Pornography consumption is highly prevalent but can develop into problematic sexual behavior with severe negative emotional consequences. Neurobiological studies indicate that compulsive sexual behaviors (CSB) are associated with altered brain structure and function in processing pornography. This study investigated the neuroaffective mechanisms underlying exposure to erotic and explicit pornographic images and their relationship to CSB-relevant symptoms. Whole-head magnetoencephalography (MEG) assessed brain activity during passive viewing of opposite- and same-sex erotic and pornographic images in healthy heterosexual and homosexual women and men (N = 50). Correlations of estimated event-related neural activity with indicators of CSB (hypersexuality, sexual sensation seeking, problematic pornography use, and time spent on pornography use), mood and anxiety, as well as with subjective picture ratings of hedonic valence and emotional arousal were analyzed. Responses of brain regions to sexual content revealed hyper- and hypoactivation and were related to problematic pornography consumption, hypersexuality, time spent on pornography use, and perceived subjective arousal. The neural activation towards erotic and pornographic content revealed further significant associations with depression and anxiety scores. The findings suggest an involvement of prefrontal and temporo-parietal cortex regions in the divergent processing of sexual content in relation to indicators of CSBD. Insight into the neurobiological factors underlying CSB can contribute to a more precise clinical conceptualization of this problem and may promote the development of more effective therapeutic interventions.
The use of pornography, while unproblematic for the majority of people from a clinical point of view, can develop into addiction-like behavior. Nowadays, individuals have fairly easy access to a wide variety of sexual stimuli; therefore, it has become a common habit to use these for sexual gratification (Binnie & Reavey, 2020). Pornography use seems to be very popular, with 40 %−90 % of people having used it for different purposes (Ballester-Arnal et al., 2023; Bridges et al., 2016; Bőthe et al., 2018; Blais-Lecours et al., 2016; Castro-Calvo et al., 2018; Döring, 2009; Martyniuk et al., 2016; Rissel et al., 2017; Zheng & Zheng, 2014). Regardless of the positive aspects, pornography consumption has been associated with several negative outcomes for sexual and mental health. One of these negative outcomes, which is associated with an elevated risk for adverse sexual behavior and emotional consequences, is the development of problematic or compulsive pornography use, which—depending on the criteria used—was recently estimated to be between 3.2% and 16.6%. (Briken et al., 2022; Bőthe et al., 2024; Cooper et al., 1999; Gola et al., 2016; Grubbs et al., 2015; Ince et al., 2021).
It is still unclear from a psychological and psychiatric perspective whether and to what extent excessive or problematic pornography use should be considered a unique disorder. For now, with the eleventh edition of the ICD-11 of the World Health Organization, published in 2019, problematic pornography consumption can be described under the term compulsive sexual behavior disorder (CSBD, World Health Organization, 2023). Many individuals seeking help for CSBD are dealing with problematic use of pornography (Reid et al., 2012). CSBD is often accompanied by excessive pornography consumption, masturbation, and/or promiscuity that are out of control, leading to distress and impairment, resulting in negative consequences in different areas such as interpersonal and intrapersonal relationships, sexual, occupational, and/or economic (Briken, 2020). From an emotional perspective, anxiety, depression, irritability, mood swings, guilt, shame, low self-esteem, and spiritual or moral discomfort are commonly experienced by individuals with CSBD (Ballester-Arnal et al., 2020; Castro-Calvo et al., 2020, 2023; Ciclitira, 2004; Hall, 2019; Patterson & Price, 2012; Wéry et al., 2016; Willoughby et al., 2014).
There is, furthermore, an ongoing scientific debate about whether CSBD can, in fact, constitute the manifestation of a behavioral addiction (Bőthe et al., 2022; Gola et al., 2022; Grubbs et al., 2020; Kor et al., 2013; Kraus et al., 2016; Potenza et al., 2017; Sassover & Weinstein, 2022), leaving many researchers unsatisfied with the current classification. Empirical data is currently lacking on whether the processes involved in the development and maintenance of CSBD are equivalent to those observed in substance use disorders or behavioral addictions such as gambling or gaming (Kraus et al., 2018). In this regard, neuroscientific findings suggest some proximity to addiction disorders (Liberg et al., 2022; Love et al., 2015; Stark et al., 2018). Recent functional magnetic resonance imaging (fMRI) studies have found dysfunctional affective modulation in prefrontal cortex regions, as well as increased activity in reward-associated regions (amygdala, dorsal anterior cingulate, ventral striatum) in response to sexual stimuli and cues, and during the anticipation of such, indicating similarities to behavioral addiction and substance use disorder cue reactivity (Liberg et al., 2022; Markert et al., 2021). Additionally, neural correlates of appetitive conditioning and neural connectivity, characterized by decreased coupling between the ventral striatum and prefrontal cortex, appear altered in patients with CSBD (Klucken et al., 2016). A review by Kowalewska et al. (2018) concludes that CSBD seems to be associated with aberrant functioning in brain regions implicated in habituation, impulse control, and reward processing. In particular, the frontal cortex, which plays an important role in higher-order cognitive functions (i.e., impulse inhibition, decision-making, prioritizing, and strategizing), as well as the reward system, has been linked to CSB. Neurobiological abnormalities of CSBD seem to share commonalities with other addictions, such as substance use and gambling disorders (Stark et al., 2018). The executive aspects of the paralimbic regions, particularly the frontal lobe and the striatum, have also been linked to behavioral addictions. For instance, in pathological gambling, higher impulsivity and functional paralimbic abnormalities have been observed in gamblers (Rømer Thomsen et al., 2013). Furthermore, studies investigating sexual rewards point toward associations between neural activity and symptoms of problematic pornography use, indicating that positive valence systems are altered in individuals with problematic pornography use (Klein et al., 2022). Nevertheless, neuroscientific studies have also reported results that appear inconsistent with some predictions made by addiction models (Prause et al., 2015).
There are two established models of addiction that have been applied to investigate pornography use: the Incentive Salience Theory (IST, Berridge, 2012; Robinson & Berridge, 1993; Robinson et al., 2016) and the Reward Deficiency Syndrome (RDS, Blum et al., 2015, 1996). Both models have been investigated in neuroscientific contexts, e.g., within an event-related potential framework whereby EEG signaling is associated with the significance and subjective salience of the stimuli, indicated by the late positive potential (LPP) in different cortical areas (Gola et al., 2016). The processing of visual sexual stimuli in pornography-related CSBD has often been examined in cue-reactivity studies (Stark et al., 2018). In this context, according to IST (Berridge, 2012; Robinson & Berridge, 1993; Robinson et al., 2016), addiction is often characterized by increased “wanting,” thus an elevated cue-related reactivity (i.e., higher LPP to the cue), but decreased “liking,” resulting in diminished reward-related reactivity (i.e., lower LPP to the target/reward). The latter is directly linked to the experienced value of the reward, while the former is related to the expected value of the reward, often carried by a predictive cue. This also leads to open questions regarding the conceptualization of visual sexual stimuli in experimental designs as cues or rewards (Gola et al., 2016). There is ongoing discussion about whether sexual stimuli are primarily cues predicting rewards (e.g., an orgasm) or unconditioned stimuli that are rather rewards in themselves (Stark et al., 2018). With lowered reactions to rewards, the IST model aligns with the Reward Deficiency Syndrome. In the RDS framework (Blum et al., 1996, 2015), genetic predispositions to lower dopaminergic responses for rewarding stimuli are expressed in diminished BOLD and electrophysiological reactivity and are assumed to be related to sensation-seeking, impulsivity, and a higher risk of addiction (Gola et al., 2016).
The aim of this magnetoencephalography (MEG) study was to further investigate neural processes related to CSB-relevant symptoms, with a particular focus on problematic pornography consumption, which constitutes a commonly reported CSB (Wéry et al., 2016). By capturing neuroelectric processes, MEG provides a higher temporal resolution than hemodynamic brain imaging, which has mainly been used so far to investigate CSBD. This higher sensitivity would, for instance, also allow for the separation of opposing effects of brain activity in consecutive processes, as shown during the perception of disorder-relevant stimuli in anxiety disorders (Wessing, Romer & Junghöfer, 2017). Compared to EEG, which is also applied in CSBD research, MEG offers a higher spatial resolution for cortical neural activity, though it has reduced sensitivity to deeper structures (Gross, Junghöfer, Wolters, 2023). Thus, MEG might facilitate the exploration of cortical structures linked to reward and self-control, which are crucial for behavioral addictions and problematic pornography consumption due to impaired self-regulation (Büsche et al., 2022; Chen et al., 2022; Miner et al., 2009).
We hypothesized that neural reactivity to sexual stimuli, especially in prefrontal regions—given the close interaction between self-control and reward processing in behavioral addictions, as well as their special involvement in saliency evaluation (Liberg et al., 2022; Goldstein et al., 2007; Markert et al., 2021)—would be associated with subjective indicators of compulsive sexual behavior (CSB). To test our hypothesis, this study investigated the early neuroaffective mechanisms underlying exposure to erotic (i.e., non-explicit sexual stimuli) and pornographic (i.e., explicit sexual stimuli) stimuli within hetero- and homosexual male and female samples by means of whole-head MEG. We determined their relationship to indicators of CSB (hypersexual behavior, sexual sensation seeking, self-reported problematic pornography use, time spent on pornography) and commonly reported comorbid anxiety and depression (Ballester-Arnal et al., 2020; Castro-Calvo et al., 2020, 2023; Ciclitira, 2004; Hall, 2019; Patterson & Price, 2012; Wéry et al., 2016; Willoughby et al., 2014). As the affective neural processing of visual sexual pictorial stimuli is strongly associated with their subjectively perceived hedonic valence and emotional arousal (e.g., Schupp et al., 2006; Leon-Carrion et al., 2007), these affective dimensions were also investigated. Furthermore, it was hypothesized that neural activation to pornographic images, rather than erotic images, would be associated with indicators of CSB, as explicit sexual stimuli could be considered more rewarding for individuals with a risk profile for developing CSBD, according to popular models of addiction (Berridge, 2012; Blum et al., 1996, 2015; Robinson & Berridge, 1993; Robinson et al., 2016).
MethodsParticipantsThe study was performed at the Institute for Biomagnetism and Biosignalanalysis (IBB) at the University of Münster, was approved by the local ethics committee and was conducted in accordance with the Declaration of Helsinki. Participants were recruited through both online and offline advertisements at the local university. All participants provided informed consent prior to assessment.
Most research on compulsive sexual behavior (CSB) has primarily focused on heterosexual men, leaving unresolved questions regarding the presence of similar pathological mechanisms in other sexual orientation groups (Stark & Klucken, 2017). Therefore, we intentionally sought to include hetero- and homosexual individuals in our recruitment process to enhance scientific understanding in this field while promoting inclusivity in neuroscientific research on sexuality. The final sample of 50 participants consisted of 12 heterosexual men (HeM), 13 homosexual men (HoM), 13 heterosexual women (HeW) and 12 homosexual women (HoW). Sexual orientation and gender were self-described by the participants. Within this sample all participants who identified themselves as women also assigned themselves female when asked about their biological sex; respectively participants who identified themselves as men also assigned themselves as male sex. The following inclusion criteria were applied: absence of current psychiatric or neurological disorders, no contraindications for MEG, and fluency in the German language. None of the participants reported of current psychiatric/neurological disorders.
Stimulus materialIn total 108 pornographic (54) and erotic (54) scenes were presented, which were controlled for physical picture properties of luminance and complexity. The pornographic images were taken from the Explicit Pornographic Picture Set (EPPS; Prantner et al., 2024b), including images of 18 male-female (MF), 18 female-female (FF) and 18 male-male (MM) performers with picture categories of masturbation, oral, vaginal, anal and group sex. These categories were deliberately chosen as most popular among pornography users (Ballester-Arnal et al., 2023; Hald & Štulhofer, 2016; Pornhub, 2023). Erotic images were selected from the NAPS ERO picture set (Wierzba et al., 2015) showing couples (18 MF, 18 FF, 18 MM) in non-nude and non-explicit settings such as kissing or hugging. The images from the EPPS and NAPS-ERO were chosen based on their expected appeal to the different sexual orientation groups (heterosexual men, homosexual men, heterosexual women, and homosexual women). To equalize the expected mean attractiveness or motivated attention across all presented pictures for the various groups of sexual orientation (heterosexual men, homosexual men, heterosexual women, and homosexual women), the pictures with the highest expected sexual appeal for each group were selected from the EPPS and the NAPS-ERO. This selection process was based on the available ratings of hedonic valence and emotional arousal, as reported by Prantner et al. (2024b) for the EPPS and Wierzba et al. (2015) for the NAPS-ERO. The selected images are reported in the Supplementary Materials.
QuestionnairesSeveral sexuality-related tendencies have been linked to CSB, including sexual compulsivity and impulsivity (Mechelmans et al., 2014; Walton et al., 2017; Wetterneck et al., 2012), sexual motivation (Klucken et al., 2016), and sexual excitation (Walton et al., 2017; Rettenberger et al., 2016). To investigate the aforementioned links, German versions of the Hypersexual Behavior Inventory (HBI; Reid et al., 2011), and the Sexual Sensation Seeking Scale (SSSS; Kalichman et al., 1994) were administered at the end of the affective ratings task. The HBI has 19-items which are rated on a 5-point Likert scale (1 =Never; 4 = Very often) designed to measure basic dimensions of hypersexuality, such as the use of sexuality to control affective states, problems in controlling or reducing sexual thoughts, urges, and behaviors, and persistence despite negative consequences. The SSSS constitutes an 11-item scale rated on a 4-point Likert scale (1 = Not at all like me; 4 = Very much like me) that assesses “the propensity to attain optimal levels of sexual excitement and to engage in novel sexual experiences” (Kalichman et al., 1994, p. 387). In our study, Cronbach's alpha values were 0.89 for the HBI and 0.73 for the SSSS, respectively.
In order to account for the participants’ pornography use, the Problematic Pornography Consumption Scale (PPCS; Bőthe et al., 2018), and the Time Spent On Pornography (TSOP) (on a scale from 1 = Never to 10 = More than 20 h per week within the last six months s. Suppl.) were assessed. The PPCS consists of 18 items answered on a 7-point Likert scale (1 = Never; 7 = Always), and its Cronbach's alpha in this study was 0.93.
Finally, participants were asked to rate each image on 9-point Self-Assessment-Manikin (SAM; Bradley & Lang, 1994) scales on the dimensions of hedonic valence and emotional arousal.
Emotional dysregulation may play a critical role in CSBD (Castro-Calvo et al., 2020; Lew-Starowicz et al., 2020; Reid et al., 2014; Schultz et al., 2014). Individuals with clinical characteristics of Compulsive Sexual Behavior Disorder (CSBD) exhibit higher scores for depression and anxiety compared to those identified as non-CSBD participants (e.g., Castro-Calvo et al. 2020). Thus, to control for individual differences in mood and anxiety, the Beck Depression Inventory-II (BDI-II; Beck et al., 1961) and the State-Trait Anxiety Inventory (STAI-T; Spielberger et al., 1971) were included. The BDI-II comprises 21 items rated on a 4-point Likert scale ranging from 0 to 3 (response categories differ for each item). The STAI-T comprises 20 items answered on a Likert scale with four response options (1 = Strongly disagree; 4 = Strongly agree). In the present study, the Cronbach's alpha values for the BDI-II and STAI-T were 0.91 and 0.90, respectively.
ProcedureAfter obtaining signed consent, the participants took a seat in the MEG scanner and looked at a monitor on which the emotional scenes were projected (Fig. 1A). Stimuli were presented using the PsychToolbox- 3 software (Brainard & Vision, 1997; Pelli, 1997; Kleiner, Brainard & Pelli 2007). To minimize MEG artifacts caused by eye movements, participants were asked to fixate but not stare on a constantly presented small central marker. Participants were instructed to passively view the scenes in two successive blocks, in each of which the complete set of 108 images were randomly presented (Fig. 1B). Following the passive viewing task, in an adjacent experimenter room, participants rated each image on 9-point Self-Assessment-Manikin (SAM; Bradley & Lang, 1994) scales of hedonic valence and emotional arousal (Fig. 1C). Thereafter demographic and questionnaire information were assessed.
Schematic representation of the procedure. A. Participants passively viewed randomized presentations of erotic and pornographic images (not blurred) while event related magnetic fields were acquired by whole head magnetoencephalography (MEG). B. Images with a small fixation cross in the center of the screen were presented for 1 s. in two blocks on the screen, with each of the 108 images shown twice in each block. The ITI was chosen randomly between 1 and 2 s. C. After the passive viewing task participants rated all 108 images on the dimensions of hedonic valence and emotional arousal with Self-Assessment-Manikin (SAM; Lang, 1980) scales followed by questionnaire assessment.
Visually evoked magnetic fields were measured using a 275-channel full-head MEG sensor system equipped with first-order axial gradiometers (Omega 275; CTF, VSM MedTech Ltd., Coquitlam, Canada). Head coordinates within the scanner were determined through landmark coils positioned on the nasion and both earlobes. MEG data were sampled at 600 Hz and later processed offline with a 48-Hz low-pass filter and a 0.1-Hz high-pass filter before being downsampled to 300 Hz. Single trials underwent editing and artifact correction following the statistical control method for artifacts in high-density EEG/MEG data (Junghöfer et al., 2000). After averaging within the experimental conditions, the neural sources underlying these recorded event-related fields were estimated using L2-minimum-norm estimates (Hämäläinen & Ilmoniemi, 1994) which were then averaged over the two blocks. The recording, pre-processing and analysis of the MEG data was done following the same procedure as described in previous publications (Kroker et al., 2023, 2024; Rehbein et al., 2023; Wessing et al., 2015).
Statistical analysisBehavioral measures were analyzed using the statistics program JMP, applying an α-level of 0.05. To test for potential influences of sample characteristics, mean scores for the CSBD-associated characteristics in the HeM, HeW, HoM, HoW groups were calculated and compared using nonparametric Kruskal-Wallis tests and pairwise Wilcoxon multiple-comparisons tests. Intercorrelation matrixes were calculated with Spearman's correlations.
To test the hypothesis that neural activation in response to stimuli with general sexual visual content, regardless of whether they were pornographic or erotic, would be associated with indicators of CSB, we averaged the estimated neural activity in response to pornographic and erotic stimuli within participants and then calculated Pearson correlations of these mean neural activations at each time point and estimated source with each of the four CSBD-associated traits (HBI, SSSS, PPCS, TSOP) across all participants (i.e., correlation of estimated neural and subjective data).
To test the hypothesis that neural activation to pornographic images rather than erotic images would be associated with indicators of CSB, we used spatio-temporal cluster permutation of the correlation coefficients of the estimated neural activation at each time point and estimated source in response to pornographic stimuli combined with negative correlations in response to erotic stimuli across all participants and again for each of the four CSBD-associated traits. A positive/negative value as result of this differential correlation would reflect a relatively more positive/negative correlation (but also relatively less negative/positive correlation) of indicators of CSB with the porn condition as compared to the erotic condition.
To test associations of neural activity with subjective ratings of arousal and valence, we averaged the respective arousal ratings for pornographic and erotic stimuli and averaged the valence ratings for pornographic and erotic stimuli for each participant and calculated correlations of these mean subjective ratings with the participants' mean neural activities following a spatio-temporal cluster permutation of correlation coefficients of the estimated neural activation across pornographic and erotic conditions (as used above). The same Pearson's correlations of mean evoked neural activations were calculated with the co-assessed questionnaire data (BDI-II, STAI-T).
The software emegs (Peyk, De Cesarei & Junghöfer, 2011) was utilized to analyze the MEG data. To address the issue of multiple comparisons, a non-parametric correction method as described by Maris and Oostenveld (2007) was used. Spatially and/or temporally neighboring values of Pearson correlation that exceeded the critical alpha level of p = .05 (first level criterion) formed a so-called spatio-temporal cluster, the mass of which was calculated as the respective spatio-temporal integral of the Pearson correlation values. This so called cluster mass was then compared against the identical analyses based on 1000 permuted drawings of neural and subjective data. When the cluster mass of the original analysis (using the correct assignment) was higher than the critical cluster mass of this permutation distribution corresponding to a p-value = 0.05 (i.e., higher than the 950 highest cluster masses found with the largest cluster of each draw of the random distribution; second level criterion), the cluster was considered significant. For visualization, L2-MNE topographies were projected onto standard 3D brain models.
Furthermore, to examine the associations in the observed neural clusters accounting for gender (men, women) and sexual orientation effects (hetero-, homosexual), multiple linear regression models were calculated for each significant spatio-temporal cluster and reported in the Supplementary Materials.
ResultsSample characteristicsThe sample characteristics are listed in Table 1. The four groups of participants did not differ with respect to Hypersexual Behavior (HBI) and Sexual Sensation Seeking (SSSS). Groups however differed in the scales assessing problematic pornography consumption (PPCS) and time spent on pornography (TSOP). Post-hoc analyses showed that homosexual men (HoM) scored significantly higher on the Problematic Pornography Consumption Scale (PPCS) than HeW (p < .001), and also spent more time on pornography than all other groups (all ps < 0.01).
Comparison of sample characteristics for HeM, HeW, HoM and HoW.
Note. HBI: Hypersexual Behavior Inventory, SSSS: Sexual Sensation Seeking Scale; PPCS: Problematic Pornography Consumption Scale; TSOP: Time Spent On Porn; Hedonic valence [1 (highly unpleasant) - 9 (highly pleasant)]; Emotional arousal [1 (very calm) - 9 (highly arousing)]; BDI-II: Beck Depression Inventory-II; STAI-T: State-Trait Anxiety Inventory.
Erotic images (M = 6.09, SD = 0.91) compared to pornographic ones (M = 5.21, SD = 0.82) were rated as more pleasant (t(98) = 6.67, p < .001), and this effect differed across groups (F(3, 49) = 3.83, p = .02). In contrast, pornographic stimuli (M = 4.70, SD = 1.29) compared to erotic images (M = 3.00, SD = 1.30) were rated as more arousing (t(98) = 6.57, p < .001), but this difference did not vary across groups (F(3, 49) = 1.31, p = .28). Group differences are further described in Table 1.
The four groups of participants did not differ in depression (BDI-II) and trait anxiety (STAI-T) scales.
Spearman's correlations between the questionnaire constructs yielded significant correlations showing that CSBD-associated characteristics (HBI, SSSS, PPCS, TSOP) mainly overlap, thus indicating incremental shares of the different constructs related to CSBD (Table 2). Valence ratings of the pornographic images correlated positively with hypersexuality (HBI) and sexual sensation seeking (SSSS). Reflecting common differences of individual reactivity to both erotic and pornographic stimuli, arousal ratings of erotic and pornographic images correlated positively with each other. While arousal and valence ratings of pornography revealed a rather strong positive correlation with each other, there was no association of arousal and valence ratings for erotic images. As expected, a strong positive correlation of individual depression and anxiety scores was found. Additionally, while individual hypersexuality (HBI) was positively correlated with both depression and anxiety scores and problematic pornography consumption (PPCS) with anxiety scores, in particular sexual sensation seeking (SSSS) and time spent on pornography (TSOP) was not associated with neither BDI-II nor STAI-T scores.
Intercorrelations of the CSBD-associated characteristics with affective ratings and co-assessed scores of depression (BDI-II) and trait-anxiety (STAI-T).
Note. HBI: Hypersexual Behavior Inventory, SSSS: Sexual Sensation Seeking Scale; PPCS: Problematic Pornography Consumption Scale; TSOP: Time Spent On Porn; BDI-II: Beck Depression Inventory-II; STAI-T: State-Trait Anxiety Inventory. Asterisks indicate significance levels: * < 0.05, ** < 0.01, *** < 0.001.
When correlating the indicators of CSB (HBI, SSSS, PPCS, TSOP), affective ratings (valence, arousal) as well as the co-assessed questionnaire data (BDI-II, STAI-T) of the participants with their respective estimated event related neural activity evoked by pornographic and erotic stimuli the analysis revealed several second level significant spatio-temporal clusters.
In the case of indicators of CSB, for Problematic Pornography Consumption (PPCS) spatio-temporal clusters with significant negative and significant positive correlations were identified (Fig. 2A and B). The negative correlation displayed a cluster between 0 and 443 ms (p-Cluster = 0.045) located at medial frontal and superior frontal areas of the right hemisphere and parietal and occipital areas in both hemispheres (Fig. 2A). The positive correlation appeared in a cluster between 150 and 600 ms (p-Cluster = 0.01) in inferior frontal, temporal and occipital regions of both hemispheres (Fig. 2B). Additionally, significant negative correlations were observed with hypersexuality (HBI) and time spent on pornography (TSOP) (Fig. 2C and D). The HBI showed a cluster between 0 and 600 ms (p-Cluster = 0.006) in the inferior, middle, and superior frontal areas of both hemispheres, and in the parietal and temporal regions, particularly pronounced in the right hemisphere (Fig. 2C). The TSOP revealed a significant correlation across the entire time interval of 0–600 ms (p-Cluster < 0.001) and extending over large frontal, parietal, temporal and occipital areas of both hemispheres (Fig. 2D). No significant associations emerged between estimated neural activity and sexual sensation seeking (SSSS).
General correlations of mean estimated neural activity in response to erotic and pornographic images with indicators of CSB. A. Negative association between evoked neural activation and Problematic Pornography Consumption (PPCS). B. Positive association between neural activity and Problematic Pornography Consumption (PPCS). C. Negative association between evoked neural activity and Hypersexuality (HBI). D. Negative association between neural activation and Time Spent on Pornography (TSOP).
Note. Topographies of effects observed in L2-MNE were projected on standard 3D brain models for visualization.
The differential analysis testing for pornographic specific associations (i.e., stronger associations for pornographic than erotic stimuli) with indicators of CSB revealed no significant clusters.
Testing for associations of neutral activity with subjective ratings of emotional arousal, we identified spatio-temporal clusters with both general (mean of porn and erotic) and differential (porn vs. erotic) correlations. A cluster between 233 and 600 ms (p-Cluster = 0.04) revealed a general positive correlation at inferior, middle, and superior frontal and parietal regions of the right hemisphere (Fig. 3A). For the differential correlation, we identified a cluster between 367 and 600 ms (p-Cluster = 0.034) in the frontal and parietal regions of both hemispheres, with a more pronounced activation in the left hemisphere (Fig. 3B). The positive values in this latter cluster of differential effects reflects the finding of relatively stronger positive correlations for pornographic compared to erotic content.
General (mean of porn and erotic) and differential (porn vs. erotic) correlations of the estimated neural activity in response to erotic and pornographic images with subjective ratings of emotional arousal. A. Positive association between estimated evoked neural activation and emotional arousal ratings to erotic and pornographic images. B. Differential correlations between neural activity and the arousal ratings to erotic and pornographic images, independently.
Note. Topographies of effects observed in L2-MNE were projected on standard 3D brain models for visualization.
For the subjective SAM ratings of hedonic valence, no significant overall or differential correlations with neural activity were found.
Regarding associations of estimated neural activity with the data from the co-assessed mood and anxiety questionnaires (BDI-II, STAI-T), we found one cluster with a general correlation of neural activation with the depression scores (BDI-II) and three clusters revealing general and one cluster revealing differential correlations for the trait anxiety scores (STAI-T). In the case of BDI-II, one cluster with negative and one with positive correlations were identified (Fig. 4A and B). The negative correlation occurred in a cluster between 0 and 137 ms (p-Cluster = 0.025) in frontal, parietal and occipital areas of both hemispheres (Fig. 4A). The positive correlation was observed between 193 and 600 ms (p-Cluster = 0.037) at occipital regions of both hemispheres (Fig. 4B).
General correlations of the estimated neural activity in response to erotic and pornographic images with depression scores (BDI-II). A. Negative association between neural responses and BDI-II scores. B. Positive association between neural activity and depression BDI-II scores.
Note. Topographies of effects observed in L2-MNE were projected on standard 3D brain models for visualization.
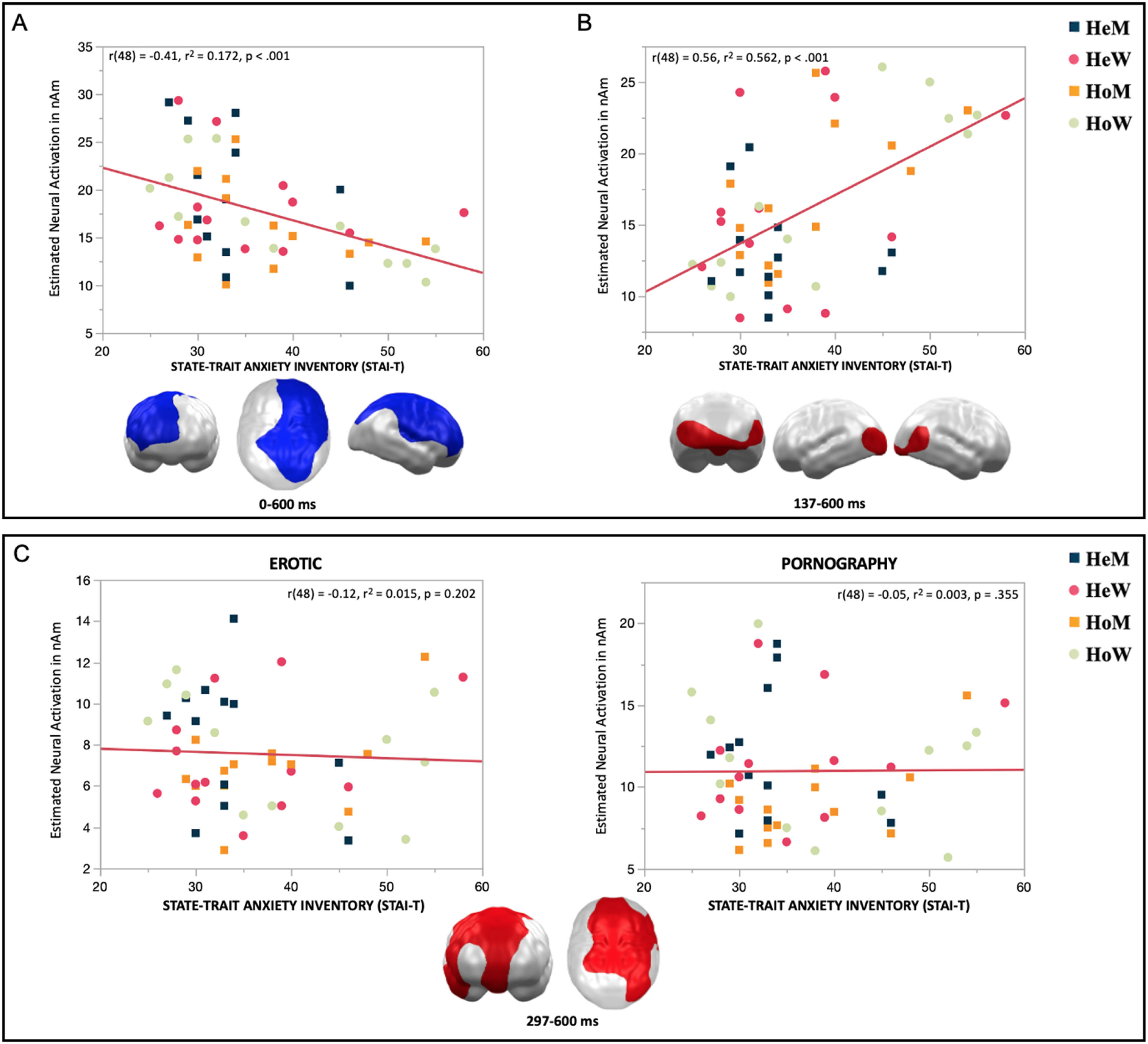
For the trait anxiety scores, a positive and a negative general correlation (Fig. 5A and B) and a differential correlation were identified (Fig. 5C). Negative correlations were observed in two clusters (Cluster 1: 0–137 ms; p-Cluster = 0.048; Cluster 2: 140–600 ms; p-Cluster = 0.023; see supplementary material) that, due to the strong similarity of their topography and temporal continuity, have been merged into one single cluster. This combined cluster was found in the frontal and temporal regions of the right hemisphere, as well as in the parietal regions of both hemispheres (Fig. 5A). Additionally, a positive correlation was identified between 137 and 600 ms (p-Cluster = 0.05) exclusively in the occipital regions of both hemispheres (Fig. 5B). In addition, we also found a differential correlation with the STAI-T that occurred between 297 and 600 ms (p-Cluster = 0.036) in inferior frontal, medial and superior frontal and temporal and parietal regions predominantly in the left hemisphere. In this cluster the negative correlations with trait anxiety scores were stronger for erotic compared to pornographic content (Fig. 5C).
General and differential correlations of the estimated neural activity in response to erotic and pornographic images with scores of trait anxiety (STAI-T). A. Negative association between neural activity and trait anxiety scores. B. Positive association between neural responses and trait anxiety scores. C. Differential correlations with the STAI-T and evoked neural activation to erotic and pornographic images, independently.
Note. Topographies of effects observed in L2-MNE were projected on standard 3D brain models for visualization.
The aim of this study was to investigate the neuroelectric correlates of cortical processes associated with selected indicators of compulsive sexual behaviors (CSBs) in the perception of non-explicit erotic and explicit pornographic pictorial stimuli. Particular emphasis was placed on investigating components of problematic pornography use, a commonly reported form of hypersexual behavior (Reid et al., 2012; Wéry et al., 2016). Additionally, given the prefrontal cortex's (PFC) critical involvement in impulse control, reward processing, and saliency evaluation (Büsche et al., 2022; Kühn & Gallinat, 2014; Chen et al., 2022; Liberg et al., 2022; Goldstein et al., 2007; Markert et al., 2021; Miner et al., 2009), the specific role of prefrontal regions in the context of CSBD indicators was explored. To this end, whole-head MEG was employed to investigate neural activation in response to erotic and pornographic visual stimuli in a healthy sample of heterosexual and homosexual men and women. Consistent with our hypotheses, associations were found between the neural processing of erotic and explicit pornographic stimuli and relevant symptoms of CSBD (i.e., problematic pornography consumption, hypersexuality, time spent on pornography, emotional arousal), as well as co-assessed depression and anxiety scores.
Relevant indicators for compulsive sexual behaviorsThe questionnaire assessments revealed strong positive correlations among all four investigated CSBD-associated characteristics (hypersexuality, sexual sensation seeking, problematic pornography consumption, and time spent on pornography; see Table 2), suggesting that these constructs provide incremental contributions to the understanding of CSBD (Castro-Calvo et al., 2020).
The higher the PPCS score, the lower the activity in an early to mid-latency neural cluster stretching over medial parietal and superior frontal areas (Fig. 2A). Conversely, higher PPCS levels were associated with enhanced mid-latency and late (150–600 ms) neural activation in the inferior frontal, temporal, and occipital cortical regions (Fig. 2B). Additionally, increased hypersexuality (HBI) and time spent on pornography (TSOP) correlated with decreased neural activation across widely distributed frontal and parietal regions throughout the entire exposure period (0–600 ms; Fig. 2C & D). In this context, the consumption of pornography and addictive media—both sexual and non-sexual—has been linked to hyperactivation and hypoactivation in brain areas typically involved in cognitive, motivational, and affective processes (Brand et al., 2016; D'Hondt & Maurage, 2017; Gola, 2016; Hilton, 2013; Kühn & Gallinat, 2014). For example, Brand et al. (2016) found that symptoms of internet pornography addiction in a subclinical male sample correlated positively with neural responses in the ventral striatum toward preferred pornographic material. Furthermore, Klucken et al. (2016) found decreased coupling between the ventral striatum and prefrontal cortex in individuals with CSB compared to controls in an appetitive conditioning paradigm. The ventral striatum has been implicated in sexual impulsivity and is crucial for reward processing, especially in its interactions with prefrontal regions (Gola et al., 2015; Haber & Knutson, 2010; Haber, 2011; Miller & Cohen, 2001; Rolls, 2004). In our study, and as predicted, specific prefrontal cortex areas exhibited strong associations with indicators of CSB, notably showing decreasing neural activation with increasing problematic pornography consumption, hypersexuality, and time spent on pornography. These results align with previous studies demonstrating that brain responses to pornographic stimuli are related to consumption frequency (Brand et al., 2016; Kühn & Gallinat, 2014). For example, functional connectivity between the right caudate and the left dorsolateral prefrontal cortex was negatively correlated with hours of pornography consumption (Kühn & Gallinat, 2014), which could suggest a decline in frontal lobe functioning (Jha & Banerjee, 2022). It can be assumed that frequent pornography viewing (i.e., time spent on pornography) and hypersexual engagement may lead to habituation effects, rendering the passive viewing of sexual stimuli more automatic and less cognitively demanding, resulting in reduced neural activation, especially in dorsal frontal and parietal regions (see Fig. 2C&D). The inverted positive correlation with problematic porn consumption in more ventral regions (Fig. 2B) might thus reflect the anti-correlation of the dorsal, so-called task-positive attention network with the more ventral, so-called task-negative attention networks (e.g., Corbetta & Shulman, 2002; Fox et al., 2006). Notably, patients with orbitofrontal cortex lesions often exhibit excessive pleasure-seeking behaviors, particularly in the sexual domain (Blumer, 1975; Miller et al., 1986).
Sexual sensation seeking (SSSS), unlike in other studies where hemodynamic correlates of brain activation were associated with sensation seeking (Cyders et al., 2016), did not reveal significant associations with evoked neural activity in this study. Given the rather strong correlations of SSSS with the other three investigated indicators of CSB (see Table 2), which showed convergent strong negative correlations with neural activity in PFC regions (see Fig. 2), this null correlation appears surprising. This might be related to the specific strong positive correlation of SSSS with valence, as well as positive trend correlations with arousal ratings of pornographic material (Table 2). Concerning the positive correlation of arousal ratings with PFC activation, which is even stronger for pornographic material (see Fig. 3), such positive correlation effects in PFC regions among sensation-seeking participants might have canceled out the expected negative correlation of SSSS as an indicator of CSB in prefrontal cortex regions. However, this interpretation is purely post-hoc and hypothetical and must be supported by further studies. Nevertheless, the findings suggest that while sexual sensation seeking and pornography consumption are often associated, the predictive power in prospective studies appears rather weak (Esplin et al., 2021).
Hedonic valence and emotional arousalThe associations of indicators of CSB with hedonic valence and emotional arousal ratings of the sexual stimuli (see Table 2) indicate that certain emotional components are involved in CSBD-associated symptoms and the visual perception of sexual content (Ballester-Arnal et al., 2020; Castro-Calvo et al., 2020; Lew-Starowicz et al., 2020). Higher hypersexuality scores (HBI) were related with higher ratings of the hedonic valence of pornographic images, but it could be that increased hypersexuality leads to greater insensitivity or dislike of purely erotic stimuli compared to explicitly sexual content. Additionally, research suggests that individuals with higher levels of sensation seeking may use pornography more intensively, primarily manifested by either an increased amount of time spent with online pornography or the development of problematic online pornography use (Bőthe et al., 2018). Higher sexual sensation seeking may not only manifest in these variables but also in the affective perception of highly explicit sexual content, indicating that sexual sensation seekers might experience the explicitness offered by pornography as more pleasant. In this sense, pornography consumers frequently report higher valence and arousal ratings for sexual stimuli than non-consumers (Emmers-Sommer et al., 2013; Kunaharan et al., 2017; Mattebo et al., 2016; Prantner et al., 2024a).
Our results indicate that there is no significant correlation between valence and arousal for erotic images, suggesting that how pleasant an erotic image appears does not necessarily relate to its arousal level. However, we found a significant positive correlation between the arousal ratings of erotic and pornographic images, suggesting that while they may evoke different emotional responses, a connection exists in how arousal is perceived across both categories. Additionally, higher valence ratings were linked to higher arousal ratings in explicit content. Overall, these results imply that while the pleasantness and arousal of erotic images are independent, possibly due to the nature of the content, there is a relationship between arousal ratings for both erotic and pornographic images, as well as between valence and arousal in pornographic images. In this context, erotic images were rated as somewhat more pleasant and significantly less arousing than pornographic content, which further highlights individual differences in the perception of different types of visual sexual stimuli (Katz et al., 2023).
Regarding affective subjective ratings of erotic and explicit pornographic images, only emotional arousal, not hedonic valence, was associated with neural activation. We believe this may be due to the lower correlation between valence ratings for erotic and pornographic stimuli compared to the greater correlation between their arousal ratings (see Table 2). Our results showed that the higher the ratings for emotional arousal, the heightened the evoked neural activity within widely distributed inferior, middle, and superior frontal and parietal regions during mid-latency to late processing steps (233–600 ms; Fig. 3A). This pattern of evoked neural activity in response to rated emotional arousal during mid-latency to late time intervals is a typical finding for passive viewing of both pleasant and unpleasant affective scenes (e.g., Sabatinelli et al., 2007; Schupp et al., 2006; Schupp & Kirmse, 2022). Moreover, the differential positive correlation observed in overlapping time intervals and brain structures (367–600 ms; Fig. 3B) was driven by the pornographic stimuli, reinforcing the assumption that frontal areas are particularly responsive to the arousal induced by pornography. Another possible explanation for this result is that a smaller signal-to-noise ratio for the generally weaker neural responses to erotic stimuli, compared to pornographic stimuli, resulted in weaker correlations.
Mood and anxietyIndividual hypersexuality was significantly positively correlated with both depression and anxiety scores, as well as problematic pornography consumption with anxiety scores; however, no further relationships were found with sexual sensation seeking or problematic pornography consumption (see Table 2). This finding aligns with assumptions that emotional dysregulation may constitute an important part of CSBD, especially regarding its hypersexual component (Castro-Calvo et al., 2020, 2023; Lew-Starowicz et al., 2020; Reid et al., 2014; Schultz et al., 2014).
The MEG analysis of neural activation in response to erotic and pornographic content revealed significant associations with depression and anxiety scores. Regarding the data from the co-assessed depression questionnaire (BDI-II), we found a negative association with neural activity in the frontal and parietal areas of both hemispheres (0–137 ms; Fig. 4A). Corresponding to the pairing of dorsal frontoparietal negative associations with positive ventral associations of neural activity related to problematic pornography consumption (Fig. 3A), we found a successive positive correlation with depression scores, which was limited to occipital regions (193–600 ms; Fig. 5B). Significant differences in brain activation between healthy and depressive participants, with distinct hypoactivations in bilateral dorsal prefrontal cortex regions that normalize after successful therapies, have frequently been reported in research on depression (Domschke et al., 2016; Ritchey et al., 2011; Zwanzger et al., 2016). Comparable differences between healthy and depressive participants have also been reported during visually evoked sexual stimulation (Yang, 2004; Yang et al., 2008).
In relation to the co-assessed anxiety questionnaire (STAI-T), we also found a negative association with neural activity in dorsal frontal and parietal areas with right hemispheric dominance, covering the entire time interval of interest (0–600 ms; Fig. 5A). Again, corresponding to the previously reported association of neural activity with individual depression scores, a positive neural association with anxiety scores in ventral occipital regions of both hemispheres was found (137–600 ms; Fig. 5B). Additionally, we identified a differential correlation driven by erotic content with the STAI-T in inferior frontal, medial, superior frontal, temporal, and parietal regions (297–600 ms; Fig. 5D). Effects of anxiety on sexual arousal have been reported, suggesting that anxiety is often implicated in sexual dysfunctions (Bradford & Meston, 2006; Laurent & Simons, 2009).
Depression and anxiety have often been associated with CSBD and problematic pornography consumption, but findings have been inconsistent (Ince et al., 2021; Lew-Starowicz et al., 2020). Additionally, the comorbidity of mood/anxiety disorders with addictive behaviors, as well as sexual behaviors, is widely reported in the clinical literature (Gola et al., 2016). Depression and anxiety levels are often elevated in CSBD patients (Castro-Calvo et al., 2020; Lew-Starowicz et al., 2020; Reid et al., 2014; Schultz et al., 2014). This may explain why higher levels of negative affect, assessed through depression (BDI-II) and anxiety (STAI-T) questionnaires, may also be associated with the processing of visual sexual stimuli. Additional research is needed to clarify the precise nature of emotional components in the etiology of CSBD.
Limitations and future researchAs most studies have focused on heterosexual men (Stark et al., 2018), limiting the generalizability to the broader spectrum of CSBs in more diverse populations, our research adds to the literature by using MEG correlates and a hetero- and homosexual sample of women and men. It provides indicators for the relationship between early cortical responses to visual sexual stimuli and CSB-associated characteristics, not only in males but also in a diverse sample that includes women and individuals with different sexual orientations. Nevertheless, the processing of visual sexual stimuli has often revealed gender and sexual orientation differences (Stolerú et al., 2012; Ziogas et al., 2023) and should be further explored in diverse samples.
In this sense, further limitations of the present study need to be addressed. First, the study included healthy individuals; thus, conclusions may not necessarily be generalizable to patients with CSBD. Second, from correlative approaches, it remains unclear whether neural correlates are consequences or causes related to indicators of CSB. Additionally, the observed negative and positive correlations of indicators of CSB with evoked neural activities may indicate a common neural activation pattern shared by various compulsive behaviors with transdiagnostic value (Robbins et al., 2024), thus not being limited to the expression of addictive behaviors. Nevertheless, our results provide further data on the involvement of addiction-like neural mechanisms in expressions of CSBD and problematic pornography consumption. Data from these neural associations suggest that the underlying mechanisms may resemble some of those involved in other addictive behaviors, supporting theory-driven hypotheses about the specific addiction-like neural components that could also be present in CSBD (Brand, 2022; Potenza, 2017).
To summarize, the observed influences on brain areas involved in emotional and reward processing, as well as habituation effects related to the assessed relevant symptoms of CSBD (i.e., problematic pornography consumption, hypersexuality, time spent on pornography, emotional arousal) and co-assessed anxiety and depression scores, align with previous research (Antons & Brand, 2021; Klucken et al., 2016; Kowalewska et al., 2018; Liberg et al., 2022; Love et al., 2015; Markert et al., 2021; Stark et al., 2018). Repetitive transcranial magnetic stimulation (rTMS) of the dlPFC has shown promising treatment results in both drug and behavioral addiction (Gay et al., 2022). Future research should consider expanding treatment options using neuromodulatory techniques, such as transcranial direct current stimulation (tDCS) and transcranial magnetic stimulation (TMS) targeting the prefrontal cortex, given its important role in sexual functioning (Spinella, 2007), to support interventions for individuals with CSBD. In this context, several reviews on sexual disorders recommend that neurostimulation be further explored as a promising avenue to improve understanding and treatment of sexual disorders (Mokros et al., 2023; Sakreida et al., 2023; Turner et al., 2022; Ziogas et al., 2023). Similarly, the impact of psychotherapeutic interventions on neurobiological changes (Barsaglini et al., 2014; Perrotta & Perri, 2022)—particularly regarding the involvement of fronto-parietal regions observed in this study—warrants further investigation in future research. The development of effective treatments will be facilitated by a fundamental understanding of the psychological and physiological mechanisms underlying CSB, particularly one of the most commonly reported behaviors by individuals with CSBD: pornography consumption (Wéry et al., 2016). Behavioral neuroscience studies, including those conducted with healthy samples, are essential for improving our understanding of the processes that contribute to the development, perpetuation, and exacerbation of CSBD, as well as for drawing translational conclusions that can aid in recovery.
ConclusionsOur study examined indicators of compulsive sexual behavior (CSB) and affective components associated with neural activation evoked by erotic and explicit pornographic images in a healthy sample of both genders with heterosexual and homosexual orientations. We found that personal characteristics mentioned as relevant symptoms for the development of CSB, such as hypersexuality, time spent on pornography, problematic pornography consumption, and emotional arousal, influenced neural responses in various cortical areas. Consistent with our hypotheses, altered activation in the prefrontal cortex was related to these indicators of CSB. Future research should investigate the full spectrum of CSB symptoms to better understand how clinically insignificant pornography use may develop into pathologically relevant behavior.