86, p < 0.001, ηp2 = 0.32). Specifically, the Post hoc LSD tests revealed the value affirmation condition (M = 3.92, SD = 0.66) was significantly higher than the emotional support condition (M = 3.46, SD = 0.64, p < 0.05) and the non-reward condition (M = 3.44, SD = 0.62, p < 0.05) in the self-value evaluation scores, whereas the emotional support condition was not significantly different from the non-reward condition (p = 0.90); On the differential emotion score, the emotional support condition (M = 3.71, SD = 0.97) was significantly higher than the value- affirmation condition (M = 2.49, SD = 0.57, p < 0.001) and the non-reward condition (M = 2.86, SD = 0.74, p < 0.001), whereas the value affirmation condition was not significantly different from the non-reward condition (p = 0.14). It suggested that the manipulation of the present study was successful for the value affirmation and emotional support reward.
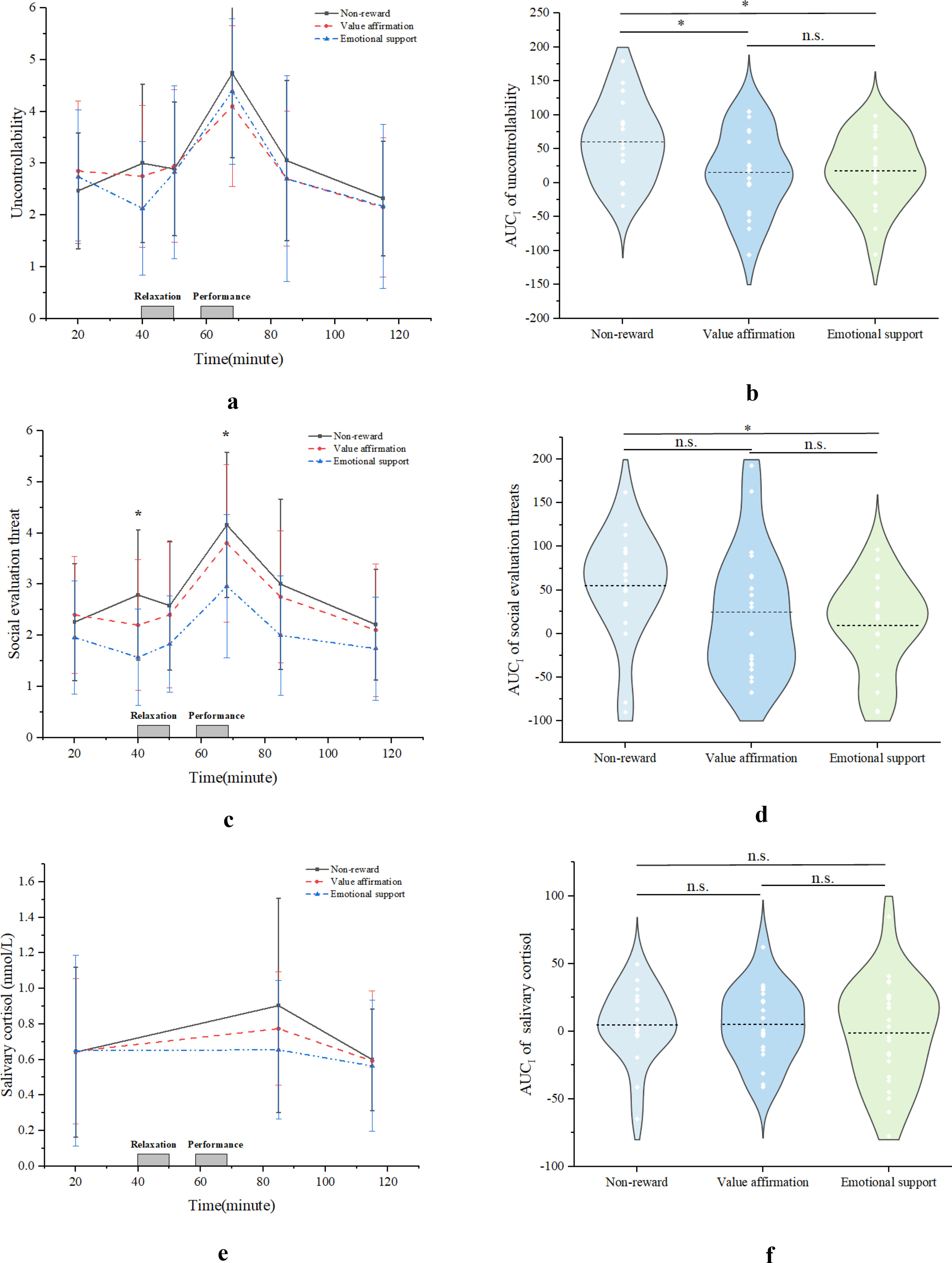
Subjective and endocrine responses to stressUncontrollability/Social Evaluation Threat Compared with non-reward condition, both value affirmation and emotional support reduced uncontrollability during stress, while only emotional support rather than value affirmation reduced social evaluation threat. Specifically, the interaction between uncontrollability time points and conditions (F (10, 112) = 1.37, p = 0.21) was not significant (see Fig. 3a), but the interaction between social evaluation threat time points and conditions (F (10, 112) = 2.02, p < 0.05, ηp2 = 0.15) was significant (see Fig. 3c). Further simple effects analyses revealed that at the peak point (t = 68), the emotional support condition (M = 2.96, SD = 0.30) had significantly lower social evaluation threat than the non-reward condition (M = 4.16, SD = 0.33, p < 0.05, 95 % CI [−2.31, −0.09]), whereas there was no significant difference between the value affirmation condition (M = 3.80, SD = 0.33) and the non-reward condition (p = 0.99, 95 % CI [−1.51, 0.79]).
Additionally, the results showed significant conditions main effect on the uncontrollability AUCI (F (2, 59) = 3.96, p < 0.05, ηp2 = 0.12), and social evaluation threat AUCI (F (2, 59) = 2.86, p < 0.05, ηp2 = 0.10). Furthermore, post hoc tests showed that both value affirmation condition (M = 15.47, SD = 13.34, p < 0.05, 95 % CI [−81.30, −8.81]) and emotional support condition (M = 17.80, SD = 10.84, p < 0.05, 95 % CI [−77.80, −7.65]) had a significantly lower uncontrollability AUCI than the non-reward condition (M = 60.52, SD = 13.41). However, there was no significant difference between the value affirmation condition and the emotional support condition (p = 0.89, 95 % CI [−36.92, 32.26]) (see Fig 2b). In terms of social evaluation of threat, the emotional support condition (M = 9.32, SD = 10.92) had a significantly lower social evaluation of threat AUCI than the non-reward condition (M = 55.29, SD = 14.39, p < 0.05, 95 % CI [−84.76, −7.16]), whereas there was no significant difference between the value affirmation condition (M = 24.75, SD = 16.20) and the non-reward condition (p = 0.13, 95 % CI [−70.63, 9.56) (see Fig 3d).
Cortisol Although the value affirmation and emotional support conditions tended to have lower cortisol responses than the non-reward condition, the interaction effect of cortisol time point (F (4, 58) = 0.66, p = 0.60, ηp2 = 0.02) and condition and the main effect of cortisol AUCI (F (2, 58) = 0.66, p = 0.52, ηp2 = 0.01) were not significant (see Fig. 3e& F).
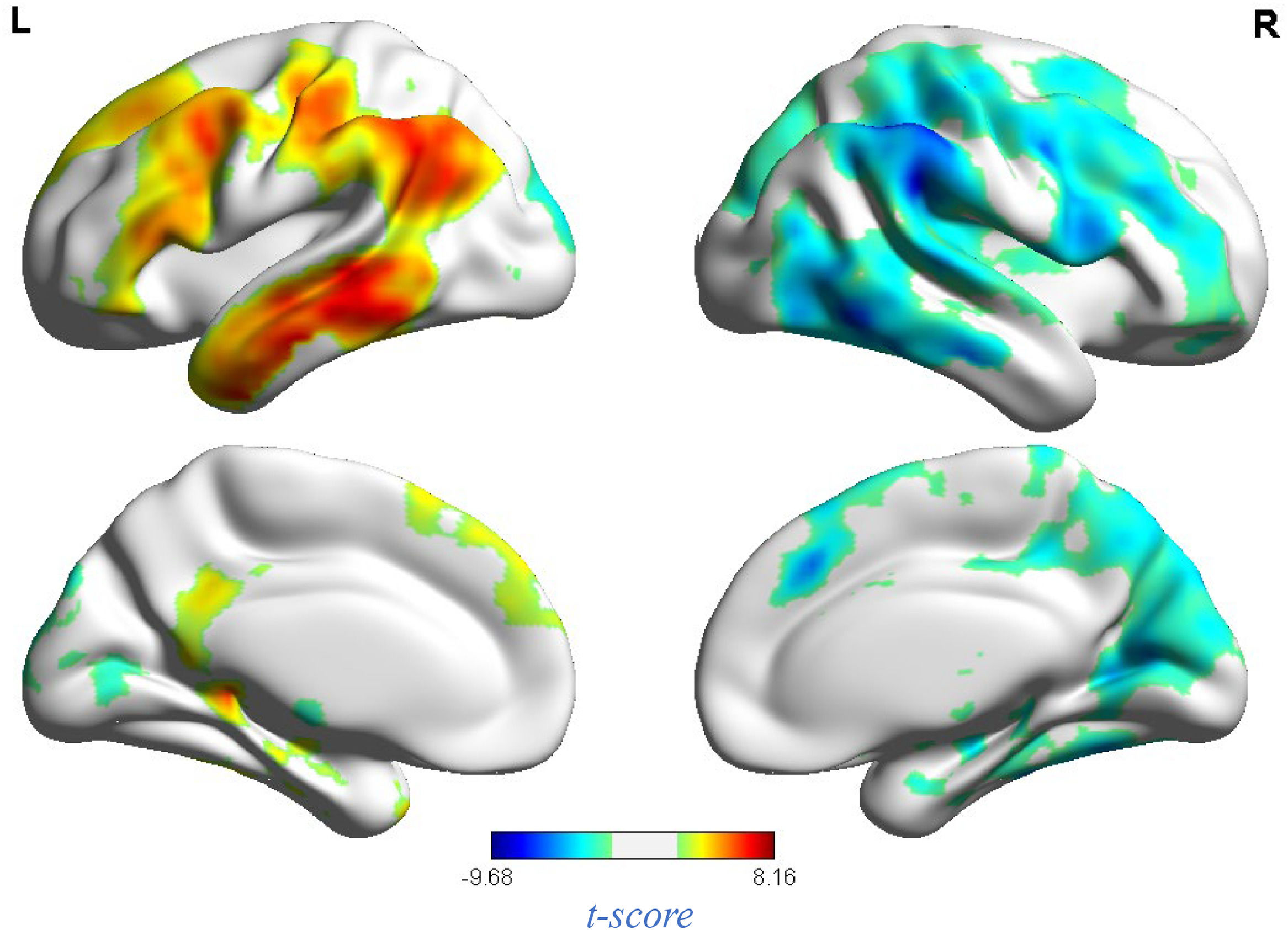
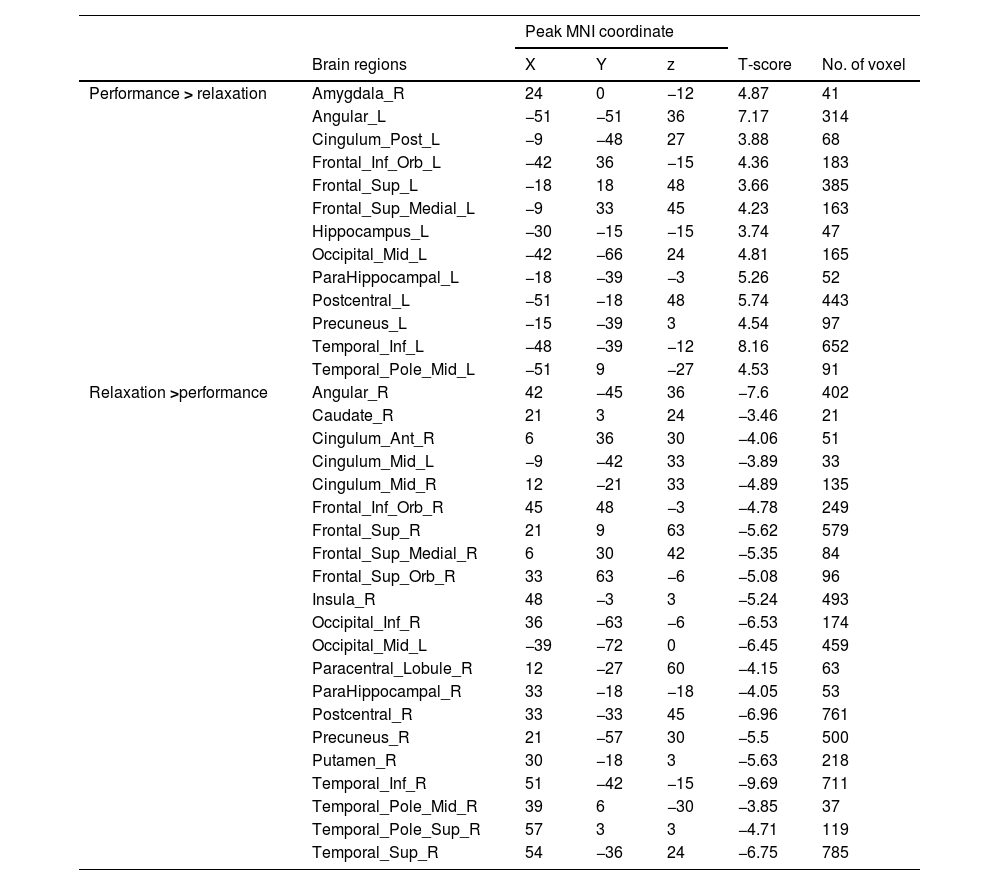
fMRI resultsNeural response to stress induction in the ScanSTRESS paradigmAt the neural level, compared with the relaxation phase, performance phase induced extensive activation, including the Amygdala, Frontal gyrus, Hippocampus, Precuneus, etc. Deactivation was detected in the Caudate, Cingulum, Putamen, temporal pole, etc. (all p values < 0.05, false-discovery rate (FDR)) corrected for the whole brain. For contiguous clusters that spread across multiple regions, the automated labeling atlas (AAL) (Tzourio-Mazoyer et al., 2002) was used to divide clusters to differentiate between structures (Table 2 and Fig. 4).
Whole brain analysis of performance versus relaxation.
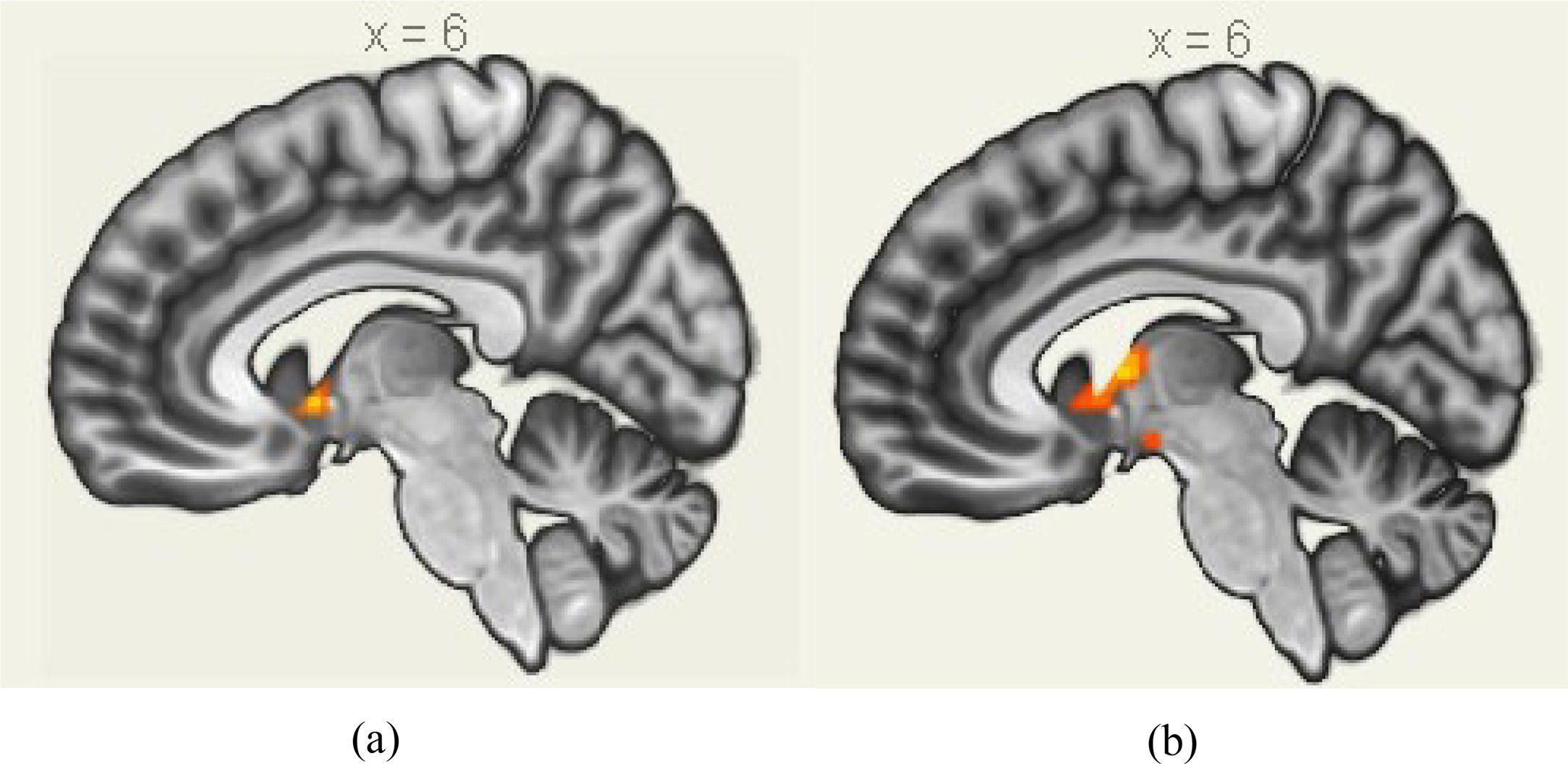
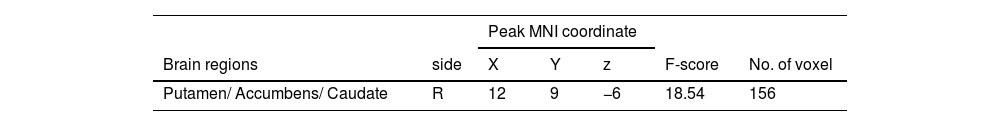
A 2 (performance versus relaxation) × 2 (value affirmation vs non-reward) ANOVA revealed a significant interaction effect (F = 18.54, p < 0.001). Simple effects analyses showed that value affirmation induced a stronger activation of putamen (MNI 12 9 − 6) relative to non-reward in the performance phase (Table 3 and Fig. 5a), but value affirmation did not significantly differ from non-reward whole-brain activation in the relaxation phase.
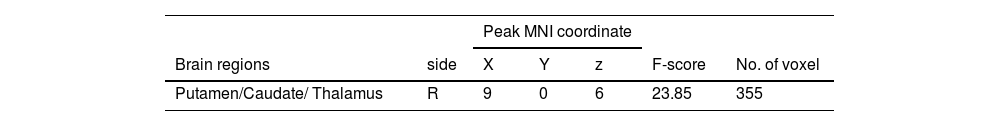
Similarly, the interaction effect of 2 (performance versus relaxation) × 2 (emotional support vs non-reward) ANOVA was significant (F = 23.85, p < 0.001). Simple effects analyses showed that emotional support induced a stronger activation of the putamen (MNI 9 0 6) relative to non-reward in the performance phase (Table 4 and Fig. 5b), but emotional support did not significantly differ from non-reward whole-brain activation in the relaxation phase.
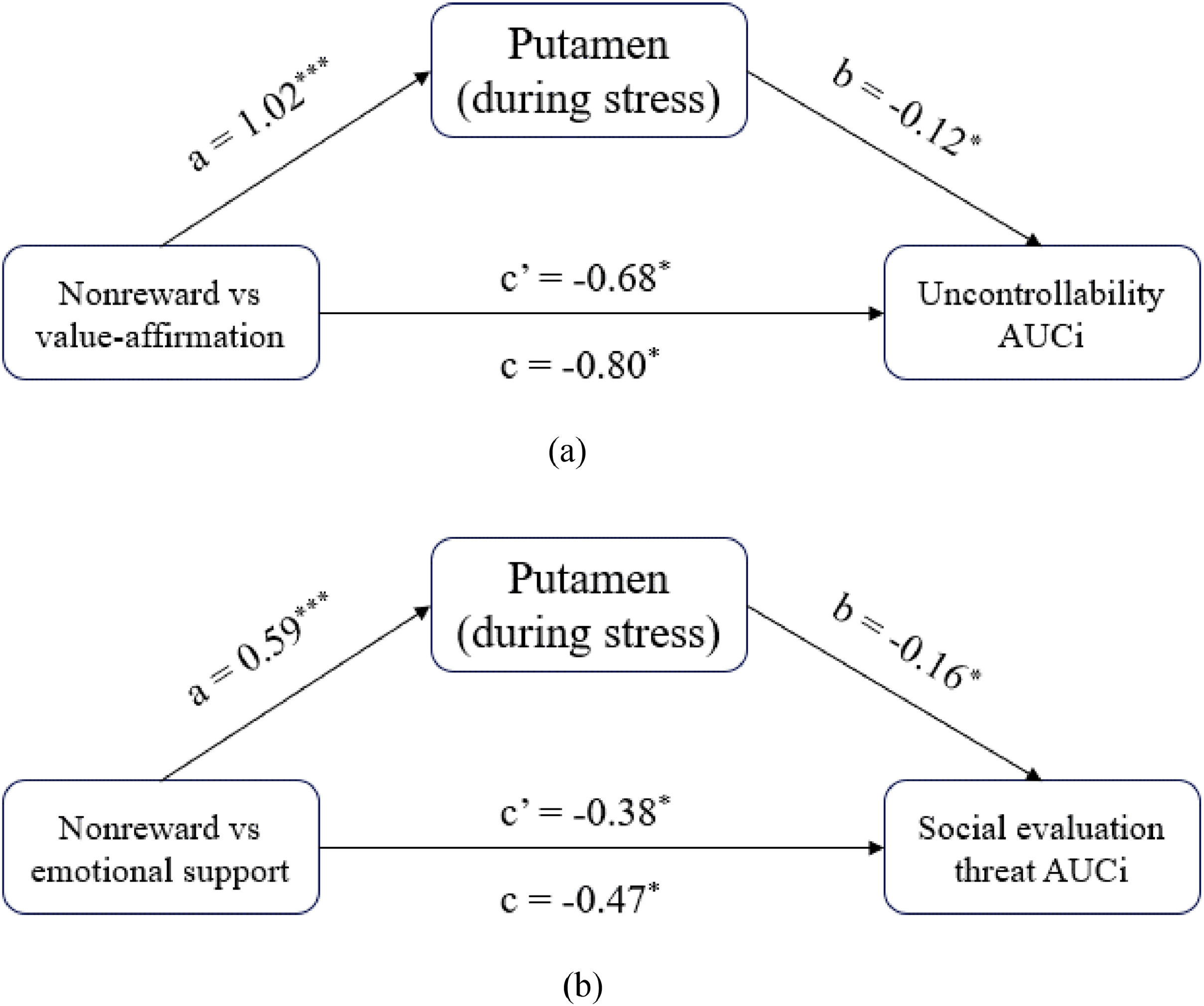
The mediation role of the putamen in the influence of social rewards on stress responsesMediation analyses found both value affirmation and emotional support reduced the subjective stress response by activating putamen. Specifically, examination of the total effect revealed that value affirmation (vs non-reward) significantly predicted uncontrollability AUCI (c = −0.80; 95 % CI [−89.27, −8.32]). The indirect effect of value affirmation on uncontrollability AUCI through putamen activity was significant (ab = −0.12; 95 % CI [−3.14, −1.68]). The direct effect of value affirmation on uncontrollability AUCI after accounting for putamen activity remained significant (c’ = −0.68; 95 % CI [−89.08, −6.37]) (Fig. 6a). Similarly, emotional support (vs non-reward) significantly predicted social evaluation threat AUCI (c = −0.47; 95 % CI [−59.36, −6.35]). The indirect effect of emotional support on social evaluation threat AUCI through putamen activity was significant (ab = −0.09; 95 % CI [−2.16, −1.77]). The direct effect of value affirmation on uncontrollability AUCI after accounting for putamen activity remained significant (c’ = −0.38; 95 % CI [−39.12, −5.46]) (Fig. 6b).
The mediation role of the putamen in the influence of social rewards on stress. (a) the mediation model of value affirmation influencing stress response; (b) the mediation model of emotional support influencing stress response. Note Total (path c), direct (path c′), and indirect (ab) with its components (paths a and b).
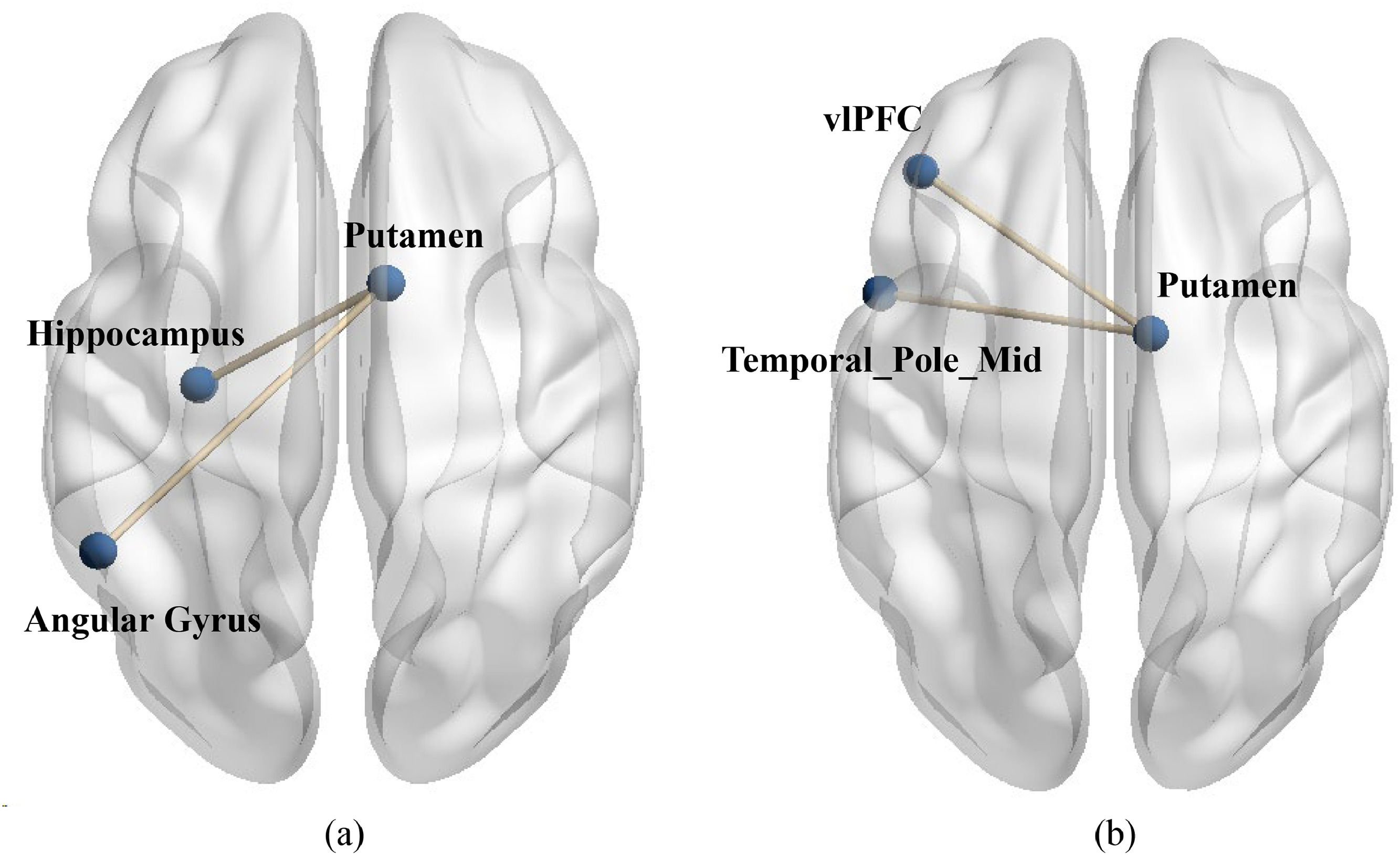
To examine differences in functional connectivity between value affirmation and emotional support, we also conducted two PPI analyses. Specifically, during the performance phase, we used the putamen activated in the two social reward conditions (value affirmation: MNI 12 9 −6; emotional support: MNI 9 0 6) compared to the non-reward condition as seed region to conduct ROI-ROI functional connectivity with all brain regions in the performance-relaxation condition. The results showed that compared to non-reward, value affirmation induced stronger functional connectivity of putamen with the left Angular gyrus (MNI-51 −51 36, t = 7.42) as well as the left hippocampus (MNI −30 −15–15, t = 4.19, see Fig. 7a). Additionally, compared to non-reward, emotional support induced stronger functional connectivity of putamen with the left vlPFC (Frontal_Inf Orb_L, MNI −42 36 −15, t = 4.46) as well as the left temporal pole mid (MNI −51 9 −27, t = 5.17, see Fig. 7b). All results’ p value < 0.001 (uncorrected), corrected by Cluster-level FDR (p < 0.05).
The functional connectivity between putamen and stress brain area during stress. (a) Compared to non-reward, value affirmation induced stronger functional connectivity of putamen with the left Angular gyrus (MNI-51 −51 36, t = 7.42) as well as the left hippocampus (MNI −30 −15–15, t = 4.19). (b) compared to non-reward, emotional support induced stronger functional connectivity of putamen with the left vlPFC (Frontal_Inf Orb_L, MNI −42 36 −15, t = 4.46) as well as the left temporal pole mid (MNI −51 9 −27, t = 5.17).
The results of the present study indicated that both value affirmation and emotional support reduced subjective uncontrollability and social evaluative threat, which is consistent with previous studies (Creswell et al., 2005; Kirschbaum et al., 1995; Thorsteinsson et al., 1998). Additionally, the present study for the first time compared the differences in the neural pathways of the two social rewards modulating the stress response. Specifically, value affirmation reduced feelings of uncontrollability by enhancing putamen activation, whereas emotional support reduced social evaluation threat by enhancing putamen activation. More importantly, during stress, value affirmation enhanced the functional connectivity of the putamen-hippocampus and putamen-angular gyrus, whereas emotional support enhanced the functional connectivity of putamen-vlPFC and putamen-temporal pole mid, compared to the non-reward condition.
Previous studies suggested that the reward system activation could reduce stress response, including changes in behavior and physiology (for a review: Dutcher & Creswell, 2018). In the present study, although both types of reward alleviated the stress response by activating the reward brain region, the putamen, it was interesting to note that the mitigating effects of value affirmation and emotional support on the stress response were manifested in different ways. A sense of uncontrollability is the doubt about one's self-competence that arises when an individual is unable to change the outcome of an event despite his or her best efforts (Thompson, 1981). According to the fundamental dimensions of social judgment, value affirmation belongs to the agency dimension, which can increase individuals' self-efficacy and competence (Taylor et al., 2003), thus effectively reducing the sense of uncontrollability during stress. However, the social evaluation threat refers to the perception of threat that arises when people are confronted with negative feedback from others, often triggering feelings of insecurity (Hughes & Beer, 2013). Emotional support, belonging to the communal dimension, is emotionally cared for by others, which can reduce insecurity by increasing people's sense of belonging (Reblin & Uchino, 2008), thus effectively reducing the social evaluation threat in stress.
Additionally, it is worth noting that while most of the brain regions activated in both conditions belonged to the putamen (value affirmation: 97 voxels; emotional support: 203 voxels), the difference was that the brain regions in the value affirmation condition contained a small portion of the nucleus accumbens (NACC, 16 voxels), whereas emotional support contained a small portion of the thalamus (36 voxels). The NACC and the thalamus play different roles in reward processing. For example, previous studies have shown that the NACC played an important role in the reward anticipation phase (Kirsch et al., 2003), whereas the thalamus was more sensitive to the subjective value of rewards (Komura et al., 2001). A question worthy of future attention is to explore whether there are differences in the neural basis of different social rewards, especially value affirmation and emotional support.
Furthermore, value affirmation led to stronger functional connectivity of putamen-hippocampus and putamen-angular gyrus in the current study. First, the hippocampus played an important role in negative feedback inhibition of the hypothalamic-pituitary-adrenal (HPA) axis in response to stressors, by which the stimulation of the hippocampus decreaseed HPA axis activity (Herman et al., 2005; Ulrich-lai & Herman, 2014). Furthermore, the stronger functional connectivity of the putamen-hippocampus was related to less stress response, especially the cortisol response (Rivera-Bonet et al., 2021). Although significant results that social rewards can reduce cortisol response were not obtained in this study, trends can be seen. This may be due to deficiencies in the selection of time points for cortisol measurements, which would be discussed in limitations. Meanwhile, several studies have shown that the angular gyrus was an important component of the default network and that a strong link existed with self-processing (Andrews-Hanna et al., 2015; Cavanna & Trimble, 2006; Leech & Sharp, 2014). Before the stress task, we triggered positive self-processing, i.e., initiated value affirmation, by having subjects recall and write about their good qualities. This was neurologically manifested as enhanced functional connectivity between the putamen and the angular gyrus, further alleviating the stress response.
Additionally, emotional support led to stronger functional connectivity of putamen-vlPFC and putamen-temporal pole mid in the current study. First, a meta-analysis suggested that vlPFC activation promotes cognitive reappraisal and plays an important role in successful emotion regulation (Buhle et al., 2014; Morawetz et al., 2017). This function of vlPFC was damaged during stress exposure (Yuan et al., 2022). It has also been studied that stronger functional connectivity of the putamen to the vlPFC can facilitate emotion regulation (Tseng et al., 2021). Therefore, in the present study, functional connectivity of the reward brain region putamen to vlPFC was enhanced by asking subjects to recall people and events that were emotionally supportive to them in their lives, which may have enhanced emotion regulation and reduced stress responses. Second, the previous study revealed the temporal pole's putative role in social and emotional processing both in non-human primates and humans (Olson et al., 2007). Emotional support, as a social reward, is a typical socio-emotional message that activates the putamen's activity and further strengthens the functional connection with the temporal pole mid. Ultimately, the stress response was relieved.
Limitation and future directionThere are a few limitations to the present work. First, the mitigating effect of social rewards on stress relief in the present study was shown only in subjective reports and not in cortisol. This may be because only three time points of saliva cortisol were collected in the current experiments, which did not respond to the entire course of the stress response, especially the point of peak cortisol response. Future studies need to increase the time points for cortisol measurements. Additionally, stress physiological responses include Sympathetic Nervous System responses, like heart rate (Kudielka et al., 2004), blood pressure (Tegenthoff et al., 2013), and skin electrical levels (Villarejo et al., 2012), in addition to cortisol secreted by the HPA axis. Only subjective reports and cortisol were focused on in this study, and future studies could further incorporate autonomic responses such as psychological, blood pressure, and galvanic skin. Second, the reward manipulation task was completed before the fMRI scanning in the present study, and the neural responses triggered by reward manipulation were not directly available. Future studies may consider recorded BOLD signals in response to social reward and further investigate the mechanisms by which reward-initiated brain activity modulates neural responses to stress. Third, all participants in this study were a group of college students, and the findings need to be generalized to other age groups with extreme caution. Last but not least, this study included more female participants. Although we controlled for the same sex ratio in each condition and included sex as the covariate, which minimizes the effect of sex on experimental results, it is necessary to include the same number of male and female subjects in the future. This would allow for further analysis of whether there are sex differences in social reward buffering psychological stress.
ConclusionsAcute stress is often accompanied by many adverse health outcomes. The present study suggested that social rewards, including value affirmation and emotional support, reduced acute stress in different neural pathways. Specifically, value affirmation reduced feelings of uncontrollability by enhancing putamen activation, whereas emotional support reduced social evaluation threat by enhancing putamen activation. More importantly, during stress, value affirmation enhanced the functional connectivity of putamen-hippocampus and putamen-angular gyrus, whereas emotional support enhanced the functional connectivity of putamen-vlPFC and putamen-temporal pole mid, compared to the non-reward condition. These findings suggested a precise categorization of social reward in the intervention of a range of adverse psychological and physiological responses caused by stress.