The “Social Motivation” hypothesis posits that social deficits in autism spectrum disorder (ASD) arise from altered reward perception. However, few studies have examined neural and behavioral responses to social reward-related cues in low functioning ASD children with limited cognitive or language abilities.
ObjectiveThis study investigated if young children with ASD show atypical gaze towards happy faces and its association with altered brain reward responses.
MethodsEye-tracking was performed in 36 ASD and 36 typically developing (TD) children (2.5–6 years) viewing happy faces of children or emoticons. Functional near infrared spectroscopy was used to record group differences in orbitofrontal cortex (OFC) activation simultaneously.
ResultsChildren with ASD showed increased pupil diameter and OFC activation compared to TD children when viewing all happy faces and gazed less at the eyes of actual faces and the mouths of emoticons. These atypical responses was associated with lower adaptive behavior scores and greater symptom severity.
ConclusionOur research reveals distinct neural hyperactivity and viewing patterns in young children with ASD when presented with reward-related facial stimuli. These results contradict the Social Motivation Hypothesis. Children with ASD exhibit heightened levels of arousal and employ less efficient facial processing strategies. This heightened demand for cognitive resources could have long-term effects on children's well-being and may hinder their ability to develop adaptive skills effectively.
Autism Spectrum Disorder (ASD) is a neurodevelopmental condition characterized by challenges in social communication and repetitive, restrictive behaviors or interests (American Psychiatric Association, 2013). Numerous studies have consistently shown that individuals with ASD have a reduced focus on and preference for social cues, while showing heightened responses to specific non-social stimuli compared to typically developing (TD) individuals (Clements et al., 2018). The social challenges observed in individuals with ASD during social information processing are believed to be primarily due to a decrease in social motivation, as proposed by the social motivation hypothesis (Chevallier et al., 2012; Clements et al., 2018).
An altered perception of social rewards, such as positive facial expressions, is seen as a key indicator supporting the social motivation hypothesis (Jack & Schyns, 2015; Frazier et al., 2017; Chevallier et al., 2012). Previous studies have shown that individuals with ASD perceive social cues, like happy faces, as less important and rewarding, which can lead to difficulties in processing social information (Chevallier et al., 2012). Additionally, individuals with ASD may not focus on pivotal social reward cues, such as the eyes when viewing faces (Kwon et al., 2019). A possible explanation for the difficulties individuals with ASD experience in social interactions and relationships could be their reduced focus on social cue-related rewards or alternatively to their different patterns of viewing them. However, some studies have shown that both TD and ASD individuals spend a similar amount of time looking at the eye rather than the mouth regions of faces, with no significant differences in attention towards these features (Vettori et al., 2020). Discrepancies in research findings may be attributed to factors such as the severity of symptoms, age differences, and variations in cognitive development.
Furthermore, it is believed that altered reward circuitry in the brains of children with ASD results in unique experiences of social stimuli compared to TD children, particularly in terms of social rewards (Zeeland, Dapretto, Ghahremani, Poldrack, & Bookheimer, 2010; Kohls et al., 2018). In terms of key neural mechanisms associated with ASD, under-activation of the orbitofrontal cortex (OFC), which is linked to difficulties in perceiving social rewards and motivation in individuals with ASD, and alterations in orbitofrontal-amygdala circuitry and the locus coeruleus-norepinephrine (LC-NE) system have been suggested (Bachevalier & Loveland, 2006; Bast et al., 2018; Dichter et al., 2012; Huang et al., 2021; Kim et al., 2022; Polzer et al., 2022; Supekar et al., 2018). Indeed, variations in the orbitofrontal-amygdala circuit, crucial for motivation, have been linked to the development of ASD (Bachevalier & Loveland, 2006). The OFC matures slightly later than the amygdala, reaching its peak early development around the second year of life, and this has drawn more attention to it by researchers (Happaney et al., 2004). Empirical investigations have revealed that individuals with ASD exhibit notably higher activation levels in the left amygdala and orbitofrontal gyrus compared to TD individuals when exposed to facial images, with amygdala activation strongly correlated with eye gaze in individuals with ASD (Dalton et al., 2005). This suggests that individuals with ASD demonstrate an elevated emotional response to faces which is likely to be associated with their fixation on the eyes, which has been highlighted in the Intense World Theory. The Intense World Theory presents a unifying perspective on autism, suggesting a neuropathology characterized by hyper-functioning of local neural microcircuits, manifesting as hyper-reactivity and hyper-plasticity. While the theory primarily focuses on the neocortex and the amygdala, its implications could extend to all regions of the brain(Markram & Markram, 2010). Recent studies employing smiling faces to explore social reward have generated conflicting findings: some reported reduced activity in the right OFC (Choi et al., 2015), while others documented increased activation in the right amygdala (Monk et al., 2010) or no significant group distinctions (Kohls et al., 2018). These contradictory outcomes underscore the necessity for further research, particularly focusing on young subgroups within the ASD population.
Given this background of previous studies we have therefore investigated differences in gaze patterns, pupil dimeter (PD) as an autonomic arousal index associated with the LC-NE system (Kihara et al., 2015) and neural responses in young children with ASD relative to TD children when viewing social rewarding stimuli in the form of happy faces and simplified happy face expressions using emoticons. We chose to include emoticons in addition to real faces since they are also processed as social information and evoke social cognitive mechanisms (theory of mind) but are only simple shapes necessitating use of minimal cognitive resources (Li et al., 2023). This may be beneficial for ASD subgroups with limited cognitive function, as it allows them to process the information more easily, overcoming the hurdle of processing real human faces. Additionally, we have also investigated if differences in children with ASD are associated with adaptive abilities and symptom severity.
We firstly hypothesized that children with ASD would show different gaze patterns to happy faces compared to TD children and that this would be most pronounced towards real faces. We secondly hypothesized that if the social motivation hypothesis is correct then the children with ASD should exhibit reduced autonomic arousal (PD) and OFC activation when presented with happy faces. If, on the other hand, children with ASD are engaged by the happy faces but perceive them in a different way then there would be no evidence for altered arousal or OFC responses. Finally, we additionally predicted that altered gaze, arousal and OFC responses to happy faces in children with ASD would be linked to their social adaptation scores and symptom severity.
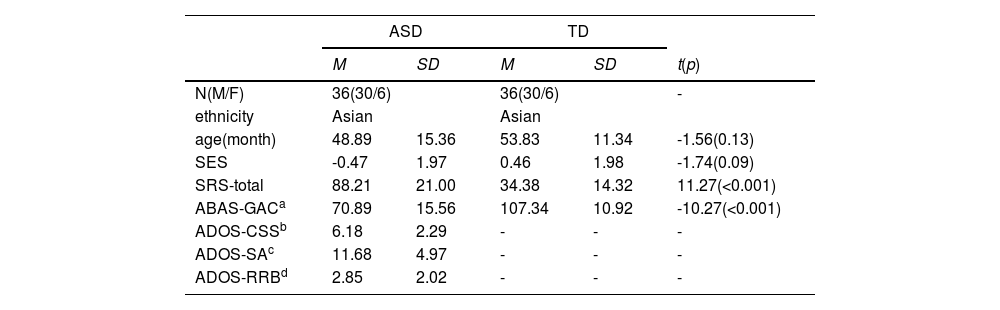
MethodsParticipantsThirty-six ASD and thirty-six TD age-matched (ASD: M = 48.89 months; SD = 15.36; TD: M = 53.83 months; SD = 11.34) and gender-matched (6 females) children were recruited for the present study. The study was conducted at the Maternal and Children's Central Hospital (MCCH) in accordance with the principles of the Declaration of Helsinki. It was approved by the Ethics Committee of MCCH (number 202,165). Written informed consent was provided by the parents or legal guardians of the children before participation. Children aged 2.5–6 years diagnosed with ASD were recruited from MCCH outpatient clinics. Participants were considered eligible if they were diagnosed with ASD according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V) (American Psychiatric Association, 2013) by clinicians. Individuals with genetic disorders, epilepsy, cerebral palsy, or other psychiatric disorders were excluded. TD children were recruited from a local Kindergarten. fNIRS data from 10 ASD and 5 TD individuals could not be included due to acquisition difficulty or poor data quality. All participants’ eye-tracking data were valid using a threshold of fixation duration for the screen being no less than 10 seconds and with successful 2-point calibration criteria (details see SI).
Clinical assessmentsThe diagnosis of ASD in participants was reconfirmed by the Autism Diagnostic Observation Schedule-Second Edition (ADOS-2) (Lord et al., 2012), administered by research-reliable trained individuals. Modules 1 and 2 were used in this study. Additionally, two questionnaires were used to assess ASD symptoms and adaptive behavior, including the Social Responsiveness Scale-Second Edition (SRS-2) (Constantino & Gruber, 2012), and the Adaptive Behavior Assessment System-Second Edition (ABAS-2) (Oakland & Harrison, 2011) (See Table 1).
Characteristics of the participants.
| ASD | TD | ||||
|---|---|---|---|---|---|
| M | SD | M | SD | t(p) | |
| N(M/F) | 36(30/6) | 36(30/6) | - | ||
| ethnicity | Asian | Asian | |||
| age(month) | 48.89 | 15.36 | 53.83 | 11.34 | -1.56(0.13) |
| SES | -0.47 | 1.97 | 0.46 | 1.98 | -1.74(0.09) |
| SRS-total | 88.21 | 21.00 | 34.38 | 14.32 | 11.27(<0.001) |
| ABAS-GACa | 70.89 | 15.56 | 107.34 | 10.92 | -10.27(<0.001) |
| ADOS-CSSb | 6.18 | 2.29 | - | - | - |
| ADOS-SAc | 11.68 | 4.97 | - | - | - |
| ADOS-RRBd | 2.85 | 2.02 | - | - | - |
SES, Socio-economic Status: Family socio-economic status = [β1 × Z (education years of primary caregiver) +β2 × Z (family income)] / characteristic root of the first factor (Z: the standard score; β1: the factor loads of education years of primary caregiver; β2: the factor loads of family income)
GAC, general adaptive composite.
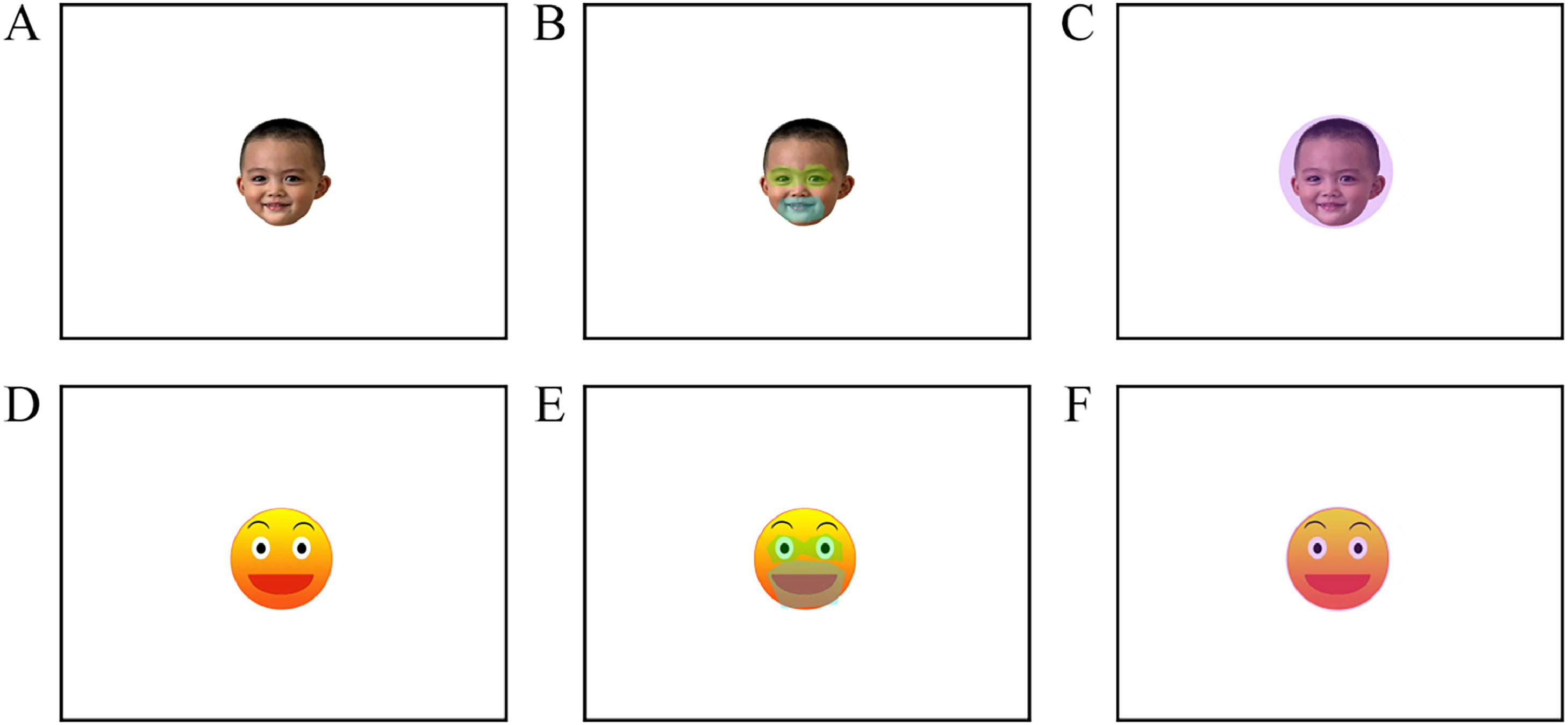
To investigate gaze patterns and neural responses, we adapted our previous emoticon versus real face task from Le et al. (2020) into a block design study. The blocks were composed of pictures of a child with a happy child face (CF) and a happy emoticon face (EF) (See Fig. 1). Areas of interest (AOIs) included the mouths and eyes of the child and emoticon faces (See Fig. 1). In this paradigm, stimuli in each block were displayed for 2 seconds, with 5 stimuli per block and 4 blocks per condition. Twenty stimuli were used in the current study. Each block was separated by a jittered interval (with an average of 8 seconds).
(A) An example in the happy complex face (CF) condition. Written consent was obtained. (B) A schematic representation of the areas of interest (AOIs) for the eyes and mouth in the CF condition; (C) The AOI of the whole face in the CF condition; (D) An example in the happy emoticon face (EF) condition; (E)The AOIs of the eyes and mouth delimited in the EF condition; (F) The AOI of the whole face in the EF condition.
The children were seated in front of a monitor with no instructions from the caregivers or investigator, so they could observe the images on the screen at their own pace. Eye-tracking data was collected using a Tobii Pro Spectrum screen-based remote eye tracker as the hardware device, with Tobii Pro Lab (version 1.142.27188) as the software to record eye gaze patterns, at a sampling rate of 120Hz. E-prime 3.0, linked with the Tobii software, was used for stimulus presentation. A standard data collection method was used, according to a previous study (Kou et al., 2019) (Details See SI).
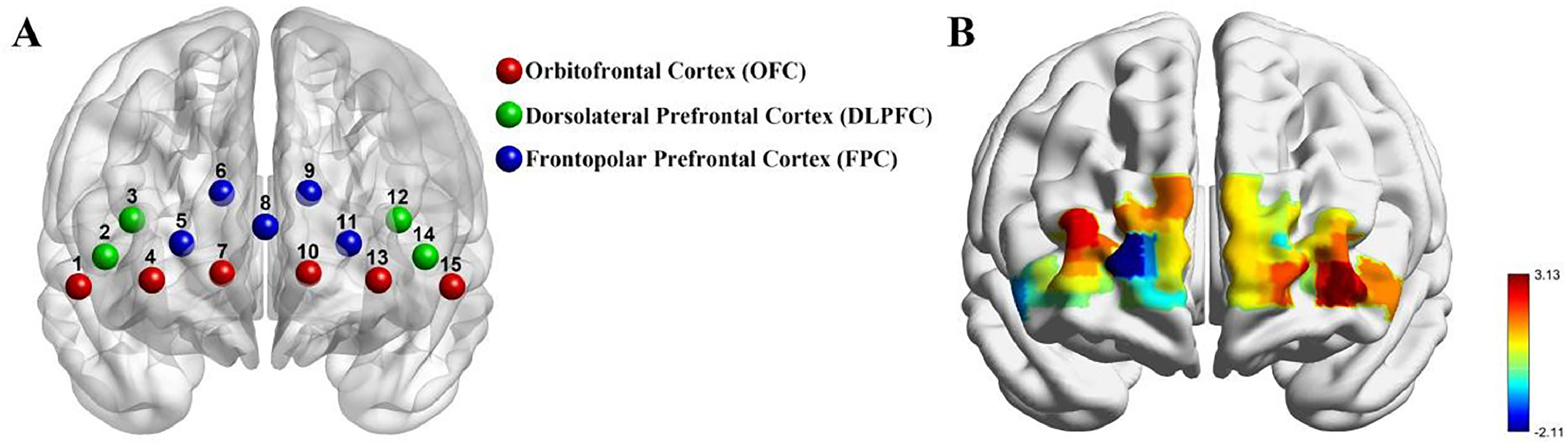
fNIRS dataWhile children viewed stimuli in the current study, we used Nirsit-Lite (Kids), a portable functional Near-Infrared Spectroscopy (fNIRS), to collect the hemodynamic changes in the OFC (OBELAB Inc., Seoul, Republic of Korea) (Bak, Shin, et al., 2022; Cho et al., 2022). The device has five dual-wavelength (780 and 850 nm) laser diodes and seven photodetectors comprising 15 channels, which are designed according to the standard International 10–20 EEG system. The optode distance is 2.5cm with an 8.138Hz sampling rate (Bak, Shin, et al., 2022; Cho et al., 2022). The alignment of channels is shown in Fig. 3A. Simultaneously, we collected eye-tracking data using the Tobbi system and measured hemodynamic changes in the OFC with Nirsit-Lite (Kids).
Data analysisEye-tracking data analysisWe plotted the areas of interest (AOIs) in Tobii Pro Lab for the CF and EF conditions, respectively. Schematic diagrams are presented in Fig. 1. In each condition, an eye area, a mouth area, and an entire face area were drawn. The entire face area was identical in size for both conditions. To ensure accurate measurement of fixations at the edge of the region, the plotted AOI was slightly larger than the corresponding actual region. Fixation data were exported from Tobii Pro Lab using the I-VT fixation filter (details can be found in the SI).
The following metrics were utilized: gaze patterns towards child and emoticon faces were quantified as the mean proportion of total fixation time or fixation counts spent on looking at the whole face and the eye and mouth regions, as in previous face processing tasks (Le et al., 2020). We measured the mean proportion of fixation counts and the mean individual fixation duration. The mean absolute pupil diameter (PD) in each condition was also measured. The proportion of the total fixation time was calculated as follows: % total fixation time spent on faces (TFD%(Whole Face)) = (time spent on faces/total looking time on stimulus screen) × 100; % total fixation time on eye-mouth gaze patterns (TFD%(Eye-Mouth)) to child faces = (time spent on eyes-mouths/total looking time on stimulus screens). The FC%(Whole Face) and FC%(Eye-Mouth) indices were calculated accordingly. Two-way ANOVA tests were conducted to investigate differences, followed by Bonferroni-corrected post hoc comparisons. Additionally, main effects between groups were also assessed.
fNIRS data analysisThe NIRS-KIT (Hou et al., 2021), a MATLAB toolbox, was used for fNIRS signal processing. Four steps were applied for pre-processing in accordance with a previous study (Hou et al., 2021): 1) trimming the signals during the non-task time to leave the actual task interval; 2) applying a linear-detrending method; 3) correlation-based signal improvement (CBSI) was used for head motion correction; and 4) applying an infinite impulse response (IIR) filter for band-pass filtering with cutoff frequencies at 0.01 Hz and 0.10 Hz. Subsequently, the general linear model (GLM) was employed to characterize the hemodynamic response of oxygenated hemoglobin (HbO) under the two conditions. HbO was chosen as the index to be analyzed since its fluctuations are more sensitive than those of deoxygenated hemoglobin (HbR).
Similarly, ANOVA tests were performed for the HbO value of fNIRS data, followed by post hoc tests for channels exhibiting significant interactions and main effects between groups. Bonferroni correction of HbO values from 15 channels to control alpha error was calculated (pBonferroni < 0.05/15 was considered significant for the interaction and main effects).
Correlation between group differences and adaptive difficulties/ autistic symptomsTo investigate the relationship between distinct neural and behavioral patterns and autistic symptoms and adaptive abilities, Pearson correlations were calculated between distinct eye-tracking and fNIRS variables and scores of SRS-2, ABAS-2 and ADOS-2. Furthermore, multiple linear regressions were performed to determine if eye-tracking and fNIRS metrics could predict adaptive behaviors and symptom severity using SRS-2 and ABAS-2 scores. All p-values from the correlations were converted to q-values with the code p.adjust in R used for correcting the correlations for multiple comparisons.
Classification markers for ASDA Support Vector Machine (SVM)-based machine learning classification test was conducted to investigate whether the eye-tracking and fNIRS data from the present task could be used as valid biomarkers to detect ASD (Details see SI). In addition to the classification accuracy of the SVM, we also evaluated the receiver operating characteristic (ROC) curves for classification based on each significant variable (Details see SI).
ResultsDemographic and clinical characteristicsAll participants in both groups were Chinese, with age, gender, and socio-economic status being matched. Significant differences were found between the two groups in terms of autistic symptom scores on the SRS-2 and adaptive capacity assessed by ABAS-2. ASD was reevaluated using the Autism Diagnostic Observation Schedule-Second Edition (ADOS-2) cut off scores for different modules. For more information on clinical assessments and demographic details, please refer to Table 1.
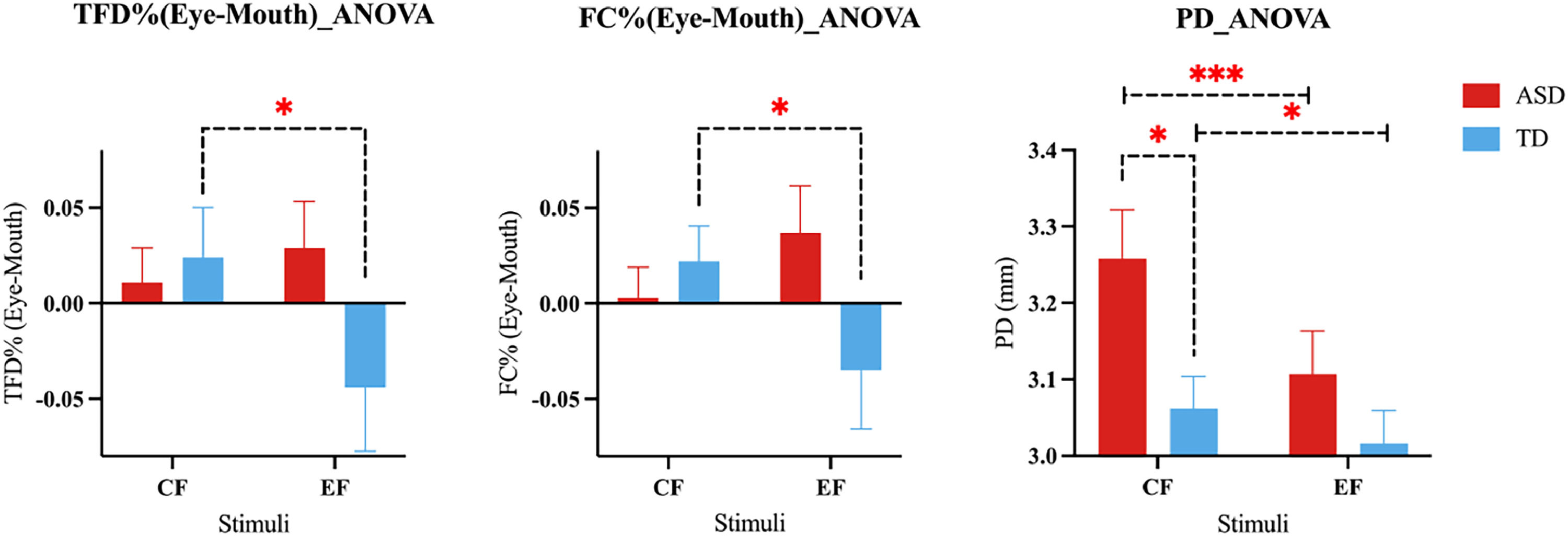
Group differences in gaze patternsOnly significant interactions were found for eye-mouth gaze patterns [TFD%(Eye-Mouth) (F1,70 = 5.237, p = 0.025,ηp2= 0.070), FC% (Eye-Mouth) (F1,70 = 6.362, p = 0.014,ηp2 = 0.083)]. Post hoc analysis showed that within the TD group there was a significant difference [TFD(Eye-Mouth) %: t35 = -2.396, p = 0.022, d = 0.399), FC(Eye-Mouth) %: t35 = -2.136, p = 0.040, d = -0.356] with children focusing more on the eyes in the CF condition and more on the mouth in the EF condition. Within the ASD group, there was no significant difference (ps > 0.170). No significant differences were found between the groups for each condition (ps ≥0.069). There was a similar trend for the EF condition in FC%(Eye-Mouth): p = 0.069, d = 0.435, and TFD%(Eye-Mouth): p = 0.083, d = 0.415. No significant main or interaction effects (ps > 0.668) were found for the remaining indices (details see SI).
The ANOVA analysis of mean PD revealed significant main effects of group (F1,70 = 3.967, p = 0.050, ηp2= 0.054) and stimuli (F1,70 = 46.143, p < 0.001,ηp2= 0.397), and a significant interaction (F1,70 = 13.059, p = 0.001,ηp2= 0.157), with post hoc tests showing that PD was larger in the ASD than TD group in the CF condition (t35 = 2.564, p = 0.013, d = 0.613) but not in the EF condition (t35 = 1.293, p = 0.200, d = 0.309). Children in both groups had larger PD looking at CF than EF [ASD (t35 = 7.359, p < 0.001, d = 1.226), TD (t35 = 2.248, p = 0.025, d = 0.375)] (see Fig. 2).
In order to manage the individual variances in baseline PD and cognitive differences, PD of triangle symbol frame and Functional Academic in ABAS-2 were taken into account as covariates. The pattern observed was consistent with the above findings.The ANOVA analysis of mean PD revealed a marginally significant main effect that the PD in ASD group was larger than TD group (F1,40 = 3.426, p = 0.072,ηp2= 0.079), and a significant interaction (F1,40 = 10.507, p = 0.002,ηp2= 0.208), with post hoc tests showing that PD was larger in the ASD than TD group in the CF condition (t42 = 3.046, p = 0.009, d = 0.941) but not in the EF condition (t42 = 0.854, p = 0.444, d = 0.264), and the PD was larger when viewing CF condition than EF condition in ASD group (t20 = 5.606, p < 0.001, d = 1.223) but not in TD group (t22 = 0.719, p = 0.476, d = 0.150) (see Figure S4).
Group differences of neural responsesANOVAs revealed no significant interactions on any of the channels after multiple comparison correction by Bonferroni correction (ps > 0.024,ηp2 < 0.090). However, as shown in Fig. 3, there was a significant main effect of group on channel 13 in HbO concentration (t56 = 3.126, p = 0.0028, d = 0.831). This indicates an increased response in the OFC for the ASD group compared to the TD group (see Fig. 3).
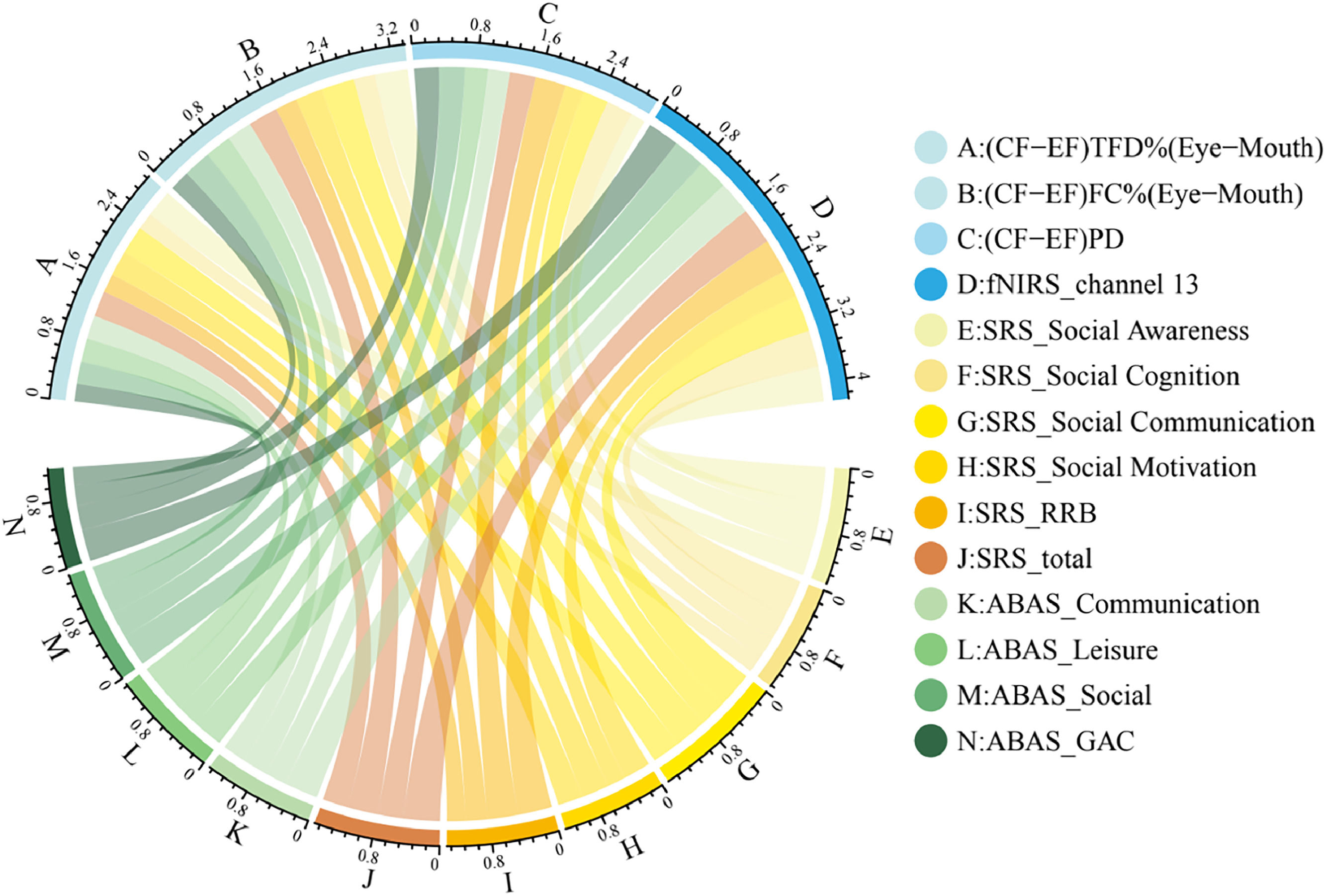
CorrelationThe results presented above for the analysis of eye-tracking and fNIRS revealed that there were significant differences in eye-mouth gaze patterns, PD and OFC activation between the ASD and TD groups. Firstly, their correlation with SRS-2 was examined and results showed both eye-mouth gaze pattern, PD measures and mean HbO values of fNIRS channels were significantly correlated with the total SRS-2 score. As shown in Fig. 4 and Figure S3, (CF-EF) TFD%(E-M) (r = -0.328, qFDR = 0.035), (CF-EF) FC% (E-M) (r = -0.383, qFDR = 0.017) were negatively correlated with SRS-2 total score. (CF-EF) PD (r = 0.330, qFDR = 0.034) and OFC activation of channel 13 (r = 0.467, qFDR = 0.012) was positively correlated with the SRS-2 total score. The detailed correlation analysis between eye-tracking, OFC activation and each dimension of SRS-2 is shown in Table S1. A similar analysis of clinical symptom severity scores on ADOS-2 in the ASD group showed a similar trend as the SRS-2 scores (Details see SI) .
As shown in Fig. 4 and Figure S3, eye-mouth gaze patterns were found to be significantly correlated with children's social skills, including leisure [TFD%(E-M) (r = 0.299, qFDR = 0.045), FC% (E-M) (r = 0.334, qFDR = 0.034)] and social [TFD%(E-M) (r = 0.279, qFDR = 0.060), FC% (Eye-Mouth) (r = 0.353, qFDR = 0.027)], as well as the "communication" dimension [TFD%(Eye-Mouth) (r = 0.299, qFDR = 0.045), FC% (Eye-Mouth) (r = 0.320, qFDR = 0.035)]. Pupil diameter difference scores of CF versus EF were negatively correlated with the "social" dimension of adaptive skills (r = -0.330, qFDR = 0.034) as well as with total scores (r = -0.313, qFDR = 0.045). Neural response data was negatively correlated with almost all dimensions (rs > -0.296, qsFDR < 0.045) of ABAS-2 as well as with total scores (r = -0.402, qFDR = 0.014). For details see Table S2. We investigated the relationship between eye gaze patterns directed towards happy real faces and happy emoticons (E-M pattern and PD) and cognitive scores derived from the cognitive-related sub-scale in ABAS-2. However, no significant correlation was found (rs < 0.166, qFDR > 0.729).
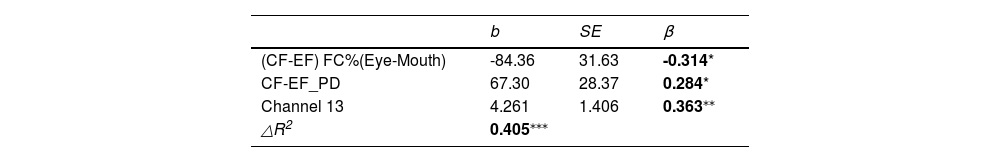
Predictors of autistic symptom and adaptive ability of childrenThe results of the above correlation analysis demonstrated that the total score of SRS-2 was significantly correlated with the eye-mouth gaze pattern, mean PD, and OFC activation. To investigate whether they can predict the total SRS-2 score multiple linear regression was conducted. To avoid the possible effects of multi-collinearity, (CF-EF) FC%(Eye-Mouth) was selected as a representative of the fixation pattern, the neural response from channel 13 of fNIRS and CF-EF for mean PD were selected as potential predictors. The results showed that all three had a good predictive effect with an R-square of 0.405(see Table 2).
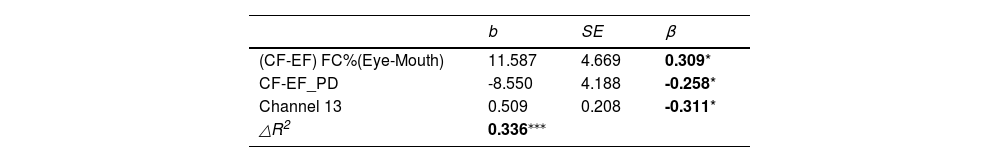
Analogous analyses were applied to the three ABAS-2 dimensions that were significantly associated with sociability as well as ABAS total scores. The results showed that (CF-EF) FC%(Eye-Mouth), (CF-EF) PD and channel 13 were significant predictors of the social dimension of the ABAS-2, with an R-squared of 0.336. The specific values are shown in Table 3. Channel 13 is also a significant predictor of the ABAS total scores, with an R-squared of 0.254, as shown in Table S4.
DiscussionOverall, our results show that although there were no significant group differences in time spent viewing the happy faces, or the total number of fixations on faces, children with ASD showed a greater mean PD and OFC activation across happy face and emoticon conditions, indicating a higher absolute arousal level and neural reward response to them. TD children showed a different trend of eye gaze patterns towards happy real faces and happy emoticons, whereas children with ASD did not. Gaze patterns towards the eyes and mouth, mean PD, and OFC activation could be used as effective predictors for social adaptive difficulties and the severity of autistic symptoms and adaptive difficulties. Higher adaptive abilities and lower autistic symptom scores were predicted by greater attention to informative reward facial features (e.g. the eyes of real faces and mouths or emoticon faces) and with reduced mean PD and OFC responses.
Greater pupil diameter and OFC activation while viewing happy faces in individuals with ASDThe greater average PD observed in the ASD group in the current study provides evidence for a hyper-arousal level to happy faces per se, which is consistent with some previous studies and the Intense World Theory but inconsistent with the Social Motivation Hypothesis. Alterations in PD and/or pupil response (pupil dilation) have both been proposed as indicators of autonomic dysfunction in autism spectrum disorder (Reisinger et al., 2020). Pupil diameter is considered an absolute measure of arousal level, while pupil dilation represents a relative arousal change. Wagner et al. (2016) reported that infants (9 months and 18 months) who were later diagnosed with ASD exhibited a greater mean PD in response to emotional faces compared to infants in a low-risk group. However, a meta-analysis of pupil response in individuals with ASD only showed a longer latency of the pupil response in ASD groups, whereas evidence on other pupil size-related indices was conflicting (de Vries et al., 2021). This highlights that other factors, such as medication use, comorbid conditions, and individual differences, may influence pupil size.
The group differences in PD between ASD and TD could be due to the involvement of the autonomic nervous system, specifically the locus coeruleus-norepinephrine (NE) system (de Vries et al., 2021; Kihara et al., 2015). Neurons in the brainstem nucleus LC are the sole source of NE (Sara, 2009), and its release throughout the mammalian brain is considered important for modulating attention, arousal, and cognition during various behaviors. The LC-NE system is closely related to the dopamine system, which is associated with the orbitofrontal-amygdala circuit (Bachevalier, 2005). In regards to the relationship between the LC-NE system and dopamine, Sara (2009) concluded that there are striking similarities between the factors that govern the activity of dopaminergic and NE neurons, suggesting that dopamine and NE are released simultaneously.
The activation of the OFC from our study suggests that children with ASD exhibit heightened neural activity when exposed to happy faces and emoticons, consistent with prior research (Styliadis et al., 2021). The adolescent ASD group in a study by Styliadis et al. (2021) demonstrated significantly heightened cerebellar activation when exposed to happy faces, further supporting our observations. The Intense World Theory posits that the increased activity of the neocortex and the amygdala could potentially extend to all areas of the brain, which is in line with our research outcomes(Markram & Markram, 2010). While our observed hyperactivity in the orbitofrontal cortex (OFC) of children with ASD contradicts earlier research that reported either hypoactivation or no difference in reward-related brain regions in response to social rewards (Kohls et al., 2018; Clements et al., 2018). Kohls et al. (2018) found no significant OFC activation disparities between youth with ASD (with intellectual ability comparable to the control group) and TD peers during tasks involving social rewards in an incentive delay task. Their task required goal-directed actions, unlike ours, which permitted free viewing of social cues and may have led to participants with ASD spending more time processing social information and possibly provoked stronger neural activation. On the other hand, Choi et al. (2015) observed a decrease in right OFC activity in autistic individuals viewing happy faces, and Clements et al. (2018) documented widespread hypoactivation in social reward circuits, with modulating effects from age, cognitive capacity, and anxiety levels.
Distinct gaze patterns(eye-mouth) without altered viewing of happy faces in children with ASDTypically developing children demonstrated a preference for focusing on the eyes rather than the mouths of children's faces, and on emoticon mouth representations rather than eyes for depictions of happy faces. Conversely, children with ASD did not show such distinctions. Moreover, the viewing pattern could predict higher adaptive abilities and was associated with lower severity scores for autistic symptoms.
In the TD group, previous research has underscored the significance of the eye region in recognizing real facial expressions (Royer et al., 2018). Studies have shown that placing a greater emphasis on the eye region is linked to improved face recognition abilities in typically developing individuals (Royer et al., 2018). Conversely, emoticons are distinguished by their use of symbolic representation, particularly in the depiction of mouth features (Cherbonnier & Michinov, 2022). While many emoticons share similar punctuation marks for the eyes (colon) and nose (dash), the symbol used for the mouth plays a crucial role in differentiating between emoticon expressions. For instance, the:-) emoticon can be used with or without the nose, maintaining the same expression, and a similar effect can be achieved by substituting the colon with the number eight (8) in 8-). Furthermore, altering the mouth of an emoticon, as seen in the shift from:-) to:-D, can signal a difference in the intensity of expression. The distinction in facial expression between:-(and:-P is attributed to a single element change, demonstrating the pivotal role of mouth punctuation in constructing distinct emoticon expressions. However, an exception to this trend is the;-) emoticon—distinct from the:-)—where the punctuation for the eyes significantly alters the facial expression. This characteristic feature of emoticons may suggest a standardized approach that aids in comprehending the construction of diverse expressions across different emoticons (Cherbonnier & Michinov, 2022). Our results are consistent with previous findings in the TD group. Nevertheless, the current patterns in Asian societies may be unique. Further research is necessary to compare cultural differences between Asian can Caucasian.
Previous research on the gaze patterns of individuals with ASD in relation to emoticons or cartoons is limited. Emoticons are simplified human faces, but they carry less cognitive and social information than human faces. They have hyperbolic features and invoke social cognitive mechanisms (theory of mind), while also being processed efficiently like simple shapes, requiring minimal cognitive resources (Li et al., 2023). Silva et al. (2015) also discovered that autistic children showed a preference for cartoon faces over human faces when presented with an emotion recognition task. Our findings revealed that the ASD group spent a similar amount of time viewing emoticons compared to real children's faces, but exhibited a different viewing pattern, deviating from previous research (Silva et al., 2015; Atherton & Cross, 2018). This discrepancy may be attributed to individual differences between the participants in our study and those in previous research. The earlier studies included participants with high cognitive function or high autistic traits, indicating potentially greater theory of mind capability compared to the lower functioning participants in our current study.
Overall, current results tend to be inconsistent with the social motivation hypothesis in ASD and instead suggest that atypical patterns of gaze towards socially rewarding stimuli may reflect differences in perceptual processing and arousal. This means that instead of a lack of motivation, some autistic children may actually feel greater attention and arousal in response to socially rewarding stimuli which might help to reduce their anxiety (Yi et al., 2022). Consistent with our observation of increased responsiveness to social reward being associated with greater adaptive behavior difficulties and symptom severity in children with ASD, a review has shown a link between increased focus on the face/head and eye regions and improved social performance and reduced autism symptom severity (Riddiford et al., 2022). However, there are some limitations in the current study. The deeper reward-related brain regions, such as the nucleus accumbens and caudate, could not be recorded using fNIRS, and therefore, they may differ from the patterns of response we observed in the OFC.
ConclusionOur study demonstrates unique neural hyperactivity and view patterns (pupil diameter and eye-mouth view) in young children with ASD when exposed to reward-related facial stimuli. These findings contradict the Social Motivation Hypothesis but are somewhat consistent with the Intense World Theory. Children with ASD seem to have heightened arousal levels and use less efficient strategies for processing faces, both real and emoticon faces. This increased resources demand could have long-term effect for the well-being of children and might impact their ability to develop adaptive capacity effectively.
LimitationsOur study did not comprehensively assess cognitive abilities, interest, familiarity, and face processing expertise. Future research should consider incorporating specific cognitive capacities and related measurements to better control the cognitive load associated with hyper neural and PD levels. Moreover, in this study, we included 30 males and 6 females, resulting in a male-to-female gender ratio of 5:1, slightly higher than the global average for autism (4:1)(Loomes et al., 2017). Subsequent studies will aim to enhance the recruitment of autistic girls to address potential sampling biases.
Declaration of generative AI and AI-assisted technologies in the writing processWhile preparing this work, the authors utilized Stork to check for language grammar errors and enhance readability. Following the use of this tool/service, the authors then reviewed and edited the content as necessary, and assume full responsibility for the publication's content.
Ethics approval statementThe study was conducted in accordance with the principles of the Declaration of Helsinki. It was approved by the Local Ethics Committee (number 202,165).
FundingThis study was supported by the National Natural Science Foundation of China [NSFC32100893], the Key Technological Projects of Guangdong Province, China “Development of New Tools for Diagnosis and Treatment of Autism” [2018b030335001], and the Natural Science Foundation of Sichuan Province China [2022NSFSC1631]&[2024NSFSC1227]. The sponsor played no role in the study design, data collection, analysis or interpretation, writing the report, and the decision to submit the article for publication.
Availability of dataOur ethics approval does not permit public archiving of individual anonymized raw data. Those who wish to access the raw data should contact the corresponding author. Access will be granted to named individuals who adhere to the ethical procedures governing the reuse of sensitive data. They must complete a formal data sharing agreement to obtain the data. The data that support the main group different finding figures related anonymized data of this study are openly available via the Open Science Framework (OSF) Repository https://osf.io/sxkhc/?view_only=6eb51e64a9b54fce909b5ff6eacdc668.