Although psychological research indicating the synchronous activities can promote interpersonal cooperation, thus far there is no direct evidence that two-person synchronous exercise effectively enhances interpersonal cooperative behaviors in Physical exercise field. This suggests that, although synchronization phenomenon is widespread in sports and is considered a potential tool for enhancing teamwork, its specific effects and functioning mechanisms still need to be clarified by further scientific research. This study intends to use two-person synchronized cycling exercise to investigate the synchronized exercise effect on interpersonal cooperative behavior and its underlying neural mechanisms.
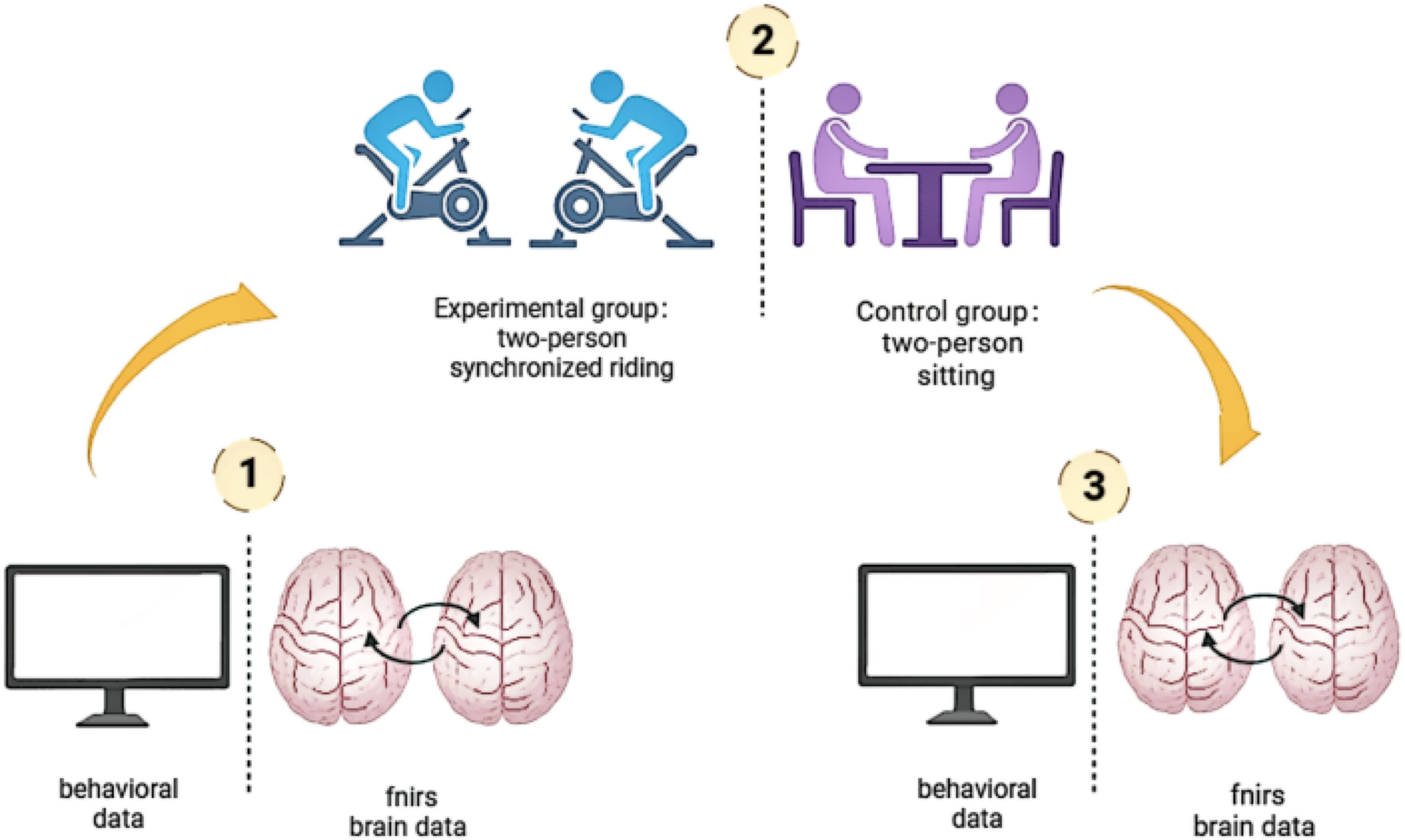
MethodsEighty college students without regular exercise habits will be randomly assigned to the experimental group (10 male dyads and 10 female dyads) and the control group (10 male dyads and 10 female dyads). During the experiment, dyads in the experimental group performed a 30-minute synchronized cycling exercise with synchronized pedaling movements; dyads in the control group rested sedentary in the same environment for 30 minutes. Interpersonal cooperative behavior was assessed with the Prisoner's Dilemma task, and the interpersonal neural synchronization(INS) data were collected in the prefrontal cortex using near-infrared hyperscanning.
ResultsThis study compared behavior and brain activity before and after synchronous exercise. Behavioral results revealed that, compared to pre-exercise, dyads in the post-exercise had higher average cooperation rates, higher cooperation efficiency and shorter cooperation response times. Compared to post-sedentary, dyads in the post-exercise had shorter cooperation response times and higher cooperation efficiency. Furthermore, brain data showed that,compared to pre-exercise, dyads in the post-exercise had stronger INS in the dorsolateral prefrontal cortex(DLPFC), whereas the dyads in the post-exercise had stronge INS in the DLPFC compared to post-sedentary. After controlling for dyads' anxiety and mood states, this study also found a marginally significant negative correlation between INS differences in the left DLPFC and cooperation response time differences.
ConclusionsThis research confirms, from both behavioral and neuroscience perspectives, that one synchronization cycle can significantly enhance interpersonal cooperative behavior, and this positive effect is closely associated with increased INS in the left DLPFC. This study provides new insights into understanding how positive interactive exercises promote interpersonal cooperation through specific neural mechanisms.
Interpersonal cooperation is a key factor in resolving social challenges and promoting societal progress. Throughout the course of human development, continuously exploring ways to improve interpersonal cooperation has always been a significant issue. Psychological research has found that synchronization, as a non-verbal social interaction signal, can influence the positive social perceptions of those involved, thereby enhancing cooperative behavior. For example, synchronized humming (Osaka and Minamoto et al., 2014), laughing together (Dezecache & Dunbar, 2012; Yun, Watanabe, & Shimojo, 2012; Vlahovic, Roberts, & Dunbar, 2012), and watching videos, reading, and gaming together (Zhou, Hong, & Wong, 2023; Tang et al., 2016) have all been shown to promote social interaction and cooperative behavior. Researchers believe that these synchronized behaviors can strengthen the bonds between members (Stoltenberg, McNeill, & Elliott, 1995), evoke positive emotions (Ehrenreich, 2006), and reduce the boundaries between self and others (Shaw et al., 2013), ultimately fostering group cohesion and enhancing cooperative behavior (Gordon et al., 2020).
In the field of sports, researchers have also explored the impact of synchronized movement on interpersonal cooperation. For example, synchronized movements (Kirschner & Tomasello, 2010; Endedijk et al., 2015; Reddish et al., 2013), synchronized clapping and tapping (Tunçgenç & Cohen, 2016), synchronized walking (Ikeda et al., 2017; Tunçgenç & Cohen, 2016), synchronized swaying (Nozawa et al., 2019; Valdesolo, Ouyang, & DeSteno, 2010), synchronized drumming (Rabinowitch et al., 2017; Hu et al., 2017), and synchronized dancing (Basso et al., 2021) have all demonstrated that synchronized movement can enhance interpersonal cooperation. However, these studies mostly involve simple actions performed by small muscle groups, lacking consideration of key exercise elements such as duration, intensity, and frequency, and do not fully reflect the effects of actual sports activities. While synchronized dancing is a typical form of sports, its synchronization process involves complex factors, making it difficult to control the rhythm and therefore not conducive to studying the causal relationship between synchronized movement and interpersonal cooperation. Recently, Li et al. (2023) found that a 30-minute moderate-intensity individual cycling exercise could enhance subjects' interpersonal cooperation, suggesting that this form of exercise intervention could be an effective means to explore the impact of exercise on interpersonal cooperation. Cycling on a power bike is a commonly used form of exercise intervention in laboratory settings, as it allows for precise control of exercise intensity, duration, and frequency, making it suitable for synchronized exercise interventions. Based on this, our study designed a 30-minute moderate-intensity synchronized cycling power bike exercise intervention to investigate the impact of synchronized movement on college students' interpersonal cooperation behaviors. This study aims to provide theoretical support and intervention measures for the prevention and treatment of mental health issues, helping individuals better cope with social isolation and interpersonal difficulties.
In recent years, an increasing number of researchers have used near-infrared spectroscopy (NIRS) hyperscanning technology to explore the neural mechanisms behind the impact of synchronized movement on interpersonal cooperation, employing interpersonal neural synchronization(INS) as a measure of cooperation quality (Pan and Cheng et al., 2017; Hu and Pan et al., 2018). For instance, studies on synchronized singing (Osaka et al., 2014), coordinated group walking (Ikeda et al., 2017), complementary and imitative joint actions (Cheng, Guo, & Hu, 2022), synchronized drumming (Liu et al., 2021), and synchronized dancing (Basso et al., 2021) have found that after synchronized activities, inter-brain synchrony in specific regions of the prefrontal cortex(PFC) significantly increases, which is positively correlated with behavioral performance. The prefrontal cortex is associated with attention processing (Hopf & Mangun, 2000), theory of mind (Shamay-Tsoory, 2007), and other social cognitive decisions (Andrews-Hanna et al., 2014), suggesting that synchronized movement may influence interpersonal cooperation by altering inter-brain synchrony in the prefrontal cortex. Although some studies have used electroencephalogram (EEG) hyperscanning technology to investigate the neural mechanisms behind the impact of synchronized movement on interpersonal cooperation—such as fingertip movement (Yun, Watanabe, & Shimojo, 2012), gesture imitation (Dumas et al., 2010), and instrument playing (Lindenberger et al., 2009; Zamm et al., 2021)—near-infrared spectroscopy imaging technology offers higher spatial resolution and better motion artifact performance compared to EEG. This technology can more precisely connect specific brain regions without requiring post-processing (Liu, Saito, Lin, & Saito, 2016). Therefore, this study aims to use near-infrared hyperscanning technology, focusing on the prefrontal cortex, to explore the impact of synchronized movement on interpersonal cooperation. Given that most current hyperscanning studies on synchronized movement involve simple actions performed by small muscle groups and lack key exercise elements, and that synchronized dancing, while a typical form of physical exercise, involves complex factors that complicate establishing a causal relationship between synchronized movement and interpersonal cooperation, this study opts for a 30-minute dual synchronized cycling exercise to explore the neural mechanisms behind the impact of synchronized movement on interpersonal cooperation. This research aims to provide new perspectives and methods for interdisciplinary studies and psychological health interventions.
Materials and methodsSubjectsIn the planned study, to estimate the required sample size, this research utilized G-power 3.1 software (Faul et al., 2009) for a priori analysis. An effect size (d) of 0.40 was set, representing a medium effect size based on Cohen's recommendation. The statistical power (1-β) was set to 0.80, implying an 80% probability of correctly rejecting a false null hypothesis. Meanwhile, the significance level (alpha, α) was set at 0.05, which is a commonly used standard of significance, indicating that the study is willing to accept a 5% risk of incorrectly rejecting a true null hypothesis. Based on these parameters, the total sample size (N) was 28 calculated in this study.
The experiment attracted a total of 40 dyads (80 individuals) of unacquainted college students, who were randomly assigned to either the experimental group or the control group, with 20 dyads (40 individuals) in each group, ensuring an equal number of male and female dyads within each group. To minimize the potential impact of fitness differences among dyads on the experimental outcomes, all dyads underwent a 20-meter round-trip running test prior to the experiment to assess their cardiorespiratory endurance, ensuring no significant differences in cardiorespiratory endurance among selected dyads.
Furthermore, considering that emotional states and anxiety levels could influence interpersonal cooperative behavior, dyads completed the Brief Profile of Mood States (POMS, Zhu Beili, 1995) and the Self-Rating Anxiety Scale (SAS, Zung, 1971) before the experiment. These scales respectively assessed dyads' mood states and social anxiety levels. The specific screening steps are as follows: the Brief Profile of Mood States Scale consists of 10 items, each scored from 1 to 5, with a total score ranging from 10 to 50. Subjects with a total score below 30 are considered to have a good mood state and are included in the experiment. The Self-Rating Anxiety Scale consists of 20 items, each scored from 1 to 4, with a total score ranging from 20 to 80. Subjects with a total score below 45 are considered to have no significant anxiety issues and are included in the experiment. This step helps control experimental conditions, allowing the results to more accurately reflect the impact of synchronized exercise on interpersonal cooperative behavior.
This study was conducted in accordance with the latest version of the Declaration of Helsinki and was approved by the Human Subjects Protection Committee of East China Normal University (HR449-2020). All dyads signed informed consent forms before participating in the experiment and received compensation upon completion of the experimental tasks.
Experimental procedureUpon arrival at the laboratory, each dyad first completed a Basic Information Questionnaire(BIQ), which collected essential details including age, sex, training experience, and other relevant questionnaires (see Table 1). Subsequently, researchers thoroughly introduced the experimental procedure (see Fig. 1) and the task paradigm (see Fig. 2) to the dyads, ensuring both had a comprehensive understanding of the experimental tasks and no remaining questions, before officially commencing the experiment. Considering the potential impact of practice effects on experimental outcomes, this study did not include a practice session.
At the start of the experiment, dyads sat facing each other at opposite ends of a table, in front of a computer screen. Researchers assisted the dyads in donning caps with 3 × 5 near-infrared spectroscopy detector patches, and explained the game rules without using any suggestive words such as "cooperation," "non-cooperation", "prosocial" or "selfish". After ensuring dyads fully understood the game rules, they were allowed a 30-second rest period of quiet rest.
Subsequently, dyads completed a round of the Prisoner's Dilemma task programmed in Eprime 2.0 software (Psychology Software Tools, Inc), during which brain activity data were collected. Following this task, dyads in the experimental group underwent a 30-minute synchronous exercise intervention, while those in the control group engaged in 30 minutes of synchronous sitting. Heart rate tests were conducted on both groups during and after the intervention to ensure their post-intervention heart rates returned to within ±10% of their resting heart rate (approximately requiring 5 minutes). After heart rate recovery, dyads once again completed the behavioral testing and brain activity data collection for the Prisoner's Dilemma task.
Finally, dyads filled out a questionnaire assessing subjective cooperativeness. Upon completing all procedures, dyads left the laboratory, marking the end of the experiment. This process aimed to explore the impact of synchronous exercise intervention on interpersonal cooperative behavior and its neural mechanisms, while rigorous design minimized potential biases to ensure the validity and reliability of the experimental outcomes.
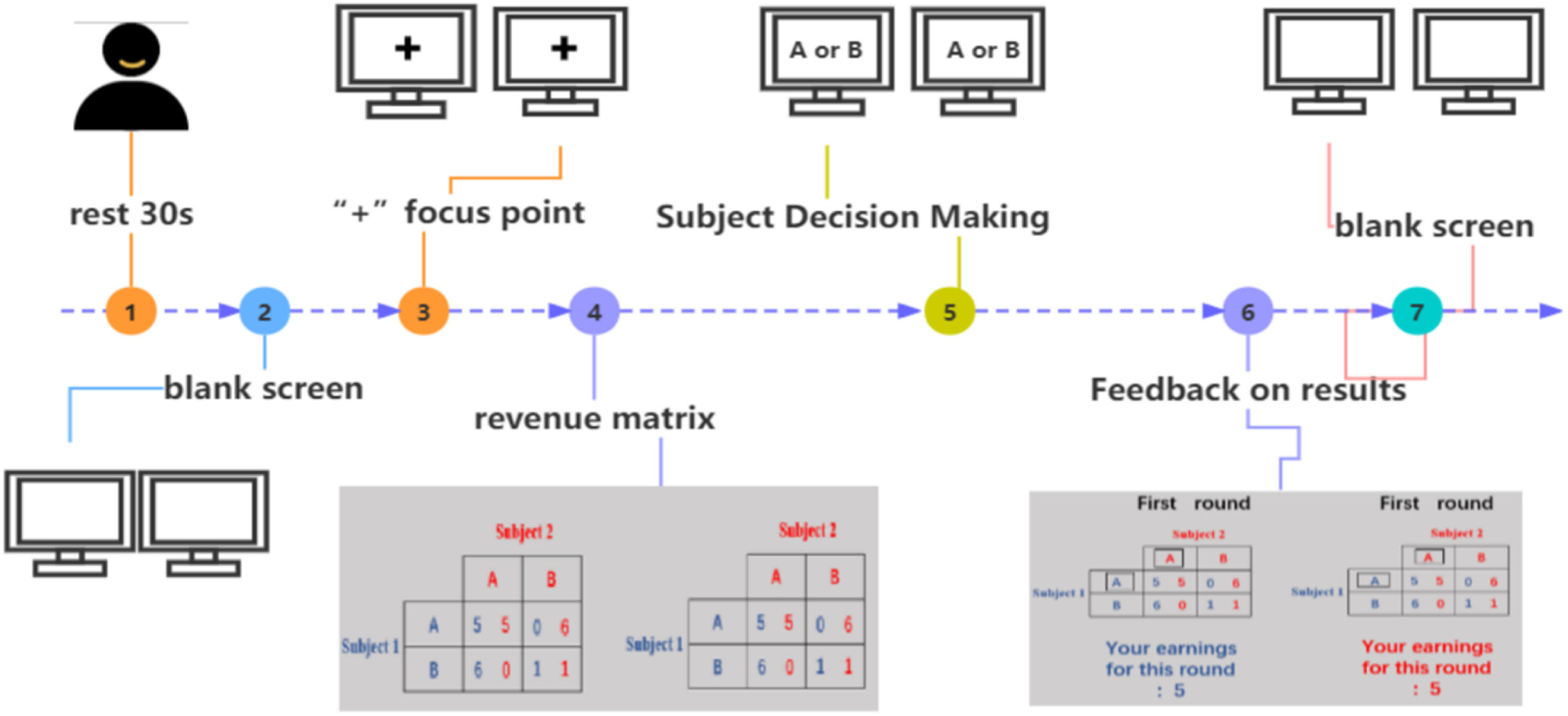
Experimental tasksIn this study, the Prisoner's Dilemma task was employed as a paradigm for investigating interpersonal cooperative behavior. The task involves two subjects, labeled as subject 1 and subject 2. Throughout the course of the game, a total of 29 rounds are conducted, with each round presenting the subjects with the decision to either cooperate with or betray the other party.
The specific payoff rules are set as follows: If both subjects choose to cooperate, each receives R units; if both choose to betray, each receives P units; if one chooses to cooperate while the other chooses to betray, the betrayer receives T units, while the cooperator receives S units. This payoff structure satisfies two conditions: (1) T (the betrayer's payoff) > R (the payoff for mutual cooperation) > P (the payoff for mutual betrayal) > S (the cooperator's loss); (2) the total payoff for mutual cooperation (2R) is greater than the total payoff when one betrays and the other cooperates (S+T).
Each round of the presentation lasts approximately 20 seconds, with the total duration of the task being around 10 minutes. Prior to the experiment, dyads were informed that their compensation would consist of a base participation fee plus a certain percentage of the amount accumulated during the task. Through repeated rounds, this task paradigm aims to simulate real-life social interaction scenarios and explore the behavioral patterns of dyads when faced with choices between cooperation and selfishness, thereby gaining a deeper understanding of the psychological mechanisms of interpersonal cooperation. Below is the procedure for a typical round in the Prisoner's Dilemma task (see Fig. 2).
Exercise interventionIn this experiment, dyads in the experimental group underwent a 30-minute moderate-intensity exercise intervention using a MONARK LC6 power bike. According to the aerobic exercise intensity classification standards for healthy adults by the American College of Sports Medicine (2006), the intensity of moderate exercise was set within a specific range. To precisely control the intensity of exercise, the method of reserve heart rate (maximum heart rate minus resting heart rate) was used, where the maximum heart rate was calculated by the formula 220 minus age, and the intensity of moderate exercise was controlled between 60% to 69% of the reserve heart rate. During the exercise intervention, dyads in the experimental group continuously wore a Polar heart rate monitor to track their heart rate in real time. They cycled face-to-face in the same room to achieve synchronized exercise. In the initial phase, dyads warmed up with 3 minutes of slow cycling, then gradually accelerated to a moderate intensity level, maintaining this intensity throughout the exercise. To ensure synchrony, the dyads' pedaling actions were required to be consistent. The entire exercise, including warm-up, lasted for 30 minutes.
Dyads in the control group were seated face-to-face in the same room and completed a 30-minute sedentary activity. During the sedentary period, dyads were instructed to avoid excessive thinking or falling asleep to maintain their level of alertness. They also wore Polar heart rate monitors to track their heart rate in real-time, ensuring that their heart rate remained within ±10% of their resting heart rate, to ensure consistency in the control group conditions.
Through this design, the experiment aimed to explore the effects of moderate-intensity synchronized exercise intervention on interpersonal cooperative behavior and its underlying neural mechanisms. The setup of the control group ensured that any observed effects could be reasonably attributed to the exercise intervention itself, rather than other external factors.
Questionnaires(1) Self-Rating Anxiety Scale (SAS, Zung, 1971): This scale consists of 20 items. The SAS uses a 4-point rating scale to primarily assess the frequency of symptoms, with the criteria as follows: "1" indicates none or a small amount of the time; "2" indicates some of the time; "3" indicates a good part of the time; "4" indicates most or all of the time. Of the 20 items, 15 are stated negatively and are scored in the order of 1 to 4. The remaining 5 items (numbers 5, 9, 13, 17, and 19) are stated positively and are scored in reverse order from 4 to 1. According to the Chinese normative results, the cut-off value for the SAS standard score is 50 points, where 50-59 points indicate mild anxiety, 60-69 points indicate moderate anxiety, and 70 points or above indicate severe anxiety.
Brief Profile of Mood States (POMS, Zhu Beili, 1995): This scale is an emotional state assessment instrument that effectively measures the mood states of healthy populations and psychiatric patients. It was designed by GROVE et al. in 1992, and the Chinese version of the POMS was revised by Zhu Beili in 1995. It includes 7 subscales: Tension, Anger, Fatigue, Depression, Confusion, Vigor, and Self-Esteem, with a total of 40 items. Item scores range from 0 to 4, with 0 indicating "not at all", 1 indicating "a little", 2 indicating "moderately", 3 indicating "quite a bit" and 4 indicating "extremely" . The total mood disturbance (TMD) score is calculated as the sum of negative emotion scores (Tension, Anger, Fatigue, Depression, and Confusion) minus the sum of positive emotion scores (Vigor and Self-Esteem) plus 100. A higher TMD score indicates more severe negative emotions, signifying a more disordered, gloomy, or dysregulated mood. The reliability of the Chinese version of POMS ranges from 0.62 to 0.82, with an average reliability of 0.71 (Baer et al., 2006). Higher scores on the mood state indicate a worse psychological state, whereas lower scores indicate a better psychological state.
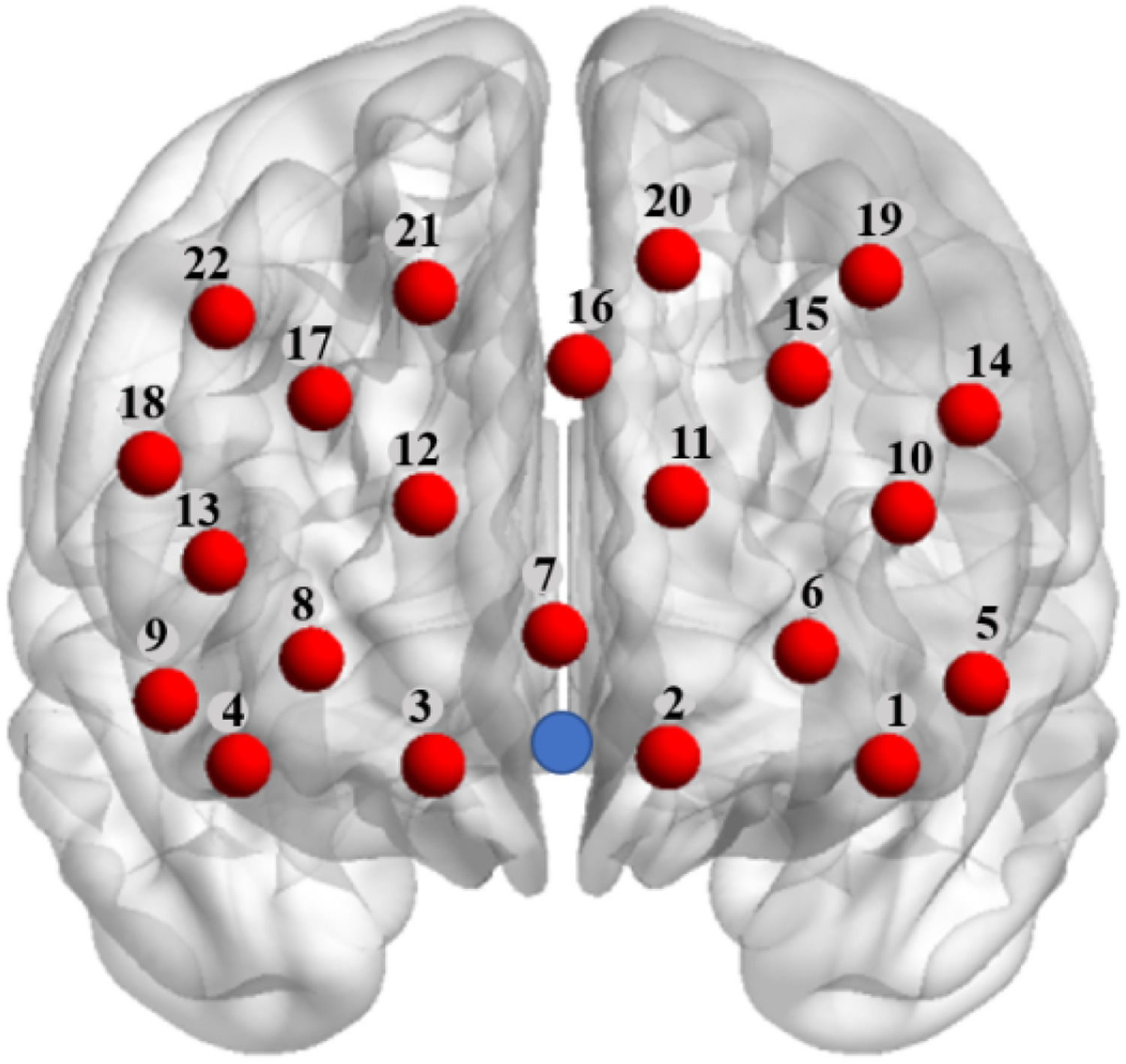
Data collectionThis study utilized the Hitachi ETG-7100 near-infrared spectroscopy imaging system (Hitachi Medical Corporation, Japan) to continuously monitor changes in the concentration of oxygenated hemoglobin (oxy-Hb) and deoxygenated hemoglobin (deoxy-Hb) in the dyads' brains during the execution of experimental tasks. The system operates at light wave lengths of 695 nm and 830 nm, with a sampling frequency set at 10 Hz. Given prior research indicating that INS during cooperative processes is primarily observed in the prefrontal cortex (de vico Fallani et al., 2010; Cui et al., 2012), this study employed two 3 × 5 near-infrared spectroscopy detector patches to cover the prefrontal cortex area of the dyads(see Fig. 3). Each optode array consists of 8 emitter probes and 7 receiver probes, spaced 3 cm apart, forming a total of 22 measurement channels.
Probe placement in the prefrontal cortex. The red nodes are channels, which are numbered as indicated by the numbers . The blue dot is the reference point Fpz (10-20 system, frontal pole midpoint). This schematic was produced using the visualization tool BrainNet Viewer (Xia et al., 2013).
For precise localization, based on the international 10-20 system for electrode placement, the bottom middle row of the optode arrays was positioned at the Fpz (frontopolar midline). The specific spatial locations of the channels were determined using the virtual localization tool from Jichi Medical University (available online at http://www.jichi.ac.jp/brainlab/virtual_registration/Result3 × 5_E.html). Moreover, channel coordinates were mapped onto the Montreal Neurological Institute (MNI) space using the NIRS-SPM software, thereby determining the exact anatomical locations of each channel.
This method allows for high spatial precision in analyzing INS during cooperative processes. By monitoring changes in oxy-Hb and deoxy-Hb concentrations, it is possible to assess real-time activity changes in the prefrontal cortex during task execution, providing a powerful tool for further understanding the neural basis of interpersonal cooperation.
Data analysisBehavioral data analysisIn this study, behavioral indicators were used to assess the dynamics of cooperation and betrayal behaviors between dyads. The specific indicators include:
*Average cooperation rate: Average cooperation rate = Number of times both subjects chose the cooperation option / Total number of trials × 100%, reflecting the frequency of cooperative behaviors between dyads.
*Average betrayal rate: Average betrayal rate = Number of times both subjects chose the betrayal option / Total number of trials × 100%, reflecting the frequency of betrayal behaviors between dyads.
*Cooperation reaction time: Cooperation reaction time = (subject 1′s cooperation reaction time + subject 2′s cooperation reaction time) / 2, measured in milliseconds (ms), reflecting the speed of cooperative behavior between dyads.
*Betrayal reaction time: Betrayal reaction time = (subject 1′s betrayal reaction time + subject2′s betrayal reaction time) / 2, measured in milliseconds (ms), reflecting the speed of betrayal behavior between dyads.
*Cooperation efficiency: Cooperation efficiency = Average cooperation rate / Cooperation reaction time × 100%, reflecting the efficiency of cooperation between dyads.
*Betrayal efficiency: Betrayal efficiency = Average betrayal rate / Betrayal reaction time × 100%, reflecting the efficiency of betrayal between dyads.
These indicators collectively reflect the quality and efficiency of cooperative and betrayal behaviors between dyads. Theoretically, a higher average cooperation rate and faster cooperation reaction time indicate a stronger willingness to cooperate; a lower average betrayal rate and slower betrayal reaction time suggest a weaker tendency to betray.
To address outliers in the data, this study adopted the criterion of mean plus or minus three standard deviations to filter out extreme values, which were replaced with the mean. Data analysis was conducted using SPSS 23.0 software, through a 2 (time points: pre-test; post-test) × 2 (groups: experimental group; control group) repeated measures analysis of variance (ANOVA). For indicators with significant interaction or main effects, further simple effects analysis and post-hoc tests were conducted to explore the impact of time and group factors on the behavioral indicators. This analytical approach aims to precisely identify the specific impacts of experimental interventions on dyads' cooperation and betrayal behaviors.
Synchronization analysis of interbrain activityThis study focuses on analyzing changes in oxyhemoglobin (HbO), as previous research has indicated that HbO is more sensitive to task-related stimuli (Wang et al., 2012). Initially, to reduce interference from global signals unrelated to the task, the HbO signal was preprocessed using a principal component spatial filter algorithm (PCA, Zhang, 2016) to eliminate the influence of global components.
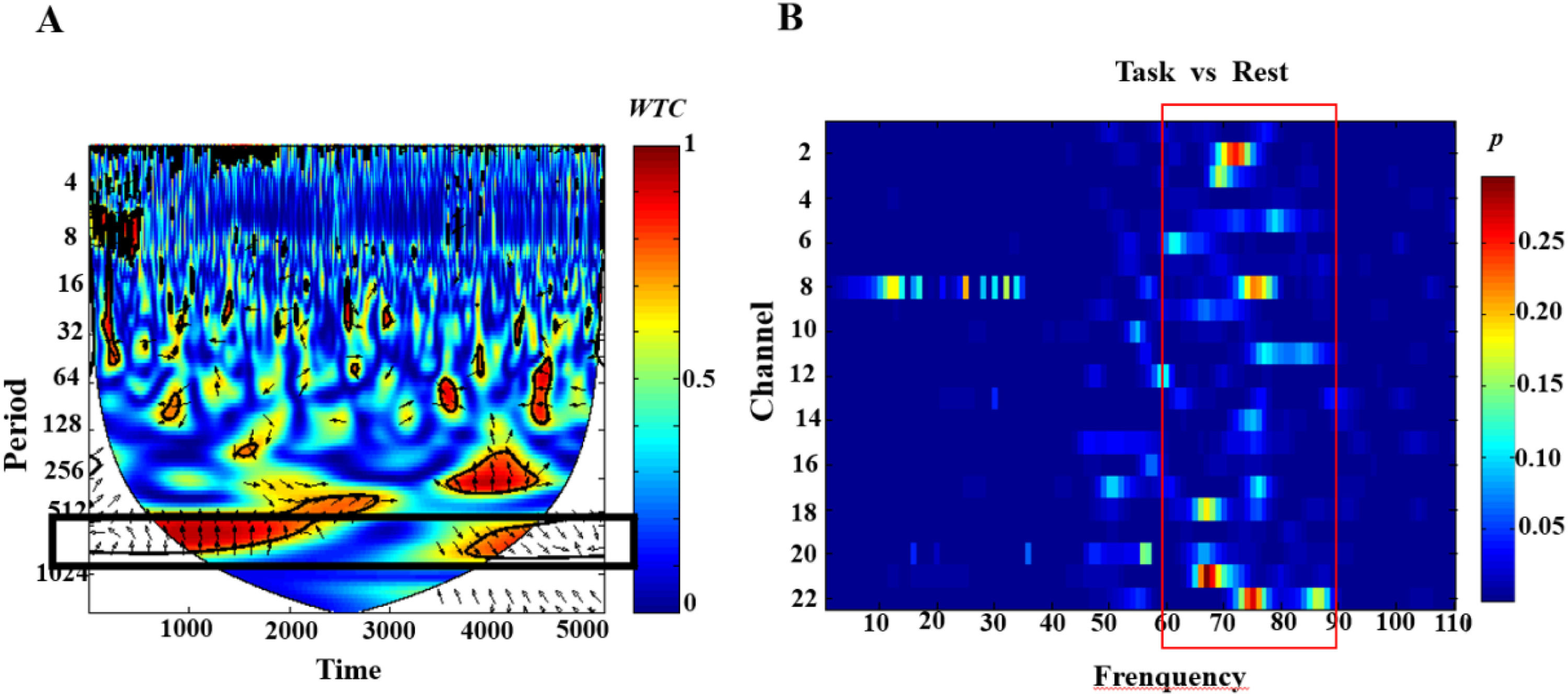
Subsequently, the wavelet transform coherence (WTC, Grinsted et al., 2004) method was utilized to calculate the correlation between each dyad of every channel signals. This method produced a time-frequency coherence map for analyzing the synchrony of brain signals among dyads. Visual inspection of the WTC frequency bands revealed higher synchronicity in the 0.010Hz to 0.019Hz frequency band (corresponding to 51.2 to 102.4 seconds, see Fig. 4A), aligning with the duration of a round of the experimental game (approximately 40 seconds). Moreover, previous studies have also supported low-frequency oscillations (typically within the 0.01 to 0.10Hz range) as a reliable indicator of neuronal synchrony within the brain (Achard et al., 2006; Zuo et al., 2010).
Example of WTC frequency-domain-time-domain coherence plots for oxy-Hb signals of channels.(A.)Spectrogram of wavelet transform coherence (WTC) of HbO signals based on the synchronization group. The color plot ranges from dark blue to dark red, and the value indicating synchronization ranges from 0 to 1, with 1 denoting the strongest synchronization between the two NIRS signals, and 0 denoting no synchronization detected. Red rectangles in the plots mark regions of significant synchronization. (B).dyaded t-test plot of task vs. rest in the full frequency band from 0.001 Hz to 1 Hz, revealing that interbrain activity synchronization in the frequency band 0.011 Hz-0.017 Hz was significantly higher during the task session than during rest, and that this frequency range covered the decision duration.
To more precisely determine the frequency range closely associated with interpersonal cooperation, this study conducted paired t-tests across the entire 0.001Hz to 1Hz frequency band during task and rest states (Nozawa et al., 2016). The results indicated that INS during the task was significantly higher than during rest within the 0.011Hz-0.017Hz(60 to 90seconds) frequency band (see Fig. 4 B). The selection of this frequency band also helps to avoid the influence of higher frequency noise, such as heartbeats (approximately 0.7-4Hz), and lower frequency noise, such as breathing (0.2-0.3Hz) (Xue et al., 2018).
Subsequently, this study calculated the change in INS(the difference between task and rest phases) and applied Fisher z-transformation to these values (Chang & Glover, 2010). A one-sample t-test (ANOVA) was used to assess whether there was a significant enhancement in INS within each group, and the "false discovery rate" (FDR correction) was applied during multiple comparisons to identify channels of interest. The synchronization results for regions of interest were visualized as 3D brain models using the xjview toolbox (http://www.alivelearn.net/xjview8/) and the BrainNet Viewer toolbox (http://www.nitrc.org/projects/bnv/).
The study employed SPSS 23.0 software to conduct a 2 (time: pre-test; post-test) × 2 (group: experimental group; control group) repeated measures analysis of variance, with simple effects analysis and post-hoc tests conducted on channels with significant interaction and main effects. Furthermore, Pearson correlation analysis was utilized to explore the relationship between behavioral changes and changes in INS, thereby enhancing the interpretability and application value of the research findings.
To ensure the observed INS was caused by actual interactive tasks and not merely by dyads engaging in similar cognitive tasks (similarity of conditions), this study implemented a permutation test to assess the statistical significance of INS (Jiang et al., 2015; Reindl et al., 2018) . The permutation test is a non-parametric statistical method that generates a random distribution of data by reallocating the labels of data samples, used to evaluate whether the observed data patterns could occur under random circumstances. Specifically, this study performed 1000 permutations for each channel to create a normal distribution reflecting the expected distribution of brain synchrony under random conditions. This method allows for a reliable test of INS for each channel without assuming a prior distribution of the data. Then, the study compared the average brain synchrony values observed in actual dyads for each channel against the distribution generated by the permutation test to determine if the observed synchrony exceeded the range of random expectation. An observed synchrony was considered statistically significant only if the p-value was less than 0.05, indicating that the observed synchrony was unlikely to have occurred by chance. This approach effectively rules out brain synchrony phenomena caused by similarity of conditions, ensuring that the analysis results accurately reflect the real INS induced by interactive cognitive tasks. Detailed permutation test results and further analyses can be found in the supplementary materials of this study. This method enhances the credibility of the research findings, providing a more rigorous scientific basis for understanding brain synchrony phenomena during interpersonal interactions. This process was applied across all channels. Results can be further explored in the supplementary files.
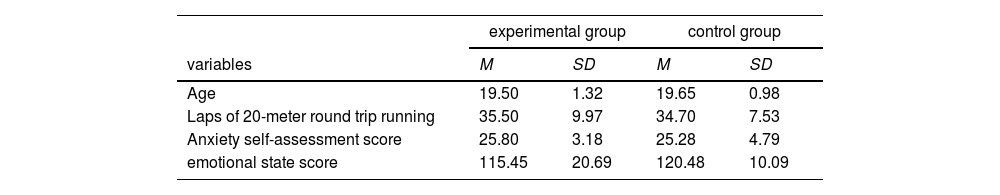
ResultsBehavioral resultsDescriptive statistics of the 20-meter round-trip running laps, anxiety self-assessment scores, and emotional state score for dyads in the experimental and control groups are shown in Table 1. An independent samples t-test on the questionnaire scores of the two groups showed that the difference between the two groups was not significant (p age = 0.565, p round trip run = 0.687, p anxiety self-assessment = 0.565, p state of mind = 0.171).
To explore the impact of synchronization cycling exercise on interpersonal cooperation, a 2 (time: pre-test; post-test) × 2 (group: experimental group; control group) repeated measures ANOVA (with FDR correction) was conducted on behavioral indices. The results revealed: for the average cooperation rate, there was a significant main effect of time (F(1, 38) = 16.24, p = 0.000, ηp2 = 0.30), a non-significant main effect of group (F(1, 38) = 0.03, p = 0.859, ηp2 = 0.001), and a significant interaction effect (F(1, 38) = 4.15, p = 0.049, ηp2 = 0.10). For cooperation response time, the main effect of time was significant (F(1, 38) = 16.10, p = 0.001, ηp2= 0.29), but the main effect of group (F(1, 38) = 0.31, p = 0.583, ηp2 = 0.01) and the interaction effect (F(1, 38) = 0.89, p = 0.350, ηp2 = 0.02) were not significant. For cooperation efficiency, the main effect of time was significant (F(1, 38) = 34.07, p < 0.001, ηp2 = 0.47), the main effect of group was not significant (F(1, 38) = 1.72, p = 0.198, ηp2 = 0.04), and the interaction effect was significant (F(1, 38) = 9.34, p = 0.04, ηp2 = 0.20). For the average betrayal rate, the main effect of time (F(1, 38) = 2.05, p = 0.160, ηp2 = 0.05), the main effect of group (F(1, 38) = 0.05, p = 0.830,ηp2= 0.001), and the interaction effect (F(1, 38) = 1.60, p = 0.214, ηp2 = 0.04) were all not significant. For betrayal response time, the main effect of time was significant (F(1, 38) = 11.18, p = 0.002, ηp2 = 0.23), but the main effect of group (F(1, 38) = 0.001, p = 0.994, ηp2= 0.001) and the interaction effect (F(1, 38) = 0.01, p = 0.912, ηp2 = 0.001) were not significant. For betrayal efficiency, the main effect of time was not significant (F(1, 38) = 0.28, p = 0.602, ηp2= 0.01), the main effect of group was not significant (F(1, 38) = 0.01, p = 0.922, ηp2= 0.001), and the interaction effect was not significant (F(1, 38) = 3.10, p = 0.086, ηp2= 0.08).
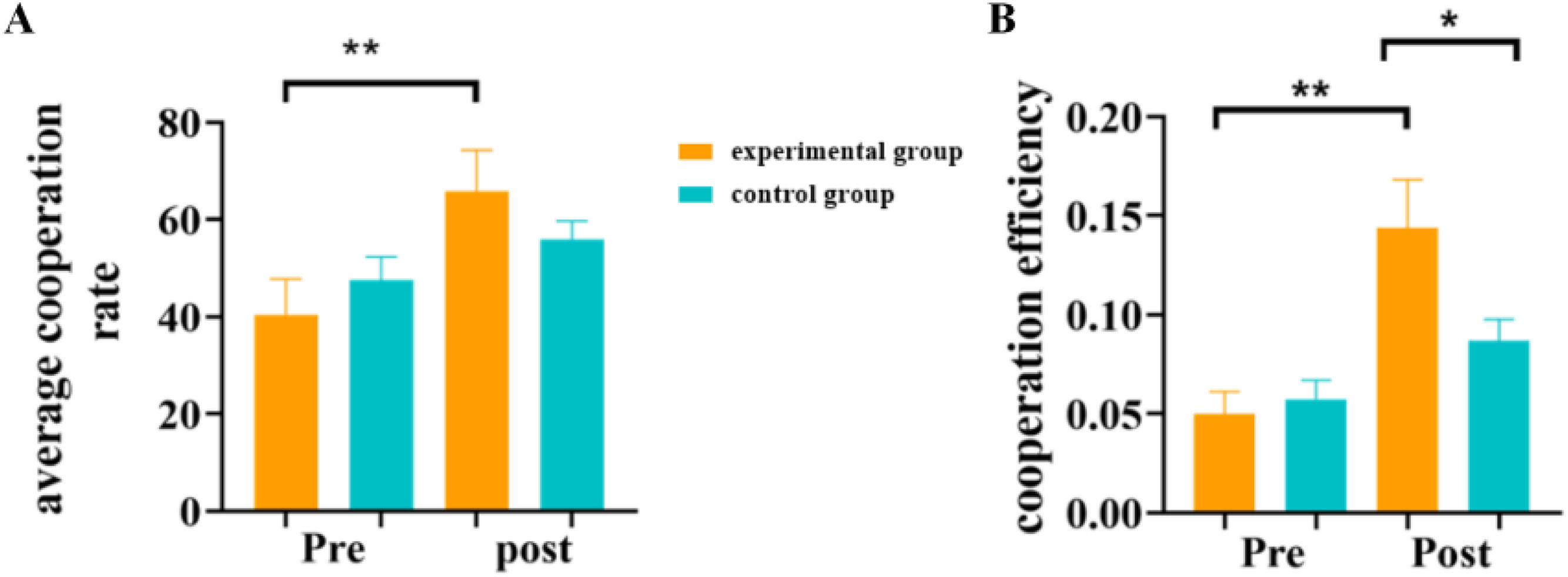
Further simple effects analyses on the behavioral indicators with interaction effects revealed that on the average cooperation rate (see Fig. 5A), there was a significant difference before and after the intervention in the experimental group (t(38) = 18.41, p = 0.001, Cohen's d = 2.95, 95% CI [-37.46, -13.44]), with the cooperation rate after synchronous cycling exercise (65.90 ± 37.53) significantly higher than before (40.45 ± 32.71). No significant difference was found before and after sitting in the control group (t(38) = 1.98, p = 0.167). There were no significant differences between the experimental and control groups either before (ppre = 0.417) or after (ppost = 0.286) the test. On cooperation efficiency (see Fig. 5B), a significant difference was found in the experimental group before and after the activity (t(38) = 39.55, p = 0.001, Cohen's d = 6.33, 95% CI [-0.12, -0.06]), with cooperation efficiency after the activity (0.14 ± 0.11) significantly higher than before (0.05 ± 0.05). No significant difference was found in the control group before and after sitting (t(38) = 3.87, p = 0.057). There was no significant difference between groups in the pre-test (t(38) = 0.27, p = 0.608), but a significant difference was found in the post-test (t(38) = 4.56, p = 0.039, Cohen's d = 0.73, 95% CI [-0.04, 0.02]), with the cooperation efficiency after the activity in the experimental group (0.14 ± 0.11) significantly higher than in the control group (0.09 ± 0.05).
fNIRS resultsGiven that the behavioral results analysis identified a significant intervention effect of synchronization cycling exercise on cooperative behavior but not on betrayal behavior, this study focused on the analysis of INS related to cooperative behavior.
To investigate whether there were significant differences in the changes in INS across all channels under each condition compared to the constant value of 0, this study utilized a one-sample t-test to investigate channels with significant INS during cooperative behavior both before and after synchronous exercise and sitting, with all results were FDR-corrected. It was found that in the experimental group pre-test, all channels exhibited significant differences (p<0.05); in the experimental group post-test, all channels except for channels 4, 8, 12, 15, 19, and 20 showed significant differences (p<0.05); in the control group pre-test, all channels except for channels 1, 12, 13, and 14 exhibited significant differences (p<0.05); and in the control group post-test, all channels except for channels 1, 7, 8, 11, 12, 13, and 14 showed significant differences (p<0.05).
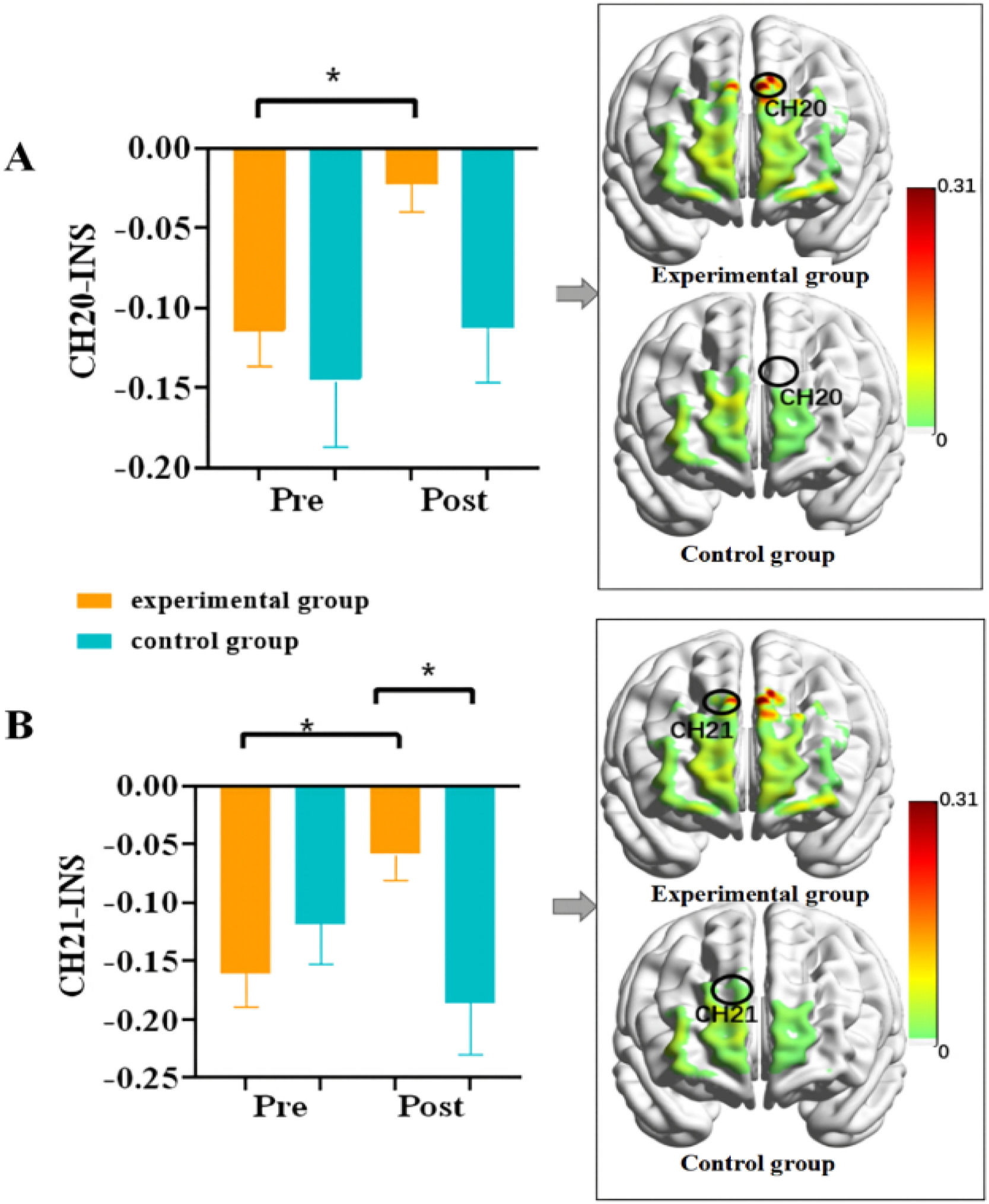
In order to investigate the effects of synchronized cycling exercise on the synchronization of interbrain activity in dyads completing cooperative behaviors before and after the exercise intervention, this study conducted a 2 (group: experimental; control) × 2 (time: pre-test; post-test) repeated measures ANOVA (all FDR corrected) on all the significant channels. The results showed significant time main effects on channels 4 (F(1, 38) = 5.39, p = 0.026, ηp2 = 0.12),7 (F(1, 38) = 7.11, p = 0.011, ηp2= 0.16),10 (F(1, 38) = 4.77, p = 0.035, ηp2 = 0.11),12 (F(1, 38) = 4.59, p = 0.039, ηp2= 0.11),13 (F(1, 38) = 4.79, p = 0.035, ηp2= 0.11) and 18 (F(1, 38) = 7.02, p = 0.012, ηp2= 0.16). Significant group main effects were found on channels 9 (F(1, 38) = 8.12, p = 0.007, ηp2= 0.18) and 19 (F(1, 38) = 6.07, p = 0.018, ηp2= 0.14). Significant interaction effects were observed on channels 20 (F(1, 38) = 4.65, p = 0.037, ηp2= 0.11) and 21 (F(1, 38) = 8.45, p = 0.006, ηp2= 0.18).
Further simple effects analyses of channels with interaction effects revealed that channel 20 (left dorsolateral prefrontal cortex) had a significant group difference on the post-test (t(38) = 4.57, p = 0.039, Cohen's d = 0.99, 95% CI [0.01, 0.24], see Fig. 6A), with the interbrain synchronization after synchronous exercise (-0.03 ± 0.13) significantly higher than after sitting (-0.15 ± 0.22). For channel 21 (right dorsolateral prefrontal cortex), there was a significant difference before and after the synchronous exercise intervention (t(38) = 6.17, p = 0.018, Cohen's d = 1.01, 95% CI [-0.19, -0.02], see Fig. 6B), with interbrain synchronization after synchronous exercise (-0.06 ± 0.10) significantly higher than before (-0.16 ± 0.13). At the same time, there was a significant difference between groups in the post-test (t(38) = 6.58, p = 0.014, Cohen's d = 1.01, 95% CI [0.03, 0.23]), with brain activity synchronicity after synchronous exercise (-0.06 ± 0.10) significantly higher than after sitting (-0.19 ± 0.20).
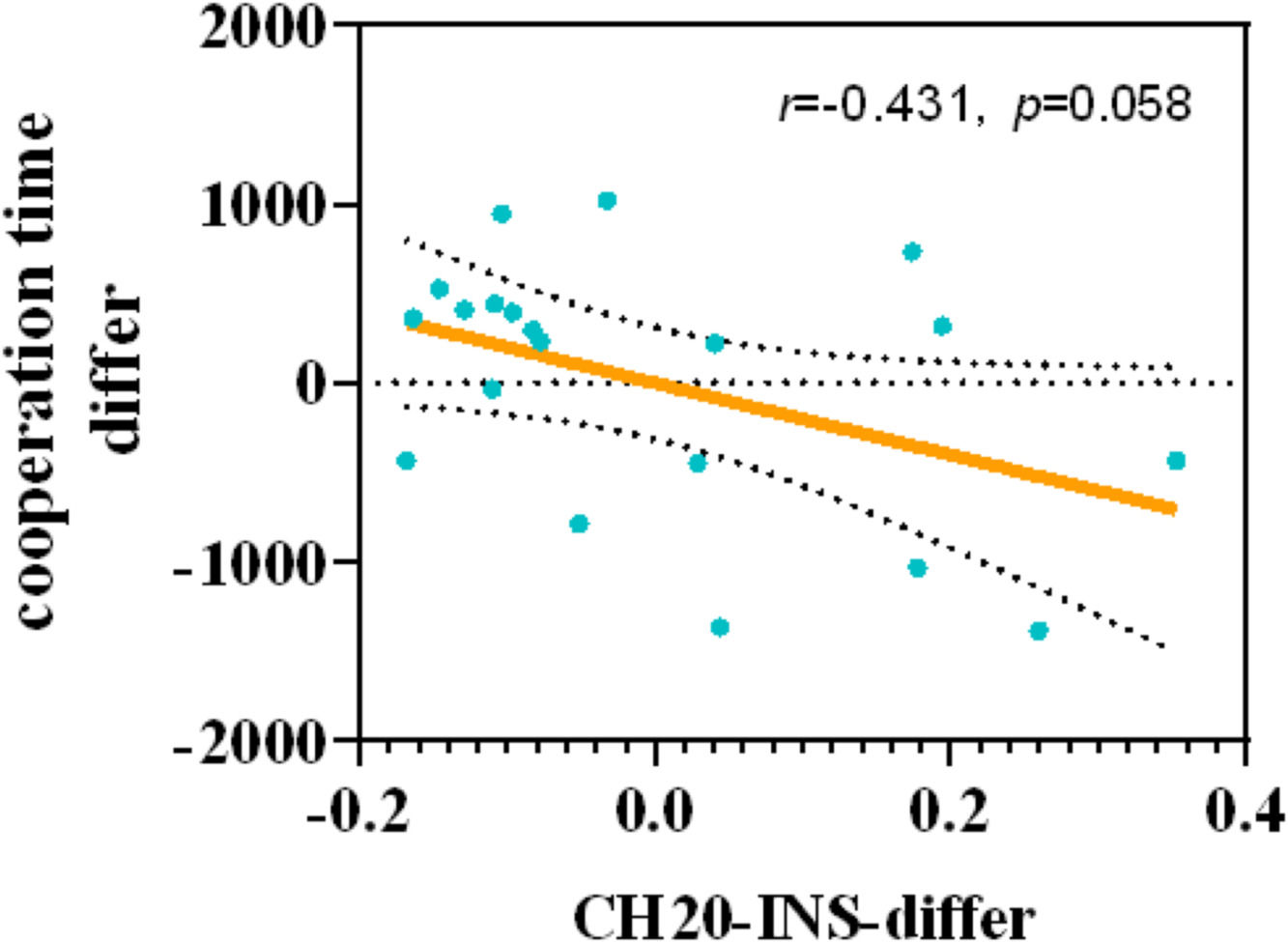
To further understand the potential relationship between the impact of synchronous cycling on the preferential response in cooperative decision-making and the functions of specific brain regions, this study conducted partial correlation tests between the pre-post changes in INS and the changes in cooperation behavioral indices, controlling for individual factors such as anxiety scores and mood state scores of the dyads in the experimental group. The results (see Fig. 7) revealed a marginally significant negative correlation between the change in INS in channel 20 (left DLPFC) and the change in cooperative reaction time(r=-0.431, p=0.058).
DiscussionThis study aimed to explore the effect of one 30-minute synchronous cycling exercise on interpersonal cooperative behavior and the changes in INS underlying this effect. The findings indicate that, compared to the control group, the experimental group showed significantly improved average cooperation rates and cooperation efficiency, as well as significantly reduced cooperation reaction times after exercise. Additionally, there was a significant increase in INS in the left DLPFC region after exercise in the experimental group dyads. Partial correlation analysis further revealed a significant negative correlation between changes in INS in the left DLPFC and changes in cooperation reaction time among dyads in the experimental group. These findings, from both behavioral and neuroscience perspectives, suggest that synchronous cycling can enhance interpersonal cooperative behavior, and this positive effect may be related to increased INS in the left DLPFC.
This study confirmed through a randomized controlled trial that a single 30-minute session of synchronous exercise can effectively enhance interpersonal cooperation. Our findings are consistent with previous research. For example, Cirelli et al. (2014) found that 14-month-old infants were more likely to help social partners with whom they had bounced synchronously compared to those in asynchronous bouncing conditions. Tunçgenç and Cohen (2016) discovered that 4- to 6-year-old children exhibited more cooperation and helping behaviors after performing rhythmic clapping and tapping in synchronous conditions compared to asynchronous ones. Rabinowitch (2017) found that 4-year-old children's cooperative behaviors were significantly enhanced under synchronous movement conditions compared to asynchronous ones.For adult studies, Wiltermuth (2009) found that synchronous walking on campus increased cooperative behavior by strengthening social bonds within groups. Valdesolo (2010) showed that synchronous swinging could enhance social cohesion and cooperation by fostering a sense of belonging and rapport. Kokal et al. (2011) found that subjects who drummed synchronously were more willing to help the experimenter. Paul Reddish (2013) discovered that subjects' cooperative behaviors significantly improved after synchronous body movements; Tarr (2016) found that synchronous dancing increased social closeness and cooperative tendencies among subjects. These studies collectively indicate that synchronous movement can effectively enhance interpersonal cooperation. However, previous research often focused on simple actions involving small muscle groups, lacking key exercise elements such as duration, intensity, and frequency. Although synchronous dancing is a typical form of physical exercise, its complex synchronization process makes it difficult to establish a causal relationship between synchronous movement and interpersonal cooperation. Therefore, this study designed a 30-minute synchronous cycling intervention scheme to explore the causal relationship between synchronous exercise and interpersonal cooperation. The results showed that synchronous cycling effectively enhanced interpersonal cooperation. This study provides reliable empirical evidence for the study of the relationship between synchronized exercise and interpersonal cooperation.
Meanwhile, this study used near-infrared hyperscanning technology and found that after a single session of synchronous cycling, subjects exhibited significantly enhanced inter-brain synchrony in the dorsolateral prefrontal cortex (DLPFC) during interpersonal cooperation. Moreover, this enhanced synchrony in the DLPFC was positively correlated with behavioral outcomes. This finding is consistent with previous research, indicating that changes in inter-brain synchrony in prefrontal regions predict cooperative behavior. Previous studies have also explored the neural mechanisms behind the relationship between synchronous movement and cooperative behavior using near-infrared hyperscanning technology. For example, Osaka (2014) found that inter-brain synchrony in the right inferior frontal gyrus increased during synchronous humming. Hu et al. (2017) discovered that, compared to asynchronous tapping, synchronous tapping led to enhanced inter-brain synchrony in the middle frontal gyrus, and this enhancement was positively correlated with increased interpersonal cooperative behavior. Liu (2021) simultaneously measured the frontal-temporal changes of 9 subjects during a drumming task using near-infrared hyperscanning technology and found that subjects who drummed synchronously exhibited stronger inter-brain synchrony and higher global network efficiency in the left temporoparietal junction and medial prefrontal cortex. These studies collectively demonstrate the critical role of enhanced inter-brain synchrony in prefrontal regions in the process by which synchronous movement influences interpersonal cooperation. Concurrently, researchers in the field of exercise cognitive neuroscience have found that the effects of a single exercise intervention are influenced by changes in the DLPFC. Specifically, studies by Hyodo (2012) and Byun (2014) both found increased activation in the left DLPFC following a single aerobic exercise intervention. The left DLPFC plays an essential role in cognitive control (Fregni et al., 2005), executive function (Decety et al., 2004), high-level judgment (MacDonald et al., 2000), intention decision-making (Richeson et al., 2003), and multitasking (Miller & Cohen, 2001). Previous research has identified the left DLPFC as a crucial brain region both for the impact of synchrony on interpersonal cooperation and for the effects of exercise interventions. Our study's results indicate that the DLPFC plays a key role in the process by which synchronous cycling influences interpersonal cooperation, likely due to the combined effects of synchrony and exercise. Unfortunately, the current experimental design and results only demonstrate the impact of synchronous movement on interpersonal cooperation, without enabling a deeper understanding of the separate roles played by synchrony and exercise factors.
We sought to explore the underlying reasons behind the improvement in interpersonal cooperation following synchronous cycling. Researchers suggest that synchronous movement, as a socialization process, enhances cooperative behavior by increasing interaction between subjects (Czeszumski et al., 2022). This view is supported by the shared intentionality theory, which posits that when individuals pursue a common goal, their actions are driven by shared intentions and objectives, thereby promoting cooperative behavior (Kirschner & Tomasello, 2010). Furthermore, the perceived similarity theory emphasizes that synchronous behavior strengthens cooperation by increasing perceived similarity between individuals, as people are more inclined to cooperate with those they perceive as similar (Fessler et al., 2014; Rabinowitch et al., 2015). The perception-cognition theory further explains the importance of non-verbal communication in conveying cooperative intentions, suggesting that foreseeing and intentionally signaling cooperation can enhance coordinated behavior. Whether it is perceiving similarity or cooperative intentions, these processes are influenced by cognitive regulation. The better the cognitive function, the smoother the synchronous coordination (Warneken et al., 2012). A meta-analysis by McNeill et al. (2018) has confirmed that exercise significantly improves cognitive functions such as cognitive control, attention, and decision-making ability. Therefore, we speculate that after synchronous cycling, subjects' cognitive functions improve due to the exercise intervention, which in turn facilitates better coordination during the synchronous process. The smoother the synchronization, the stronger the perceived similarity and cooperative intentions between subjects, leading to enhanced interpersonal cooperation behavior.
In summary, this study combined behavioral results and near-infrared hyperscanning brain data to validate the facilitative effect of synchronous cycling on interpersonal cooperation. Compared to previous research, this study designed a structured synchronous exercise intervention program and employed a rigorous randomized controlled trial design alongside innovative neuroimaging techniques. This approach minimized the interference of confounding variables on the results, providing more precise and direct evidence of the positive impact of synchronous cycling on interpersonal cooperation. These findings hold significant implications for clinical and health psychology Healthy social interactions and interpersonal cooperation play a crucial role in maintaining good mental health. Supportive social networks can help individuals alleviate anxiety, depression, and feelings of loneliness, thereby improving their quality of life and sense of well-being. Our study found that a single 30-minute session of synchronous cycling effectively enhances interpersonal cooperation behavior. This finding provides a scientific basis and specific intervention measures for the prevention and treatment of mental health disorders, helping individuals better cope with social isolation and interpersonal relationship difficulties. This is particularly important in the treatment of depression, anxiety, and other psychological disorders (Kawachi & Berkman, 2001). Currently, exercise interventions have become an important means in clinical settings for improving chronic psychological conditions in both the general population and patient groups. Compared to long-term pharmacological treatments, the 30-minute synchronous cycling aerobic exercise intervention proposed in this study is more cost-effective, convenient, widely applicable, and acceptable, with no side effects such as drug dependency.
Of course, this study has several limitations. First, the research primarily focused on the prefrontal cortex, which may lead to the neglect of other potentially important brain regions. Future studies should consider including a broader range of brain regions to comprehensively understand the neural mechanisms of how group exercise influences interpersonal cooperation. Second, although this study successfully explored the causal relationship between synchronous exercise and interpersonal cooperation behavior, it did not analyze in detail which specific types of exercise are most effective in promoting interpersonal cooperation. Subsequent research could further explore the differential effects of various forms of exercise on interpersonal cooperation behavior, thereby providing deeper insights for theoretical and practical research on the relationship between exercise and interpersonal cooperation. Lastly, this study selected college students without regular exercise habits as subjects to explore the impact of synchronous cycling on interpersonal cooperation. We recognize that this sample may not fully represent the broader population. The effects of exercise interventions can be influenced by age and demographic structure, thus affecting interpersonal cooperation behavior. Future research should consider expanding the sample range to include individuals of different age groups, occupational backgrounds, and exercise habits to verify the generalizability of the findings. In conclusion, although this study has taken an important step in understanding the neural mechanisms by which synchronous exercise promotes interpersonal cooperation, more research is needed to overcome these limitations and deepen our understanding of the relationship between exercise and interpersonal cooperation.
ConclusionThis study utilized near-infrared hyperscanning technology to delve into how synchronous cycling between two individuals affects interpersonal cooperative behavior. The results showed that after one synchronous cycling exercise, dyads exhibited significantly enhanced interpersonal neural synchronization in the left dorsolateral prefrontal cortex area during cooperative decision-making, thereby promoting interpersonal cooperative behavior. This finding reveals that synchronous cycling may enhance interpersonal cooperation by enhancing interpersonal neural synchronization in the left dorsolateral prefrontal cortex. Hence, this study not only enriches the theoretical research on the relationship between team sports and interpersonal cooperation but also provides new neuroscientific evidence for promoting interpersonal cooperation through positive interactive exercise methods. These insights offer a reliable neural mechanism explanation for further exploring the impact of exercise on social behavior, highlighting the potential value of synchronous exercise in enhancing interpersonal relationships and social interactions.
FundingThe study was funded by the Open Program of Key Laboratory of Adolescent Health Evaluation and Exercise Intervention, Ministry of Education, East China Normal University, China
Informed consent statementInformed consent was obtained from all subjects involved in the study.