Reduced inhibitory control is a general characteristic of smokers and becomes increasingly pronounced in smoking-related contexts. However, research has rarely considered differences in the effects of various smoking-related cues. To fill this research gap, this study compared the effects of smoking object-related and smoking social-related cues on inhibitory control in smokers.
MethodsWe used a visual Go/NoGo paradigm with three types of long-lasting backgrounds (neutral, smoking object, and smoking social background) to record the error rates, reaction times, and amplitudes of the N2 and P3 event-related potentials (ERPs) by 25 smokers and 25 non-smokers.
Results(1) Smokers displayed smaller NoGo-N2 amplitudes than controls under the neutral background; (2) smokers displayed smaller NoGo-N2 amplitudes under the smoking social background and smoking object background than they did under the neutral background; (3) relative to neutral and smoking object backgrounds, smokers displayed higher commission error rates, shorter reaction times, and larger NoGo-P3 amplitudes under smoking social background.
ConclusionSmoking-related stimuli impair inhibitory control in smokers, especially when these stimuli are socially related.
Tobacco use causes more than seven million deaths annually from various life-threatening disorders, such as heart disease, different types of cancer, pulmonary disease, and exacerbation of multiple chronic health conditions (Land et al., 2022). Despite awareness of its consequences on health and intensive prevention and treatment efforts, a large proportion of the population still initiates and continues to smoke (Agaku et al., 2020). Therefore, targeting the neuropsychological mechanisms of processes associated with the onset and development of smoking is vital.
According to several contemporary models of addiction, dysfunctional changes in cognitive control networks potentially affect the onset and development of addiction (Brand et al., 2019; Wise & Robble, 2020). Inhibitory control is defined as the ability to adaptively suppress inappropriate behaviors, which is an essential component of cognitive control (Brand et al., 2019). Empirical and neuroimaging studies from several people who abuse substances have supported these theoretical perspectives (e.g., Akkermans et al., 2018). However, little is known about the psychophysiological course of inhibition in smokers. Given the sensitivity of event-related potentials (ERPs) to dynamic cognitive processes, the ERP technique may provide a well-established electrophysiological index of reduced inhibitory control and help further investigate the neuropsychological mechanisms of smoking behaviors (Liu et al., 2019).
Previous studies generally assess inhibitory control by a Go/NoGo task, in which participants should respond as quickly and accurately as possible to the frequent “Go” stimuli and inhibit response to the infrequent “NoGo” stimuli (Gao et al., 2020). Responses to infrequent NoGo stimuli reflect the individual's inhibitory control level. Two major ERP components are enhanced for “NoGo” stimuli compared with the “Go” stimuli. The first is the NoGo-N2, a negative waveform emerging approximately 200–300 ms after the stimuli with a frontocentral distribution. The NoGo-N2 is an index for the early stages of the inhibitory process, which reflects the conflict monitoring of irrelevant information (Buzzell et al., 2014). The second is the NoGo-P3, a positive waveform emerging approximately 350–550 ms after stimuli with a parietal-central distribution. The NoGo-P3 is an index of the late stage of the inhibitory process response, which is related to the success of evaluation/decision or response inhibition (Luijten, 2016).
Previous studies have compared the differences in behavioral and electrophysiological data between smokers and non-smokers to investigate whether an impairment of inhibitory control occurs in smokers (Buzzell et al., 2014; Luijten et al., 2011; Yin et al., 2016). For example, Buzzell and colleagues (2014) found that the NoGo-N2 of smokers is significantly smaller than that of the controls, while behavioral performance and NoGo-P3 did not differ between groups. Yin et al. (2016) also found that more NoGo error rates and smaller NoGo-P3 amplitude were observed in smokers relative to non-smokers. Although the results of previous studies are mixed, most seem to suggest that impairment of inhibitory control is a general characteristic of smokers.
According to the Incentive-Sensitization Theory (IST; Berridge & Robinson, 2016; Robinson & Berridge, 2008), inhibitory control is further reduced in special external contexts, such as those involving drug-related cues. Owing to classical conditioning, repeated exposure to drug and drug-related cues in the context of long-term substance use may change the dopaminergic systems involved in reward-based learning (Wise & Robble, 2020). These changes result in markedly increased salience and responsivity to drug-related cues, even when the consequences are deleterious (Berridge & Robinson, 2016). Thus, when smokers are exposed to smoking-related cues, their inhibitory control is further reduced, resulting in increased cravings and smoking behaviors. To support these theoretical assumptions and demonstrate the weakening effect of smoking-related cues on inhibitory control, researchers have used modified Go/NoGo tasks to investigate whether inhibitory control is weaker in smoking-related contexts than in neutral contexts among smokers (Detandt et al., 2017; Kräplin et al., 2019; Li et al., 2021; Luijten, 2016; Luijten et al., 2011; Tsegaye et al., 2021; Wilcockson et al., 2021). In modified Go/NoGo tasks, participants respond to “Go” or “NoGo” signals in a drug-related context (i.e., exposed to a lighting cigarette) or neutral context (i.e., exposed to a toothbrush). Some behavioral studies have shown that smokers have shorter Go reaction times (RTs) and more NoGo response errors in smoking-related contexts than in neutral contexts (Li et al., 2021; Tsegaye et al., 2021). However, some behavioral evidence has not shown that impairment of inhibitory control in smokers is more pronounced under smoking-related backgrounds (Kräplin et al., 2019; Wilcockson et al., 2021). Moreover, electrophysiological evidence has rarely demonstrated such effects. Luijten and colleagues (2011, 2016) used a similar Go/NoGo task and found that NoGo-N2 and NoGo-P3 under two types of cues do not differ significantly. Detandt et al. (2017) showed that smokers made fewer mistakes and displayed a larger NoGo-P3 amplitude in smoking-related backgrounds than in other contexts. Given these inconsistent findings, this study further investigates smokers' behavioral and electrophysiological performance under smoking-related and neutral cues.
Moreover, previous studies have rarely considered the differences in the effects of different types of smoking-related cues on inhibitory control in smokers. Many smokers not only consider their smoking as an individual behavior driven solely by nicotine dependence but consider smoking as a social activity that facilitates social interaction between individuals (Agaku et al., 2018; Mao et al., 2014). Thus, smoking social factors, such as other smokers or environments, may serve as cues that induce an intense craving to smoke through repeated exposure to addictive drugs (Conklin et al., 2013). Owing to the social reward of smoking, smoking social cues may trigger more intense cue reactivity and more frequent smoking behavior than smoking object cues (Martin & Sayette, 2018). A manipulation study in a laboratory suggested that the presence of other smokers exerted a crucial influence on smoking behaviors, showing that smokers increased their smoking to match that of other smokers in a social smoking environment (Reymarova et al., 2015). Further, a cue reactivity to pictures study indicated that smokers responded more impulsively to cues depicting human interaction with cigarettes than cues representing smoking-related objects alone (Haight et al., 2012). However, most studies have focused only on smoking object cues to explore smokers' inhibitory control under smoking-related cues. Few studies investigated the difference in the effect of smoking object and social cues on smokers' inhibitory control. This study included smoking object cues and smoking social cues as smoking-related cues which aims to investigate whether smoking social cues would further impair inhibitory control in smokers relative to smoking object cues. This study will contribute to the comprehension of the effect of social factors on impaired inhibitory control in smokers, and explore the underlying mechanisms of increased smoking behavior in social smoking contexts.
To summarize, the current study aims to investigate the different effects of various smoking-related cues on inhibitory control in smokers. To this end, we adopt a modified visual Go/NoGo task with three types of backgrounds (neutral, smoking object, and smoking social-related pictures) to assess behavioral and electrophysiological responses. We hypothesize that at the behavioral and electrophysiological levels (1) smokers' inhibitory control would display a general deficiency compared with non-smokers; (2) smokers' inhibitory control would be further impaired in a smoking background relative to a neutral background; and (3) smoker's inhibitory control would be especially impaired in a smoking social background relative to a smoking object background.
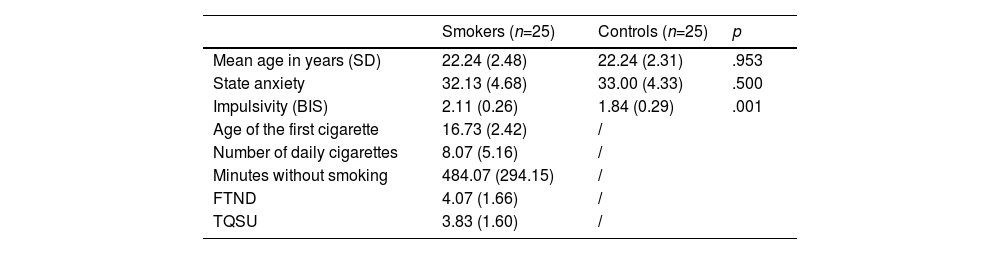
MethodsParticipantsWe recruited 25 Chinese male daily smokers and 25 non-smoking controls (see Table 1 for demographic data) through advertisements on social networking sites. The exclusion criteria included (a) the current presence of a physical or psychiatric illness; (b) past or current drug consumption (except nicotine for the smoking group); and (c) left-handedness. The inclusion criteria for smokers were (a) smoking daily and (b) not currently enrolled in a treatment or program. Smokers were asked to abstain from smoking for two hours before participating in the experiment to avoid floor or ceiling effects from the urge to smoke during the task (Li et al., 2021). We conducted a priori power analysis with G*Power 3.1.9.2. Based on the experimental design and previous study, the power analysis revealed that a total of 48 participants would be required to achieve a power of 0.8 with alpha of 0.05 and a medium effect size (Kräplin et al., 2019). The Institutional Review Board approved the study protocol of the university and all participants provided informed consent before participating. This study was preregistered at: https://osf.io/8kdw4.
Characteristics of the study participants: mean scores (±standard deviation) of the clinical characteristics of smokers and controls.
Participants were asked to report basic information about their smoking behavior, including smoking age of their first cigarette, number of daily cigarettes, and the number of minutes since their last cigarette. Additionally, participants completed the state trait anxiety inventory (STAI) to assess self-reported anxiety (Gauthier & Bouchard, 1993). The Fagerström test for nicotine dependence (FTND) was used to assess nicotine dependence levels (Heatherton et al., 1991). A brief version of the Questionnaire for Smoking Urges (QSU) was used to assess subjective craving for a cigarette (Sanderson et al., 2001). The short form Barratt Impulsiveness Scale (BIS) was used to assess the frequency of several common impulsive or non-impulsive behaviors/traits (Spinella, 2007).
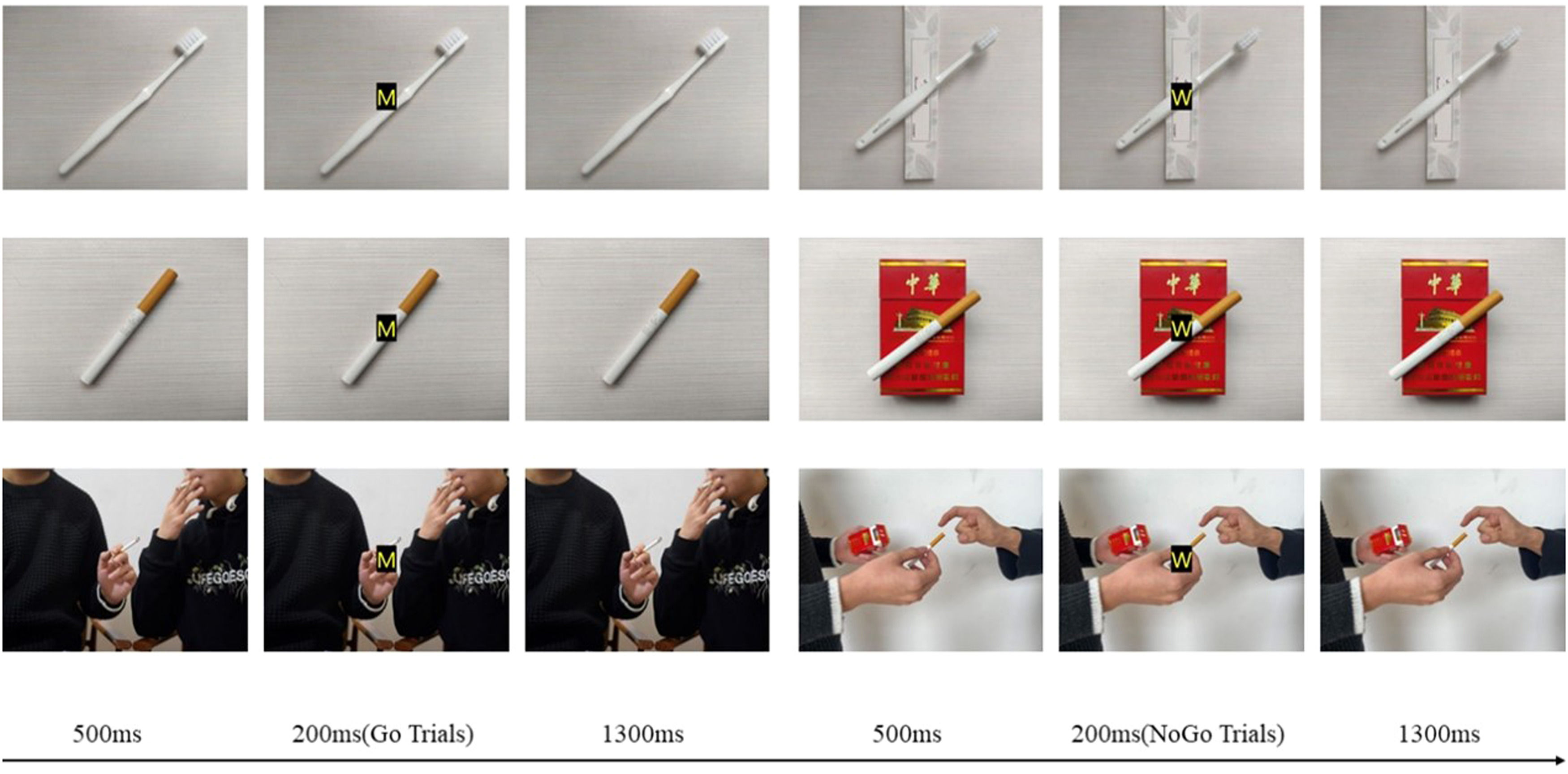
Background cue in Go/NoGo taskThe stimuli consisted of two red capitalized letters (“M” and “W”; the font size is 60, and the typeface is Microsoft elegant black) with boldface to make them as visible as possible, superimposed on three different types of background pictures (displayed on a 15.6-inch monitor).
A trained photographer photographed 30 background pictures in three categories (i.e., ten neutral, ten smoking objects, and ten smoking social) (960 × 720 pixels). Ten smoking objects and ten neutral pictures were common smoking-related/neutral items (e.g., cigarette, toothbrush) and matched in color and shape. Ten social pictures of smoking presented two actors (two males) demonstrating social smoking behaviors. Then, we asked 30 male smokers who did not participate in the formal experiment to assess the background pictures for cigarette-relatedness, social-relatedness on a scale from one (“not at all”) to seven (“extremely”), and emotional level on a scale from zero (“very unpleasant”) to ten (“very pleasant”). Based on the results, we selected six pictures with higher scores as the neutral, smoking object, and smoking social background.
ProcedureParticipants were tested individually and seated in a pleasantly-lit testing room where they completed questionnaires about demographic information. Subsequently, a Go/NoGo task, adapted from Li et al. (2021) was performed, in which the backgrounds were continuously presented on screen for the duration of the task (see Figure 1). During the Go/NoGo task, we instructed participants to respond by making a keypress as fast and accurately as possible when the letter M (Go trials) was presented and to refrain from pressing the button when the letter W (NoGo trials) was presented. The “M” or a “W” was laid over long-lasting backgrounds. During each trial, first, a background was presented without a letter for 500 ms. Then, the letter M or W appeared on the background screen for 200 ms, after which the initial background screen was shown (1300 ms). Thus, the subjects could press the button for a maximum of 1500 ms before the letter appeared.
The formal experiment comprised three blocks (neutral background block, smoking social object background block, and smoking social background block). Two different pictures of the same background type were presented in a block. Each block had 200 trials, divided into 150 Go trials (75%) and 50 NoGo trials (25%). The order of the blocks was counterbalanced across participants. Each block began with the presentation of a red fixation cross for 1000ms, requiring attention to focus. We asked participants to keep watching the center of the screen and avoid moving and blinking during the task to reduce the interference caused by movements. Participants were encouraged to take short breaks (approximately one minute) between the blocks.
Electroencephalographic recording and analysisElectroencephalographic (EEG) data were recorded using a 64-channel amplifier (based on the 10–20 system; Brain Products, Gilching, Germany) with a sample rate of 500 Hz. The reference electrode was set as FCz. A vertical electrooculogram (EOG) was collected from the external canthi of the right eye. Scalp impedances were maintained below 10 kΩ. The EEG data were analyzed using a Brain Vision Analyzer (v.2.1, Brain Products, Gilching, Germany). In offline analyses, the reference electrode was converted to an average reference. The data were then filtered with a band-pass of 0.1–30 Hz. An independent component analysis was performed to correct eye movements and blinks. Then, the data were segmented into epochs of 1600ms. The data epochs exceeding ±100μV were removed. The mean 200ms pre-stimulus (“W” or “M”) period was used as the baseline. Finally, the signals related to the target stimulation for each scalp site were averaged. Hence, the mean number of accepted trials exceeded 30 for each target stimulation.
This study focused on the maximum peak amplitudes and latencies of the N2 and P3 components associated with inhibitory control. The most negative peak value was approximately 200∼300ms after stimulus onset for N2, and the most positive peak value was approximately 350∼550ms for P3. The peak amplitudes and latencies of N2 and P3 in the three sets of data in the time windows of N2 and P3 respectively were automatically measured.
Statistical analysisAll analyses were performed using SPSS 22.0 and repeated-measures ANOVA were employed to analyze the behavioral outcomes of performance (omission error rates, commission error rates, and reaction times (RTs)) on the Go/NoGo task, and ERP (N2 and P3) as the index of inhibitory control. Analyses were applied to the average peak amplitudes or peak latencies at the frontocentral electrodes (i.e., Fz, FCz, Cz, and CPz) (Gao et al., 2020; Luijten et al., 2011). The group was the between-subject factor, and background (neutral, smoking object, and smoking social background) and condition (Go, NoGo) were within-subject factors. All F-ratios associated with repeated-measures factors were assessed using the Greenhouse-Geisser procedure. Bonferroni adjustments were performed as post hoc analyses.
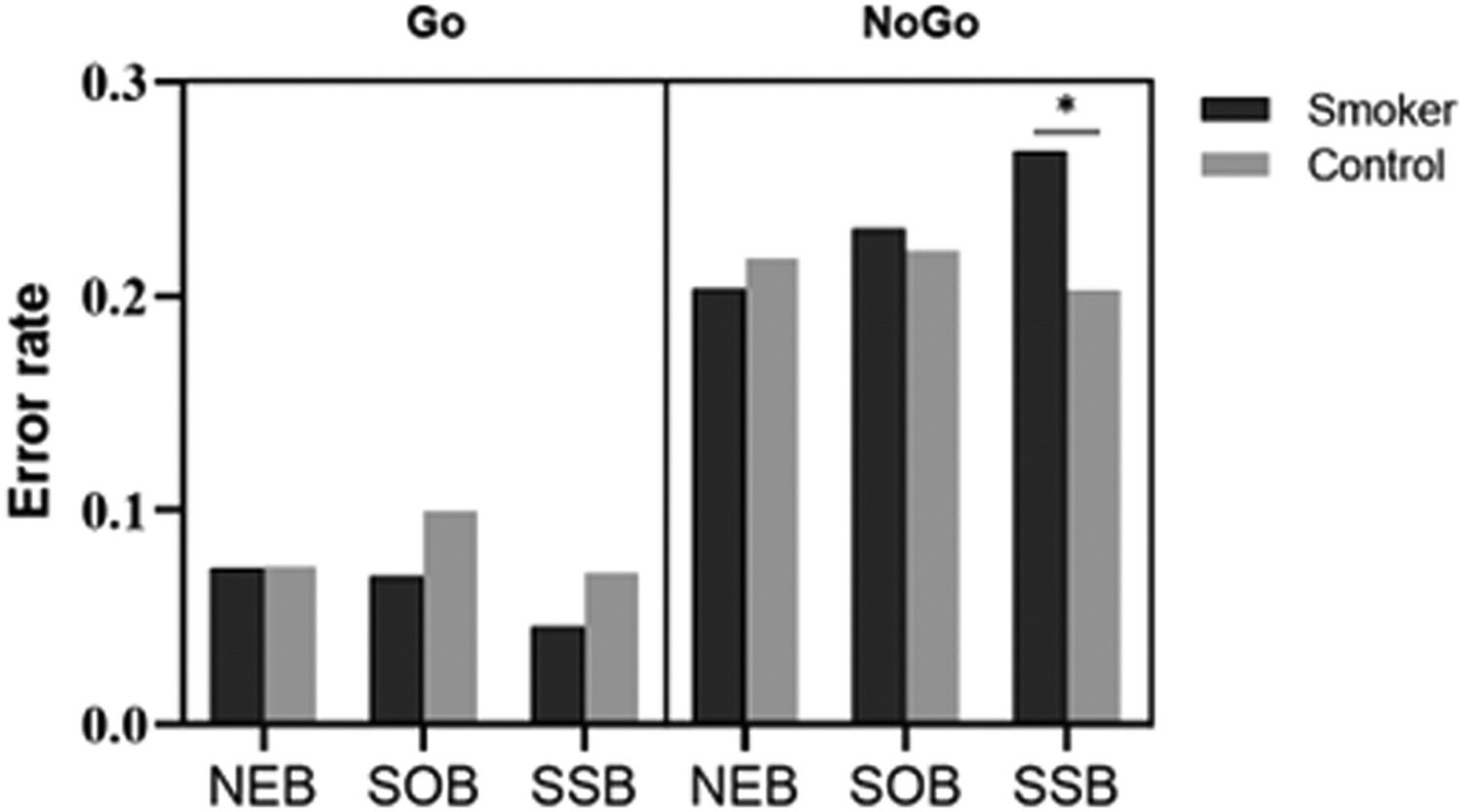
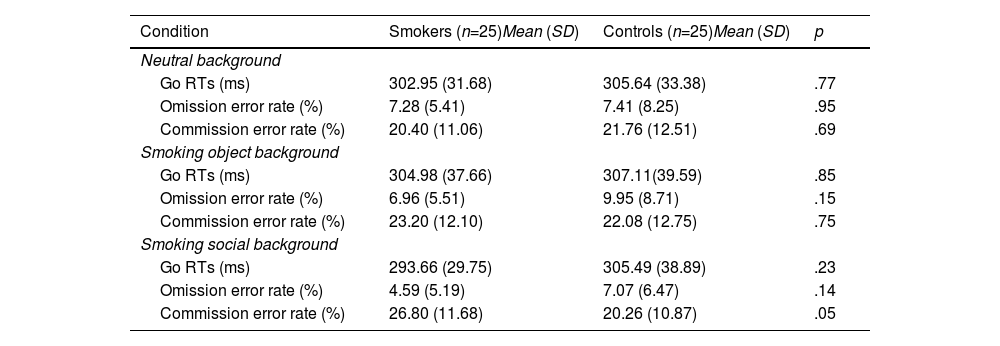
ResultsBehavioral resultsFigure 2 and Table 2 report the behavioral results (RT on Go trials and error rates) for smokers and controls in the neutral background (NEB), smoking object background (SOB), and smoking social background (SSB).
Reaction times on Go trials, omission, and commission error rate for two groups in the three backgrounds.
*Commission error rate in smoking social background is significantly different (p <.05) from the two other backgrounds for smokers.
For RT, the ANOVA revealed a significant main effect for condition (F(2,47)=127.21, p=.01, ηp2=.182). An interaction of group × background was significant (F(2,47)=3.43, p=.04, ηp2=.127). Post hoc analyses showed that only smokers responded faster in smoking social background (MSSB=293.67ms) than in other backgrounds (p=.01, MSOB=305.00ms and MNEB=302.95ms), and there was no difference between the RT of controls in different backgrounds (MSSB=305.49ms, MSOB=307.11ms and MNEB=305.64ms).
For error rates, the ANOVA revealed a significant main effect for condition (F(1,48)=127.21, p<.001, ηp2=.726). An interaction of condition × background was significant (F(2,47)=6.08, p<.001, ηp2=.206). Post hoc analyses showed that participants made fewer omission errors in smoking social background (MSSB=5.83%) than in other backgrounds (ps<.01, MSOB=8.45%, and MNEB=7.35%), and there was no difference between the omission error rates of neutral and smoking object backgrounds (p=.10).
A triple interaction of condition × group × background was significant (F(2,47)=4.36, p=.02, ηp2=.156). Post hoc analyses showed that smokers incurred a higher commission error rate in smoking social background (MSSB=26.80%) than in other backgrounds (ps<.05, MSOB=23.20%, and MNEB=20.40%), and there was no difference between the error rates of neutral and smoking object backgrounds (p=.38). We found no difference among the commission error rates of controls with different backgrounds (MSSB=20.56%, MSOB=22.08%, and MNEB=21.76%). In addition, smokers incurred higher commission error rates than controls only in the smoking social background (p=.05, Msmokers=26.80%, and Mcontrols=20.56%).
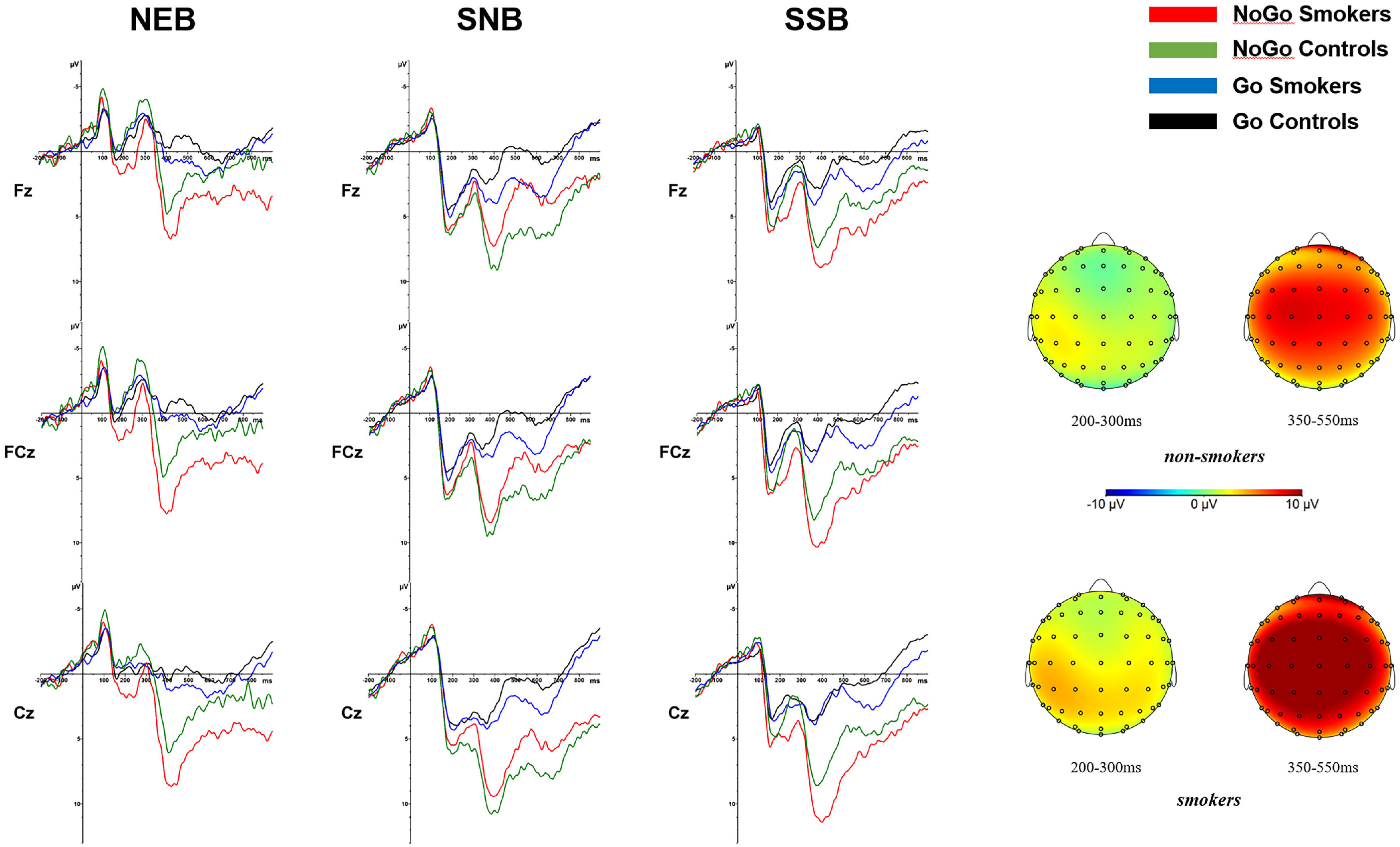
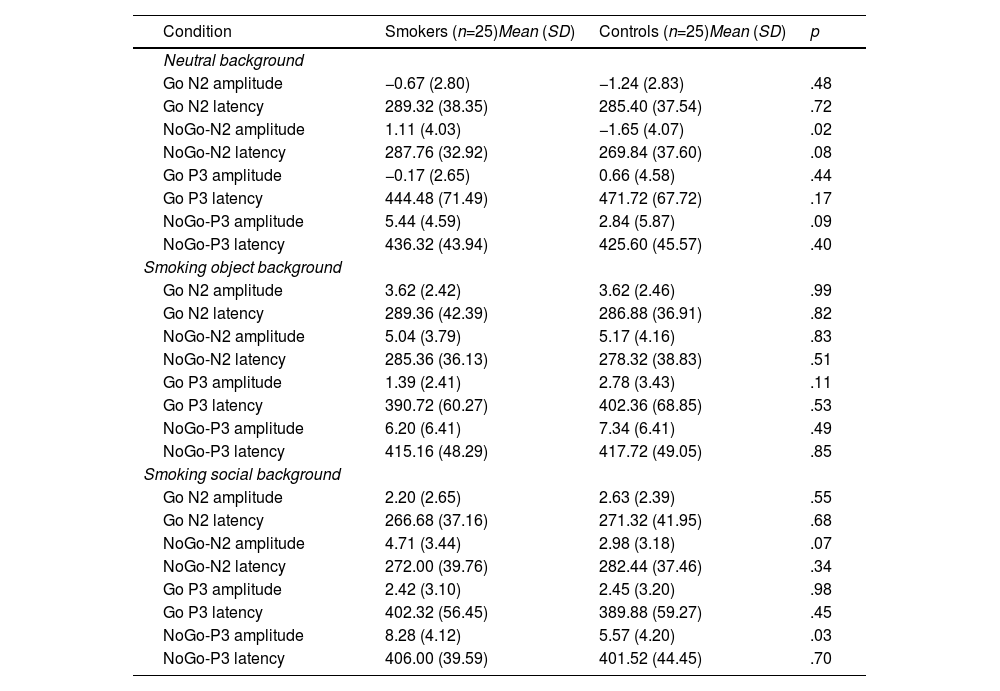
ERP resultsFigure 3 and Table 3 show the ERP results (N2 and P3 amplitudes and latencies) for smokers and controls in the neutral background (NEB), smoking object background (SOB), and smoking social background (SSB).
N2 and P3 amplitude and latency by condition (Go, NoGo) for two groups in the three backgrounds.
*NoGo P3 amplitude in smoking social background is significantly different (p <.05) from the two other backgrounds for smokers.
For the N2 amplitude, the ANOVA revealed a significant main effect for condition (F(1,48)=16.05, p<.001, ηp2=.251) and background (F(2,47)=67.48, p<.001, ηp2=.742). An interaction effect of condition × group was significant (F(1,48)=5.03, p=.03, ηp2=.095). Post hoc analyses showed that in smokers, the negative deflection of N2 amplitude in the NoGo condition (MNoGo =3.62 mV) was smaller than that in the Go condition (p<.001, MGo=1.71 mV), while the difference between the N2 amplitudes of the NoGo and Go conditions was not significant (p=.22, MNoGo=2.21 mV, MGo=1.67 mV) in the control group. A triple interaction effect of condition × group × background was significant (F(2,47)=4.38, p=.02, ηp2=.157). To understand the background effect of the triple interaction effect, post hoc analyses showed that the negative deflection of NoGo N2 amplitudes for smokers in the smoking object and smoking social background (MSOB=5.05 mV and MSSB=4.72 mV) was smaller than that in the neutral background (p<.001, MNEB=1.11 mV), and the difference between the NoGo N2 amplitudes of the smoking object and smoking social background was not significant (p=.66). For the group effect of the triple interaction effect, the negative deflection of NoGo N2 amplitude for smokers was smaller than that of controls in the neutral background (p=.02, Msmokers=1.12 mV, and Mcontrols=-1.65 mV), and the difference between the NoGo N2 amplitudes of the two groups was not significant in smoking object and social backgrounds (ps>.10).
For the N2 latency, the ANOVA only revealed a significant main effect for the background (F(2,47)=5.42, p=.008, ηp2=.187), showing that N2 was shorter in the smoking social background (MSSB =273.11ms) than in other backgrounds (ps<.05, MNEB=283.08ms and MSOB =284.98ms).
P3 effectsFor the P3 amplitude, the ANOVA revealed a significant main effect for condition (F(1,48)=68.60, p<.001, ηp2=.588) and background (F(2,47)=12.94, p<.001, ηp2=.355). An interaction effect of condition × group interaction was significant (F(1,48)=4.12, p=.05, ηp2=.079). Post hoc analyses showed that the P3 amplitude in the NoGo condition (MNoGo =6.64 mV) was larger than that in the Go condition (p<.001, MGo=1.21 mV) for smokers. An interaction effect of group × background was significant (F(2,47)=5.47, p<.01, ηp2=.189). Post hoc analyses showed that P3 amplitude in the smoking social background (MSSB=5.35 mV) was larger than that in the smoking object and neutral background (ps<.05, MSOB=3.79 mV and MNEB=2.63 mV) in smokers, and there was no difference between the P3 amplitudes of the smoking object and neutral background (p=.09). A triple interaction of condition × group × background was significant (F(2,47)=3.49, p=.04, ηp2=.129). For the background effect of the triple interaction effect, post hoc analyses showed that the NoGo-P3 amplitude of smokers in the smoking social background (MSSB=8.28 mV) was larger than that in the smoking object and neutral background (ps<.05, MSOB=6.20 mV and MNEB=5.44 mV), and the difference between the NoGo-P3 amplitudes of the smoking object and neutral background was not significant (p=.44). For the group effect of the triple interaction effect, the NoGo-P3 amplitude for smokers was larger than that of controls in the smoking social background (p=.03, Msmokers=8.28 mV, and Mcontrols=5.57 mV), and the difference between the NoGo-P3 amplitudes of the two groups was not significant in neutral and smoking object backgrounds (ps>.10).
For the P3 latency, the ANOVA only revealed a significant main effect for background (F(2,47)=21.80, p<.001, ηp2=.481), showing that P3 appeared later in the neutral background (MNEB=444.53ms) than in other backgrounds (ps<.001, MSSB=399.93ms and MSOB =406.49ms). An interaction effect of the condition × background was significant (F(2,47)=5.58, p<.01, ηp2=.192). Post hoc analyses showed that NoGo-P3 appeared later in the neutral background (MNEB=430.96ms) than in other backgrounds (ps<.05, MSSB=403.76ms and MSOB =416.44ms).
DiscussionThe present study investigated the effects of smoking social and object cues on smokers' inhibitory control by using a modified Go/NoGo task combined with ERP recordings. The results indicated that, first, the NoGo N2 amplitudes of smokers were smaller than that of controls in the neutral background. This result supports hypothesis 1, indicating a general impairment of inhibitory control in smokers compared with non-smokers. The N2 is a particularly sensitive index of early conflict detection, and a smaller N2 amplitude reflects the reduced monitoring and detection processes of inhibitory control (Buzzell et al., 2014). Previous studies have also shown that compared with controls, a reduced N2 amplitude was displayed for smokers in Go/NoGo tasks (Buzzell et al., 2014; Detandt et al., 2017; Luijten et al., 2011). These results demonstrate that a deficiency in inhibitory control is an essential characteristic of nicotine dependence.
Second, the results showed that smokers exhibited higher commission error rates, shorter RTs, larger NoGo P3 amplitudes, and smaller NoGo N2 amplitudes in the smoking-related background relative to the neutral background. This result supports hypothesis 2, indicating that smoking-related cues could impair inhibitory control in smokers. The P3 component represents a later stage of the inhibition process of the motor system in the premotor cortex. Usually, P3 amplitudes increase with the number of cognitive resources needed for inhibitory control (Luijten, 2016). The more intense the inhibitive processes in the paradigm of measuring inhibitory control, the larger the P3 amplitudes (Luijten, 2016). Previous studies have also reported inhibitory control in smokers would be impaired in a smoking-related context. From a neurophysiological perspective, Detandt et al. (2017) found that smokers displayed a larger NoGo P3 amplitude in a smoking-related context than in a neutral context. The IST (Berridge & Robinson, 2016; Robinson & Berridge, 2008) may explain the effect of smoking-related cues on control-related process. Long-period repeated drug-taking behavior leads to changes in the reward and executive control system, resulting in a bias of attentional processing toward drug-related stimuli and an inability to control the pathological motivation to approach drug and drug-related stimuli (Wise & Robble, 2020). Thus, when smokers are exposed to smoking-related cues, automatic activation of the impulsive system contributes to dysfunctional changes in inhibitory control and approach behaviors toward smoking-related cues.
Third, the results showed that smokers had higher commission error rates, shorter RTs, and displayed larger NoGo-P3 amplitudes in the smoking social background than the smoking object background. This result supports hypothesis 3, indicating that smoking social cues further impaired smokers' inhibitory control compared to smoking object cues. Previous studies have also provided ERP evidence supporting the view that different drug-related cues produce different cue-induced reactivities. In line with our results, Zheng et al. (2020) found that heroin addicts displayed a larger P3 amplitude in response to cues of drug action. For smokers, other smokers and smoking environments may serve as smoking-related cues, directly affecting their cue-induced reactivity (Conklin et al., 2013). Owing to the socializing function of smoking, smokers appeared to experience more difficulties inhibiting the urge to smoke in smoking social contexts than in object contexts (Shiffman et al., 2015). An ecological momentary assessment study suggested that smokers were more likely to smoke in contexts where they received a cigarette from other smokers than in contexts where no such event occurred (Waring et al., 2020). The present study, combined with the results from the studies mentioned above, showed that the larger facilitatory effects of smoking social contexts on smoking behaviors compared to object contexts might be owing to the smoking social cues further weakening smokers' inhibitory control.
The further weakening effect of smoking social cues may be explained as follows. First, smoking social cues may be more rewarding than smoking object cues (Shiffman et al., 2015). Smokers typically engage in social smoking to meet their physiological (nicotine dependence) and sociability requirements (Moran et al., 2004). Smoking with others fosters relationships between family members, peers, and business associates (Agaku et al., 2018). Thus, smoking social cues not only provide a physiological reward but also exist as social rewards, while smoking object cues are only associated with physiological rewards that satisfy nicotine dependence (Conklin et al., 2019). Second, stimuli with higher rewarding value can attract and capture attention better in substance use disorders (Volkow & Morales, 2015). According to Social Motivation Theory (Chevallier et al., 2012), in much the same way that negative signals (e.g., threats) capture attention, potentially beneficial or rewarding information is prioritized. Behavioral studies have shown that smokers react faster to smoking-related cues depicting humans interacting with smoking-related objects than smoking-related objects alone (Haight et al., 2012). Third, attentional bias toward smoking-related social stimuli consumes cognitive resources and weakens cognitive control (Wilcockson et al., 2021). According to the strength model of self-control, cognitive processes such as attention would occupy the self-control resources, resulting in larger P3 amplitude in control-related tasks (Baumeister et al., 2007; Luijten, 2016). Thus, smoking social cues with higher reward values capture attention and impair inhibitory control more than smoking object cues with lower reward values.
This study contributes to the literature and theoretical models. Many smoking-related factors, such as smoking objects, smoking partners, smoking social environments, can serve as smoking-related cues which induce robust craving to smoke. (Conklin et al., 2013). To investigate the smoking-related cues on cognitive process, previous research has indicated the significant weakening role of smoking-related context cues on smokers' inhibitory control (e.g., Brand et al., 2019; Robinson & Berridge, 2008). However, most previous research has focused on the effect of smoking object cues on inhibitory control in smokers. Little is known about the effect of smoking social cues and the differences in the effects of different types of smoking-related cues. This study has investigated the electrophysiological mechanisms underlying the effect of smoking social and object cues on smokers' inhibitory control and indicated the further weakening effect of smoking social cues. The results may demonstrate that apart from pharmacological factors, social factors would also affect the cognitive process in smokers.
This study has practical implications for smoking cessation interventions. First, it suggests that individuals attempting to quit smoking may benefit from being protected from smoking-related cues, mainly smoking social cues including smoking partners and contexts. Second, smoking cessation clinics could consider implementing strategies to enhance inhibition control among smokers under smoking-related backgrounds, which may potentially strengthen their resistance to cigarettes. Third, it is recommended that government and social media propagate reasonable socializing methods to potentially decrease the prevalence of unreasonable beliefs for smokers, such as the belief that "smoking contributes to socializing". However, further research is needed to establish the effectiveness of these interventions.
Despite its contributions, this study has some limitations. First, the participants are young male smokers with light-to-medium dependency levels. Future research should consider gender and nicotine dependency differences to improve the generalizability of the results. Second, the social situation setting of smoking social cues is rudimentary. It is undeniable that the smoking social situation has a non-negligible effect on cue reactivity in smokers (Conklin et al., 2019). Thus, increasing diversity of smoking social cues is should be addressed in future research. Third, only the effect of external context stimuli on smokers' inhibitory control is examined. According to the Addiction Circuity Model (Volkow & Morales, 2015), inhibitory control in addicts may have been simultaneously affected by the external context and individual factors (e.g., emotion and motivation). Hence, future studies should verify the combined effect of smoking social cues and individual factors such as smoking social motivation on smokers' inhibitory control.
ConclusionThis study is the first to employ electrophysiological measures to investigate the effect of smoking social cues on inhibitory control in smokers. Based on addiction theories such as the Incentive-Sensitization Theory, inhibitory control in smokers is not only generally impaired but also further weakened in smoking-related cues. However, previous theories and research have rarely compared the effects of different types of smoking-related cues on inhibitory control. This study employs ERP technology to test inhibitory control for smokers and non-smokers in neutral, smoking object and smoking social backgrounds. The results support existing addiction theories and, importantly, reveal that smoking-related cues with social stimuli further impair the inhibitory control in smokers relative to cues with only smoking-related objects. This study suggests that social factors of smoking behaviors should be considered in the formulation and promotion of tobacco control policies in the future.
CRediT authorship contribution statementBoqiang Zhao: Conceptualization, Methodology, Software, Formal analysis, Data curation, Writing – original draft, Writing – review & editing. Haide Chen: Conceptualization, Supervision, Project administration, Funding acquisition.
Project supported by fund for building world-class universities (disciplines) of Renmin University of China, and National Natural Science Foundation of China under Grant (number 31800946).