Interoception, which refers to sensing, interpreting, and integrating internal bodily signals, has been suggested to be associated with emotion regulation. Previous research has demonstrated individual differences in interoception and its impact on emotion regulation. However, the priming effect of interoception on emotion regulation and the underlying neural mechanisms remain unknown. This study aims to examine how interoception primes different strategies of cognitive reappraisal, using electroencephalography (EEG). Thirty-seven healthy participants completed an interoceptive priming task. We found that interoception increased the amplitudes of the late positive potential (LPP) during both interpretation and detachment strategies. The priming effect of interoception in enhancing LPP amplitudes lasted longer for interpretation than for detachment. A decrease in alpha power during reinterpretation was observed after interoceptive priming, but not during detachment. The results revealed that interoception enhanced attention to bodily signals associated with negative emotions during cognitive reappraisal. Interoception showed distinct effects on different strategies of cognitive reappraisal, with different underlying neural mechanisms. Interoception-based programs may be an effective way to enhance the capacity for cognitive reappraisal.
Interoception refers to the process by which the nervous system senses, interprets, and integrates signals originating from within the body and contributes to the maintenance of homeostatic functioning (Jeganathan et al., 2024; Khalsa et al., 2018; Nord & Garfinkel, 2022). Dysfunction of interception is increasingly recognized as an important component of different mental health conditions like anxiety disorders and mood disorders (Domschke et al., 2010; Paulus & Stein, 2010). While another hot topic in mental health research is emotion regulation, and the deficits in emotion regulation are related to mental disorders including anxiety, depression, and a variety of other psychopathological symptoms (Gross & Muñoz, 1995; Kraiss et al., 2020). However, limited research has explored the association between interoception and emotion regulation, and the neural mechanisms underlying this relationship remain largely unexplored. Identifying the mechanism of interception during emotion regulation is crucial for guiding the prevention and treatment of mental disorders.
Interoception has been proved to be associated with emotional feeling states. The James-Lange theory of emotion states that the perception of activity within the body constitutes feelings of emotions (Cannon, 1987; James, 1884). Based on this theory, Schachter and Singer (1962) proposed that an emotion occurs when general physiological arousal is perceived, which is labelled cognitively according to contextual social cues. The somatic marker hypothesis emphasizes that generated bodily arousal responses induced by external or internal events feed back to the brain to influence emotional processing (Damasio, 1994). Both theories highlighted that interoception contributes to emotional processing, which is consistent with the modern theory of constructed emotion (Barrett, 2017) and predictive coding model (Seth, 2013). Notably, interoception highlights the importance of the internal environment, and points out that emotional experience is determined by the combination of physiological change and cognitive appraisal, which conflicts with the process model of emotion regulation pioneered by Gross (2015) that focuses on emotion generation in response to external situation. While the process of emotion regulation is also accompanied by physiological changes and cognitive appraisal. However, how interoception influences the physiological and psychological changes during emotion regulation remains largely understudied.
Neuro-anatomical overlap in brain regions involved in interoception and emotion regulation provides a foundation for studying how interoception influences the dynamic physiological changes associated with emotion regulation. A recent neuroimaging meta-analysis of interoception and emotion regulation indicated that anterior insula and connection with anterior cingulate cortex (ACC) were recruited during interoception and emotion regulation (Tan et al., 2022). Moreover, the anterior insula receives bodily signals from the mid- and posterior insula and sends predictions to the hypothalamus and brainstem (Barrett & Simmons, 2015; Seth, 2013). Additionally, the anterior insula is closely connected structurally and functionally with ACC as part of a cortical ‘salience network’ which is believed to detect emotional saliency (Menon & Uddin, 2010; Uddin, 2015). Therefore, interoception may influence the dynamic processing of negative emotion during emotion regulation.
According to the process model of emotion regulation (Gross, 2015), various strategies can be employed to achieve emotion regulation, including situation selection, situation modification, attentional deployment, cognitive change, and response modulation. Among these strategies, cognitive reappraisal, which focuses on altering one's appraisal of emotional events (Buhle et al., 2014), has been shown to be more effective in reducing negative emotions compared to other strategies such as suppression and distraction (Chen, Long, et al., 2023; John & Gross, 2004; Webb et al., 2012). Functional magnetic resonance imaging study revealed that ACC and insular cortices are activated during cognitive reappraisal (Dörfel et al., 2014; Picó-Pérez et al., 2019), which further support the idea that interoception would play a critical role in cognitive reappraisal. However, cognitive reappraisal can be implemented through the tactics of detachment and reinterpretation (Powers & LaBar, 2019). Detachment involves distancing oneself from an emotional event, adopting a non-involved observer perspective, while reinterpretation refers to construing an emotional stimulus in a positive light (Ochsner et al., 2012). Whether interoception has varying effects on the two cognitive reappraisal strategies still remains unknown.
While imaging studies have investigated the common neural basis of both interoception and cognitive reappraisal, there is limited research exploring the temporal dynamics of how interoception impacts cognitive reappraisal. Electroencephalography (EEG) with high temporal resolution allows for insights into how the interoception influences the time course of emotion processing during cognitive reappraisal. To date, limited research has utilized event-related potential (ERP) technology to investigate the effect of interoception on cognitive reappraisal. Pollatos et al. (2007) first explored the differences in emotional experience using EEG for participants with high or poor interoceptive awareness, which revealed that the late positive potential (LPP) amplitudes to pleasant and unpleasant pictures were enhanced for subjects with high interoceptive awareness. In addition, interoceptive awareness is related to an enhanced activation in insula, anterior cingulate, and prefrontal cortices. These results were subsequently confirmed by Herbert et al. (2007). Füstös et al. (2013) first examined the association between interoception and the LPP amplitude during cognitive reappraisal. The positive association between interoception and the decrease of LPP amplitude during cognitive reappraisal was observed.
These limited studies have revealed the association between interoception and emotion processing. However, several issues remain unexamined. Firstly, critically, Füstös et al. (2013) only explored the association between interoception and reinterpretation, one of the strategies in cognitive reappraisal. It is unknown whether interoception relates to detachment. Secondly, the level of interoception was measured using the heart count task and classified into high or low groups in studies by Herbert et al. (2007) and (Pollatos et al., 2007), which revealed individual differences in interoception but did not reflect the effect of interoceptive priming on emotion regulation. Additionally, the effect of exteroception remains unknown. Thirdly, the potential neural communication mechanisms, such as neural oscillations, underlying the effect of interoception on emotion regulation have not been examined. Fourthly, the findings between Füstös's and Pollatos's study were confilicting. Interoception enhanced the LPP amplitude in Pollatos's and Herbert's study (Herbert et al., 2007; Pollatos et al., 2007), while interoception showed higher decrease of the LPP amplitude in Füstös's study (Füstös et al., 2013). Therefore, it is necessary to further explore the effect of interoception on cognitive reappraisal.
The present study aims to replicate previous findings and further examine the effect of interoceptive priming on cognitive reappraisal, focusing on priming effects rather than individual differences in interoception. Additionally, this study will investigate the effect of interoceptive priming on both strategies of cognitive reappraisal: reinterpretation and detachment. Previous research has demonstrated that interoception modulates the LPP in response to unpleasant content (Füstös et al., 2013; Herbert et al., 2007; Pollatos et al., 2007). Therefore, LPP is considered an appropriate candidate for assessing the effects of interoception on the time course of cognitive reappraisal. Building on the findings that individual differences in interoception enhanced the amplitudes of LPP in response to unpleasant stimuli (Herbert et al., 2007; Pollatos et al., 2007), we hypothesize that interoceptive priming will similarly increase LPP amplitudes during both interpretation and detachment strategies. Given that prior studies indicated distinct neural bases for these two strategies (Dörfel et al., 2014), we predict that the underlying neural mechanisms linking interoception to reinterpretation and detachment will also differ. Thus, the neural oscillatory mechanisms that underlie these effects will be investigated in the current study.
Material and methodsParticipantsThirty-seven healthy right-handed participants (33 females, mean age = 21.30 ± 1.84 years) were recruited. All the participants completed Beck Anxiety Inventory (BAI) and Beck Depression Inventory (BDI). Nobody met the criteria for anxiety (mean BAI = 5.05 ± 4.65, cut-off score <45) and depression (mean BDI = 4.05 ± 3.47, cut-off score <21). Primary criteria for inclusion were having normal or corrected to normal vision, reporting no history of psychiatric or neurological disorders and being fluent Chinese speakers. They all gave their written informed consent prior to the experiment. Each participant received 50 Chinese Yuan for finishing the study. The experiment procedures were approved by the Ethics Committee of the Department of Applied Psychology in the author's university and the experiment was conducted in accordance with approved guidelines.
A post-hoc power analysis using G*Power (Kang, 2021) indicated that with a final sample size of n = 32 and α = 0.05, the study achieved a power of 0.82 to detect a small effect size (f = 0.20).
Stimulus materialsTwo hundred and forty pictures (180 negative and 60 neutral) were selected from the International Affective Picture System (IAPS) (Lang, 2005). According to the valence and arousal ratings in the IAPS datasets, when compared with neutral pictures, negative pictures had lower normative valence t(164.89) = 33.35, P < 0.001 (negative: M = 2.50, SD = 0.68, neutral: M = 4.99, SD = 0.42) and higher arousal t(101.13) = 29.38, P < 0.001 (negative: M = 6.00, SD = 0.67, neutral: M = 3.07, SD = 0.67). The 180 negative pictures were divided into three groups (60 for detachment, 60 for reinterpretation, and 60 for negative view), which did not differ in valence and arousal (Ps > 0.55). Stimuli were presented on a black background on a 21-inch monitor, and participants were seated in a separate room approximately 70 cm from the screen. The task was presented using E-prime 2 stimulus presentation software (Schneider et al., 2002).
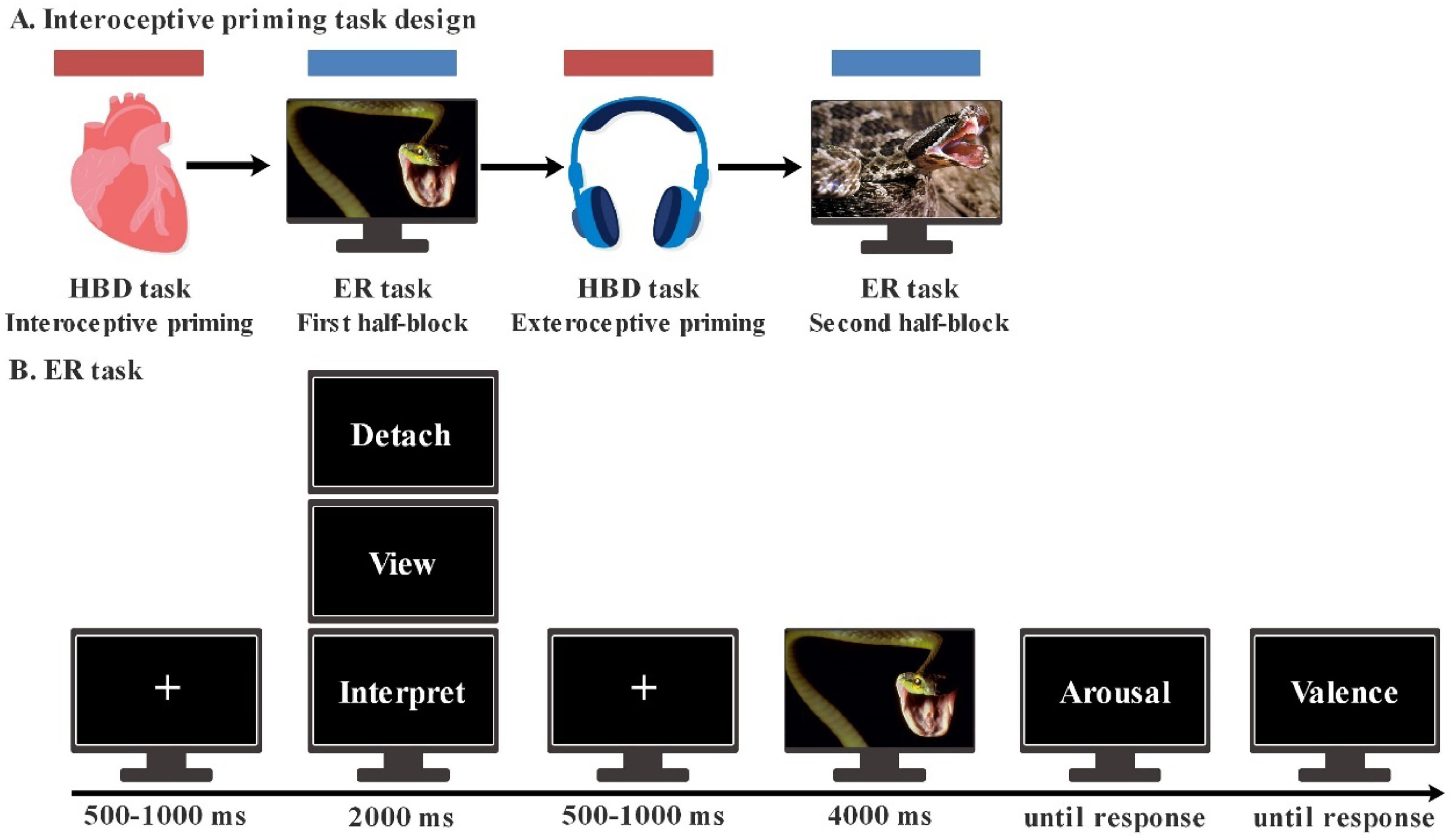
Interoceptive priming taskInteroceptive priming task (IPT) was developed to reveal novel links between interoception and emotion in the current study (Fig. 1), which included two phases: (1) a tapping-phase, in which participants have to follow their own heartbeats (interceptive condition [Intero]) or a simulated heartbeat audio (exteroceptive condition [Extero]); and (2) a subsequent ER phase, in which subjects were requested to regulate the emotion elicited by pictures. The tapping-priming phase consisted of four blocks of 4 min each, each block included both Intero and Extero condition of 2 min each, which were counterbalanced across participants. Immediately after each tapping-priming condition, a block of the ER phase was administered. Before the test, participants had a 2 min tapping practice phase and an ER practice phase. EEG recordings were obtained during the whole IPT.
Interoceptive priming task design and the trial structure of emotion regulation task. A. Interoceptive priming task. The priming phase included interoceptive and exteroceptive condition. One block of ER task was departed into first and second half-block and was performed immediately after heartbeat detection (HBD) task. B. emotion regulation task. Illustration of timing and sequence of stimuli on screen.
For the tapping-priming phase, a heartbeat detection (HBD) task was used (Garfinkel et al., 2015; Richter et al., 2021; Salamone et al., 2021). Previous studies have revealed that HBD were based more on sensations generated by heartbeats rather than on personal beliefs or accurate knowledge of heart rate when compared to the heartbeat counting task (Ring & Brener, 2018). HBD has been proved with significant interoceptive priming effect in emotional processing (Salamone et al., 2021). Following the instruction in previous research (Garfinkel et al., 2015; Salamone et al., 2021), participants were required to tap a “Z” key along with their own heartbeats (Intero) or simulated heartbeat sounds (Extero). Four blocks were presented for each condition. In each block, for Intero condition, participants were instructed to focus on their heartbeat and tap along with each perceived beat without using any external aids or visual cues. Each participant was provided with the following instructions: “Tapping task following your own heartbeat. Press Z every time you consider your heart beats. Not allowed to abuse the system by pressing your veins or any other kind of cheat.”. In Extero condition, subjects were asked to close eyes and follow an audio recording of a simulated heartbeat for 2 min. Each participant was provided with the following instructions: “Tapping task following an external heartbeat. Press Z every time you hear the heart beating.”.
For the ER phase, a classical cognitive reappraisal task was used in the current study based on previous research (Qi et al., 2017; Thiruchselvam et al., 2011) . The ER task comprised 240 trials, divided into 4 blocks of 60 trials each. Four types of ER trials were conducted: viewing neutral pictures (View-neutral), viewing negative pictures (View-negative), reinterpreting a negative picture in a positive way (Reinterpretation), and detachment from the negative image (Detachment). To decrease the likelihood that participants mixed the two different strategies, each block contained 15 View-neutral trials, 15 View-negative trials, and 30 Reinterpretation or 30 Detachment trials following previous studies (Chen, Long, et al., 2023; Chen, Oei, et al., 2023; Thiruchselvam et al., 2011). Each ER block was departed into two parts to match with the Intero or Extero condition in tapping-priming phase. The order of the ER blocks was counterbalanced across Intero and Extero condition as well as subjects. Participants were asked to look at an image directly and allow themselves to feel emotions in view neutral or negative trials. In the Reinterpretation trials, participants were required to reinterpret the image in a positive way to decrease the negative feelings that they experienced. In the Detachment trials, participants were told to look at an image directly but try to take the position of a neutral, noninvolved observer.
The trial structure for the ER task was presented in Fig. 1B. Each trial began with a white fixation cross for 500-1000 ms, followed by the instruction cue (View, Interpret, or Detach) displayed in white text on a black background for 2 s. After this, participant viewed a white fixation cross for 500-1000 ms, prior to seeing picture for 4 s. After picture offset, participants were instructed to rate their level of valence (until response) and arousal (until response) from 1 to 9 point (Lang, 1980). Higher numbers indicated more arousing and more pleasant pictures.
EEG recording and data reductionContinuous EEG was recorded using an Ag/AgCl electrode Brain Products EasyCAP (ActiCHamp plus, Brain Products, GmbH, Gilching, Germany). Sixty electrode sites were used based on the 10/20 system. Data were collected using Brain Vision Recorder and actiCHamp amplifier at 1000 sapling rate, using FCz as a digital reference. The impedances were set at ≤10 KΩ for all apparatuses. Data were re-referenced offline to TP9/TP10, which located approximately at the mastoids.
ERP analysisFor event-related potential analysis, EEG data were processed offline using EEGLAB toolbox (Delorme & Makeig, 2004), an open access toolbox running in Matlab environment. EEG data was filtered with a 0.1 Hz high-pass filter and 30 Hz low-pass filter. The data was segmented into epochs with a time window from -500 to 2000ms. Baseline correction was performed by subtracting the mean of the 500ms before picture onset. To identify and remove trials containing nonstationary artifacts, we considered a trial to be bad if its absolute z-score across trials exceeds 3 on any of the following metrics: 1) the mean across channels of the voltage range within the trial, 2) the mean across channels of the variance of the voltages within the trial, and 3) the mean across channels of the difference between the mean voltage at that channel in the trial in question and the mean voltage at that channel across all trials.
To identify and remove stationary artifacts such as eye blinks, eye-movements, muscle movements, and the cardiac field artifact, the pruned EEG data was subjected to independent component analysis (ICA) with the runica function of EEGLAB. And the IClevel toolbox was used (Pion-Tonachini et al., 2019). A threshold of more than 90% was set for eye blinks and muscle movement artifacts, and a threshold of more than 10% was applied for cardiac field artifact (Pion-Tonachini et al., 2019). Finally, each of the components automatically marked for rejection was verified by visual inspection.
Previous studies demonstrating that LPP is typically the highest at the centroparietal sites (Paul et al., 2013; Thiruchselvam et al., 2011). LPP was quantified as the average signal amplitude collapsed across the sensors within the centroparietal region (CP3, CP1, CPz, CP2, CP4, P1, Pz, and P2) between 300 and 1100 ms after image onset. To explore the nuanced differences throughout the LPP and the dynamic effects of interoceptive priming on reinterpretation and detachment, the waveform was split into four 200-ms time windows following previous LPP studies (Paul et al., 2013; Qi et al., 2017).
Time frequency decompositionFieldTrip was used for time–frequency analysis of the EEG data (Oostenveld et al., 2011). The EEG data was re-segmented into epochs with a time window from -500 to 4000ms. The preprocessing procedure was similar to ERP analysis in removing stationary or nonstationary artifacts. Data were converted to the time–frequency domain using a multi-taper transformation based on multiplication in the frequency domain (cfg.method = ‘mtmconvol’). A time window of 500 ms was used for each frequency (1 Hz steps between 1 to 30 Hz) and time point (50 ms steps). The decomposed time-frequency representation was normalized by baseline activities from -500 to 0 ms relative to the picture onset. These normalized time-frequency representations were averaged across trials for each condition for each participant. To examine the effects of the interoceptive priming on different ER types, we implemented cluster-based permutation test for each frequency band. All cluster-based permutation test were conducted as follows: First, a within-subject t-test were applied to compare Intero and Extero conditions for each time point and electrode; neighboring data points with an alpha value of less than 0.05 were clustered. A minimum cluster size of the two electrodes was imposed to identify the neighbor electrodes. Then, a permutation test with 1,000 random draws using the Monte Carlo method were applied to establish a reference distribution and identify the clusters. The final t-values of the clusters were calculated by summing all t-values within the corresponding clusters. The cluster's Monte Carlo P value was considered to be significant at α = 0.05.
Statistical analysesAll statistical analyses were conducted in R (version 4.2.1) (R Core Team, 2022)and data figures were made using ggplot2 package (Wickham et al., 2016). The level of significance was set to α = 0.05. To examine the effect of interceptive priming on different ER strategies, a within-subjects design was used in the current study, and a 2 (condition: Intero, Extero) × 4 (ER types: View-neutral, View-negative, Reinterpretation, Detachment) repeated measures analysis of variance (ANOVA) was performed for behavioral outcome (arousal and valence rating) and LPP data. The afex package in RStudio were used in the current study (Singmann et al., 2015). All ANOVAs were reported with partial eta-squared (ηp2) as measure of the effect size (Kelley & Preacher, 2012). When the sphericity assumption was violated, Greenhouse-Geisser correction was used. Cohens’ d was used to measure the effect size for simple effect or post-hoc test. Effect sizes for planed comparisons were computed with effectsize package (Ben-Shachar et al., 2020). Multiple comparisons were adjusted using the Hommel correction (Hommel, 1988).
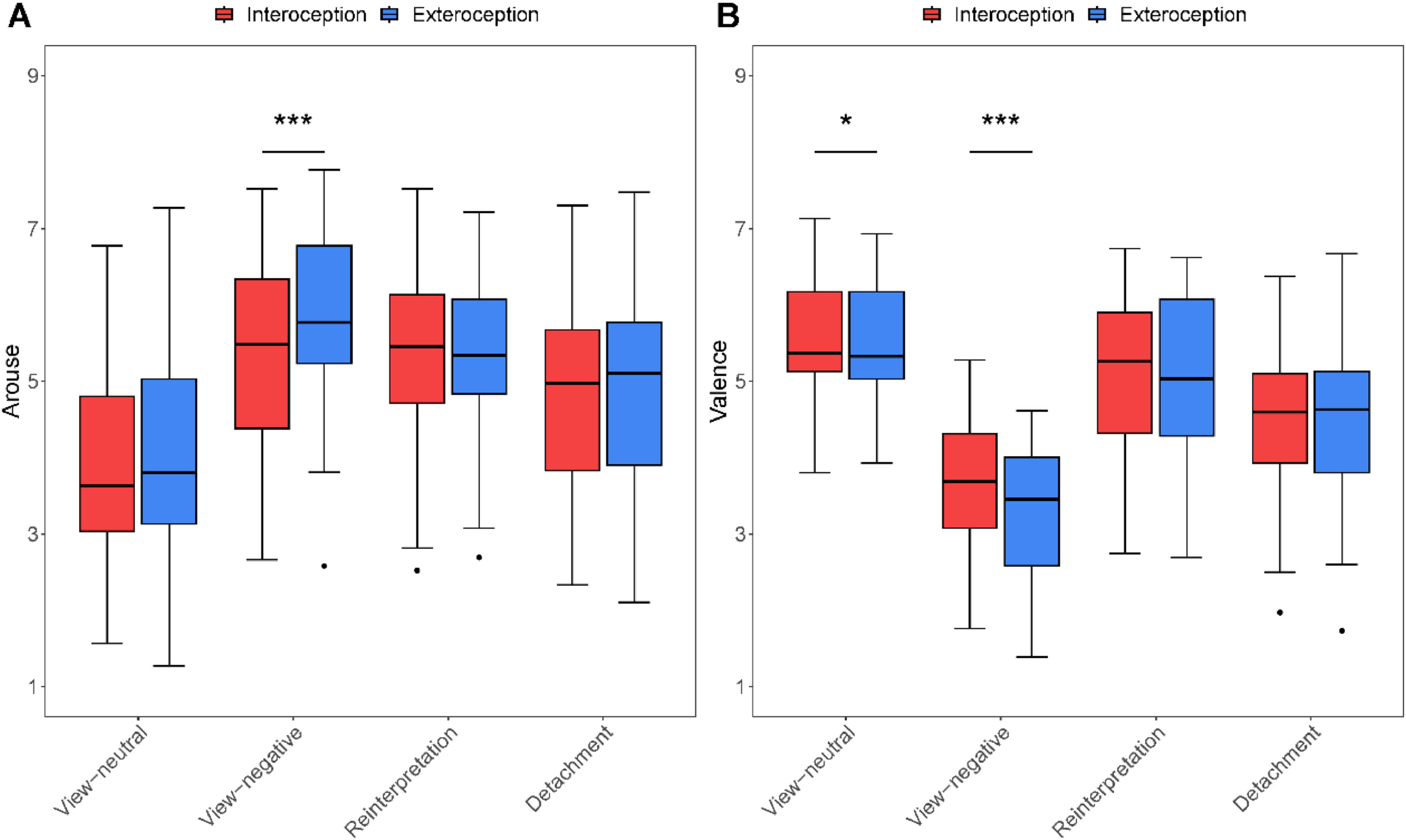
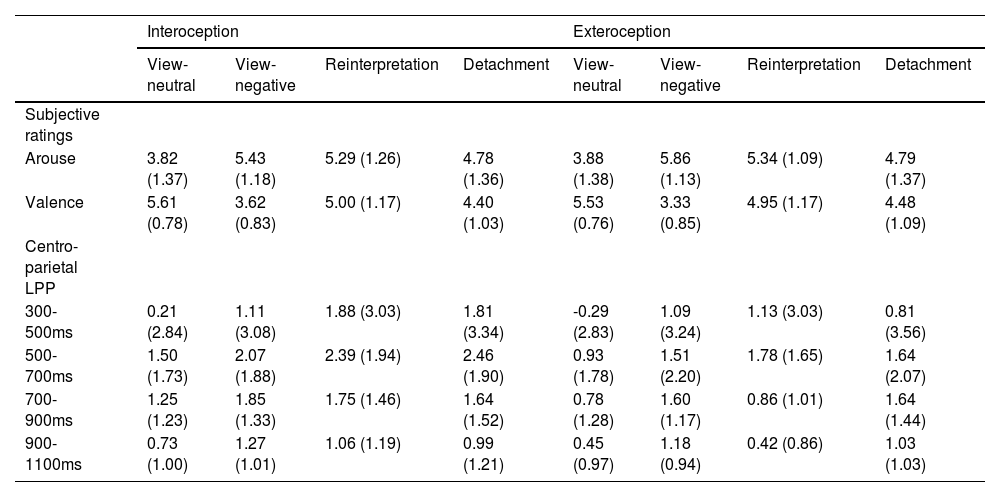
ResultsBehavioral resultsThe main effects of condition [F(1,36) = 9.71, p = 0.004, ηp2 = 0.21] and ER types [F(3,108) = 54.86, p < 0.001, ηp2 = 0.60] were observed as significant for arouse. There was also a significant interaction between condition and ER types [F(3.108) = 8.03, p < 0.001, ηp2 = 0.18]. Simple effect test revealed that interoceptive priming (M = 5.43, SD = 1.18) showed lower arouse than exteroceptive priming (M = 5.86, SD = 1.13) in viewing negative pictures [t(36) = -6.72, p < 0.001, d = -1.15, Fig. 2A, Table 1].
Mean (SD) subjective ratings and centro-parietal LPP.
For valence, The main effects of condition [F(1,36) = 5.94, p = 0.020, ηp2 = 0.14] and ER types [F(3,108) = 71.87, p < 0.001, ηp2 = 0.67] were significant. The interaction term between condition and ER types [F(3.108) = 7.17, p < 0.001, ηp2 = 0.17] was also significant for valence. Simple effect test revealed that interoceptive priming presented higher valence (view-neutral: M = 5.61, SD = 0.78; view-negative: M = 3.62, SD = 0.83) than exteroceptive priming (view-neutral: M = 5.53, SD = 0.76; view-negative: M = 3.33, SD = 0.85) in viewing neutral pictures [t(36) = 2.20, p = 0.034, d = 0.27, Fig. 2B, Table 1] or negative pictures [t(36) = 4.60, p < 0.001, d = 0.95, Fig. 2B, Table 1].
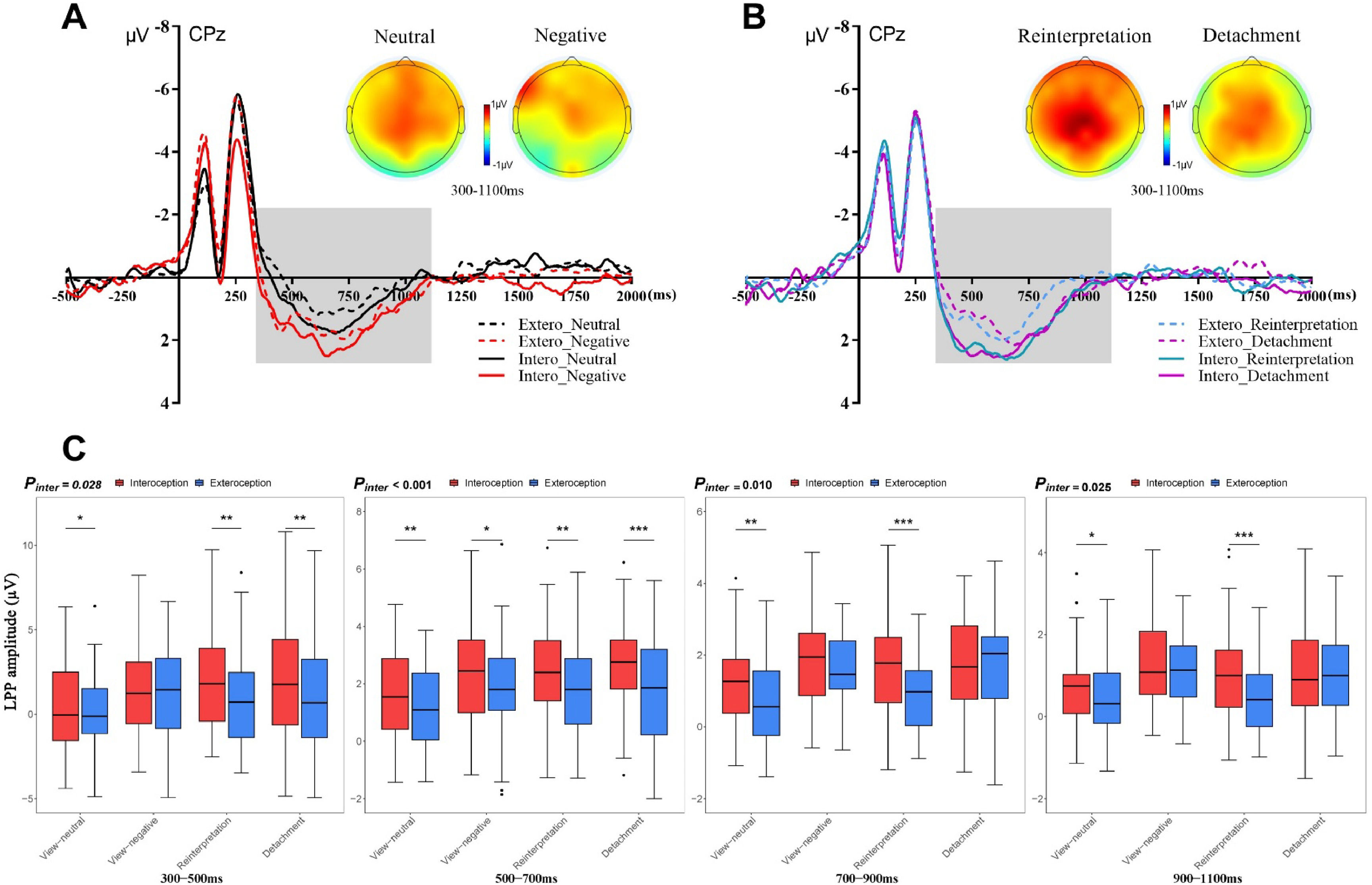
EEG resultsERPFig. 2 depicts grand-averaged waveforms for viewing neutral or negative pictures (Fig. 2A) and reinterpretation or detachment of negative pictures (Fig. 2B) at the centro-parietal areas after interceptive and exteroceptive priming. Fig. 2C showed the LPP differences between interoceptive and exteroceptive priming for four types of ER task in four different time windows. For the LPP in a 300-500 ms time window, the significant main effects of condition [F(1,36) = 15.63, p < 0.001, ηp2 = 0.30] and ER types [F(3,108) = 22.88, p < 0.001, ηp2 = 0.39] were observed. The interaction item between condition and ER types was also significant [F(3,108) = 3.23, p = 0.028, ηp2 = 0.08]. Simple effect test indicated that interoception primed larger LPP amplitude than exteroception on view-neutral [Intero: M = 0.21, SD = 2.84; Extero: M = -0.29, SD = 2.83; t(36) = 2.11, p = 0.042, d = 0.40], reinterpretation [Intero: M = 1.88, SD = 3.03; Extero: M = 1.13, SD = 3.03; t(36) = 3.26, p = 0.003, d = 0.61], and detachment trials [Intero: M = 1.81, SD = 3.34; Extero: M = 0.81, SD = 3.56; t(36) = 3.54, p = 0.001, d = 0.81, Fig. 3C, Table 1].
Grand-averaged waveforms elicited by pictures in viewing (A) and reappraising (B) condition at centro-parietal areas, and the LPP differences in four different time windows across four types (C). *p<0.05, **p<0.010, ***p<0.001, significant difference between interoception and exteroception.
For the LPP in a 500-700 ms time window, Only the main effect of condition [F(1,36) = 32.13, p < 0.001, ηp2 = 0.47] and the interaction between condition and ER types were significant [F(3,108) = 11.92, p < 0.001, ηp2 = 0.25]. Simple effect test demonstrated that interoception primed larger LPP amplitude than exteroception on view-neutral [Intero: M = 1.50, SD = 1.73; Extero: M = 0.93, SD = 1.78; t(36) = 2.90, p = 0.006, d = 0.74], view-negative [Intero: M = 2.07, SD = 1.88; Extero: M = 1.51, SD = 2.20; t(36)= 2.28, p = 0.029, d = 0.13], reinterpretation [Intero: M = 2.39, SD = 1.94; Extero: M = 1.78, SD = 1.65; t(36) = 3.21, p = 0.003, d = 0.79], and detachment trials [Intero: M = 2.46, SD = 1.90; Extero: M = 1.64, SD = 2.07; t(36) = 3.88, p < 0.001, d = 1.07, Fig. 3C, Table 1].
For the LPP in a 700-900 ms time window, there were the significant main effects of condition [F(1,36) = 13.76, p < 0.001, ηp2 = 0.28] and ER types [F(3,108) = 9.42, p < 0.001, ηp2 = 0.21], as well as the significant interaction between condition and ER types [F(3,108) = 4.02, p = 0.010, ηp2 = 0.10]. Simple effect test revealed that interoception primed larger LPP amplitude than exteroception on view-neutral [Intero: M = 1.25, SD = 1.23; Extero: M = 0.78, SD = 1.28; t(36) = 3.12, p = 0.004, d = 0.50] and reinterpretation trials [Intero: M = 1.75, SD = 1.46; Extero: M = 0.86, SD = 1.01; t(36) = 4.69, p < 0.001, d = 0.96, Fig. 3C, Table 1].
For the LPP in a 900-1100 ms time window, there were the significant main effects of condition [F(1,36) = 7.53, p = 0.009, ηp2 = 0.17] and ER types [F(3,108) =13.43, p < 0.001, ηp2 = 0.27], as well as the significant interaction between condition and ER types [F(3,108) = 3.38, p = 0.025, ηp2 = 0.09]. Simple effect test revealed that interoception primed larger LPP amplitude than exteroception on view-neutral [Intero: M = 0.73, SD = 1.00; Extero: M = 0.45, SD = 0.97; t(36) = 2.10, p = 0.043, d = 0.37] and reinterpretation trials [Intero: M = 1.06, SD = 1.19; Extero: M = 0.42, SD = 0.86; t(36) = 3.64, p < 0.001, d = 0.85, Fig. 3C, Table 1].
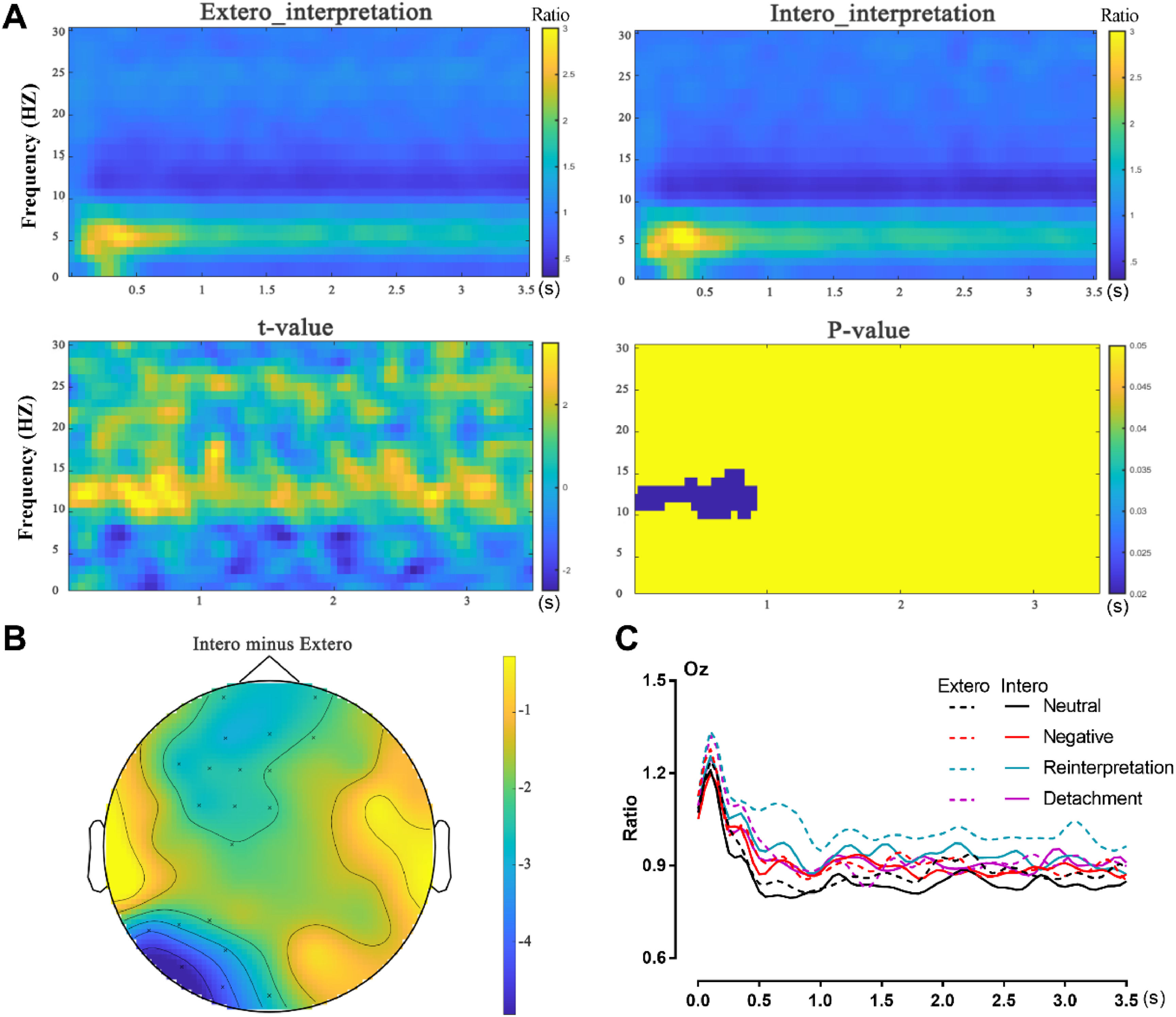
Time frequency resultOnly the significant results of time frequency decomposition for reinterpretation after interoceptive or exteroceptive priming were presented in Fig. 4. Panel A on Fig. 4 depicts spectral perturbations in different frequencies recorded in this study. The plot revealed that differences between Intero and Extero condition occurred in alpha range lasting for ∼1 s from the stimulus presentation (Fig. 4A, p = 0.018). The follow-up analysis comparing the differences between Intero and Extero condition revealed one significant negative cluster on the frontal sites and left occipital area (Fig. 4B, p = 0.020), indicating that the alpha power evoked significantly lower by interoceptive than exteroceptive priming. The relative power of all conditions in occipital area was also presented in Fig. 4C.
Time frequency representation of reinterpretation after exteroceptive or interoceptive priming (top panel A), and the contrast between exteroceptive and interoceptive attention and significant alpha cluster (bottom panel A). The topographic distribution of the alpha band, asterisk indicates the significant cluster in frontal and occipital area (B). The relative power of all conditions in occipital area (C).
Despite ongoing research concerning the important role of interoception in emotion regulation (Kever et al., 2015; Tan et al., 2023), there is limited exploring the neural mechanisms behind their association, especially the association between interoception and different strategies of emotion regulation. To examine the effect of interoception on emotion regulation, the present study employed EEG and an interoceptive priming task to explore the psychophysiological mechanisms underlying the effect of interoceptive priming on two tactics of cognitive reappraisal. In self-reported emotional ratings, interoception decreased arousal ratings and increased valence ratings when viewing negative pictures, while it did not show significant effects on reinterpretation or detachment. ERP results showed that interoception enhanced LPP amplitudes for both reinterpretation and detachment in the early time window, but only increased LPP amplitudes for reinterpretation in the late time window. Additionally, interoceptive priming evoked significantly lower alpha power for reinterpretation but not detachment compared to exteroceptive priming at the frontal sites and occipital area. These results indicate that the effect of interoceptive priming differs between the two strategies of cognitive reappraisal and is supported by distinct neural mechanisms.
One main finding was that interoception decreased arousal and increased valence when viewing negative pictures but did not affect the reinterpretation or detachment of these pictures. This suggests that interoceptive priming can enhance self-reported emotional feelings regardless of the use of cognitive reappraisal strategies. Concerning the valence, our data first indicated that interception increased the valence of negative stimuli, which extended previous findings that arousal may be more closely tied to interoception than valence (Füstös et al., 2013; Herbert et al., 2007; Pollatos et al., 2007). Furthermore, valence and arousal might be impacted by interoception the differences between them might be due to the distinct processes in interoceptive pathways (Feldman et al., 2024). In addition, the finding about arousal is inconsistent with previous research on the impact of individual interoception habits on emotional arousal ratings (Pollatos et al., 2007). One possible reason is that different interoception tasks were used in the two studies. The heartbeat counting task employed in Pollatos et al. (2007) may be less effective in priming interoception (Ring & Brener, 2018), which could result in a reduced ability to decrease arousal associated with negative emotions. Additionally, the interoceptive priming task focuses on short-term effects on emotional processing, whereas individual interoception habits are more relevant to emotion processing in daily life. Prior research has shown that brief mindfulness meditation can improve emotional intensity (Wu et al., 2019), suggesting that interoception-based mindfulness training could be an effective method for enhancing emotion processing.
Our ERP findings in the current study extended previous research on the relationship between interoception and emotion processing. The results showed that interoception increased LPP amplitudes during interpretation, revealing that interoception enhances detection to self-embodied emotional states during emotion regulation (Critchley & Garfinkel, 2017). Previous emotion regulation research, particularly based on Gross (2015) process model, has primarily focused on LPP during the regulation phase rather than the emotion generation phase. The possible reason for the interoception-related enhancement of LPP amplitudes may be the increased interoceptive attention during the emotion generation phase. Recent research using Bayes factors to deconstruct the brain bases of emotion regulation revealed that a fronto-parietal-insular system engaged by emotion generation (Bo et al., 2024), which demonstrated that interoceptive priming increased interoceptive attention during the emotion generation phase. Thus, the process model of emotion regulation should consider not only how to regulate the influence of external situations but also changes in internal bodily states. Additionally, this finding contrasts with Füstös et al. (2013). One possible explanation is that the HBD task was employed in the current study instead of the heartbeat counting task. Previous research has shown that the HBD task is more effective than the heartbeat counting task in detecting heartbeat based on sensation rather than belief (Ring & Brener, 2018). Moreover, the significant interoceptive priming effect on emotional processing was demonstrated using the HBD task (Salamone et al., 2021). Additionally, the ER task was conducted immediately after the HBD task, with a much shorter interval between the HBD and ER tasks compared to the interval between the heartbeat counting and ER tasks in Füstös et al. (2013). In other words, this study emphasizes the priming effect of interoception rather than individual differences. Future research should further explore the differences between interoceptive priming and individual interoceptive habits, as well as their combined roles.
The effect of interoception on detachment was first observed in the current study. Detachment, by definition, involves distancing oneself from an emotional event, suggesting that the process of detachment includes both emotion generation and regulation phases. The increased LPP following interoceptive priming during detachment may be due to enhanced detection of self-embodied emotional experiences. Additionally, detachment is associated with spatial attention (Ochsner et al., 2012; Powers & LaBar, 2019). During HBD task, the participants were instructed to focus on their heart region. Thus, the spatial attention would be enhanced in HBD, which could facilitate the interoception attention to internal emotional states during detachment. Notably, the duration of interoceptive priming for detachment was shorter than for reinterpretation, supporting previous findings that reinterpretation is more effective than detachment (Chen, Long, et al., 2023). Studies investigating the neural correlates of interpretation and detachment have revealed different brain region activations. The insula is more engaged during reinterpretation than detachment (Dörfel et al., 2014), which may explain the differing durations between reinterpretation and detachment.
To our knowledge, this study was the first to observe a decrease in alpha power during reinterpretation after interoceptive priming, demonstrating that interoception enhances individual attention to internal bodily signals during reinterpretation. This finding contrasts with previous research on attentional mechanisms during heartbeat tasks (Villena‐González et al., 2017). Villena‐González et al. (2017) found that interoception was associated with an increase in posterior alpha band activity. Notably, different tasks were used in both studies. Villena‐González et al. (2017) employed a heart count task, while the current study used an HBD task. Additionally, the observed decrease in alpha power during reinterpretation in our study occurred spontaneously, without requiring participants to focus on their hearts as in Villena‐González et al. (2017) study. This requirement may have increased cognitive demand, indexed by the increase in alpha power. Previous research has shown that suppression of alpha-band activity is related to improved detection performance (Klimesch, 2012), suggesting that interoceptive priming may enhance detection of emotional feelings during reinterpretation. However, we did not find any significant time-frequency results for detachment. A plausible reason for this could be the difference in neural mechanisms between reinterpretation and detachment. Functional magnetic resonance imaging (fMRI) studies have shown that the insula is activated during reinterpretation (Dörfel et al., 2014), indicating that interoceptive changes may occur during reinterpretation rather than detachment. The neural mechanisms underlying the effect of interoception on different strategies of emotion regulation need to be further explored in future fMRI studies. In addition, modulating alpha oscillation with transcranial electric stimulation may be a plausible way to promote individual interoception and cognitive reappraisal.
These innovative findings provide empirical evidence for a deeper understanding of the psychophysiological mechanisms underlying interoception and emotion regulation. In theory, these innovative findings extend the process model of emotion regulation (Gross, 2015), which emphasizes the need to consider both external and internal situations. Exploring the body-brain interplay of interoception in response to external situations may provide new insights into the processes of emotion generation and regulation. However, there are several limitations. First, while heartbeat counting task and HBD are commonly used to measure interoception (Ring & Brener, 2018), only the HBD task was performed in the current study. The effect of interoception on emotion regulation in this study needs further validation using other interoceptive tasks like heartbeat counting task to determine if similar effects are observed. Second, To observe the effect of interoceptive priming, the duration of interoceptive priming was controlled based on the research by Salamone et al. (2021), aligning the length of the emotion task with the priming task as closely as possible. The exact duration of the priming effect needs further examination. Third, the sex imbalance in the sample may limit the generalizability of the findings in the current study. Given that sex differences in interoception have been reported (Prentice & Murphy, 2022), future research should examine the role of sex in the relationship between interoception and emotion regulation. Fourth, this study only explored the effects of interoception on negative emotions. Investigating the effects on positive emotions could be an interesting and valuable topic for future research.
ConclusionIn conclusion, the findings of the present study replicate and extend those of previous studies investigating the relationship between interoception and emotion regulation (Füstös et al., 2013; Herbert et al., 2007; Pollatos et al., 2007; Tan et al., 2023). We highlight the role of internal bodily states during cognitive reappraisal, enriching the process model of emotion regulation that traditionally focuses on external situations (Gross, 2015). The distinct effects and neural mechanisms of interoceptive priming on different strategies of cognitive reappraisal provide evidence for the potential efficacy of mindfulness or interoception-based training programs. Future research should further investigate the neurobiological underpinnings of interoception in emotion regulation using fMRI, as well as the effects of tailored interoception-based interventions—such as virtual reality or neurofeedback—on promoting emotion regulation in clinical practice.
CRediT authorship contribution statementLC: Methodology, Writing- Original draft preparation, Writing- Review & Editing; FC: Conceptualization, Investigation, Supervision, Writing- Original draft preparation; KB: Methodology, Investigation; JS: Supervision; RZ: Writing- Original draft preparation, Writing- Review & Editing.
Ethics approval statementThe project was approved by the Ethics Committee of the Nantong university and carried out in accordance with the approved guidelines.
FundingThis work was supported by National Natural Science Foundation of China (32400875), Humanities and Social Science Fund of Ministry of Education in China (24YJC190003), Natural Science Foundation of Jiangsu Province (BK20230609), Research on Philosophy and Social Sciences in Higher Education Institutions in Jiangsu Province (2023SJYB1698). We are grateful to the study participant and their families.
Data availabilityThe datasets analyzed during the current study are available at https://osf.io/b8j24/.