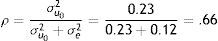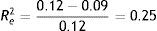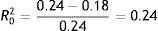The purpose of this study was to investigate the efficacy of neurofeedback, pharmacological treatment and behavioral therapy in Attention Deficit Hyperactivity Disorder (ADHD) through a controlled, randomized, multigroup design, with pre-, post- and follow-up treatment phases. The objectives of this study are: a) to analyze individual trajectories over time of each child in treatment, from specific measures of EEG (theta/beta ratio/TBR) considering age and sex and b) to determine the therapeutic effect on attentional and behavioral variables evaluated through the Integrated Visual and Auditory Continuous Performance Test. A total of 57 children (7-14 years) diagnosed with ADHD, were randomly assigned to one of the following experimental conditions: 1) 30 Theta/Beta training sessions, 2) Methylphenidate treatment and, 3) Behavior therapy administered according to a cognitive-behavioral protocol based on manuals. Data were analyzed using a Multilevel Longitudinal Regression Model. Results show that administered treatments are effective and cause similar effects on TBR variable, with no differences between them. However, significant differences were observed in the global attention (p=.002), auditory attention (p=.017) and visual attention (p=.028).
Se investiga la eficacia del neurofeedback, tratamiento farmacológico y terapia de conducta en el Trastorno por Déficit de Atención con Hiperactividad (TDAH) mediante un diseño multigrupo, aleatorizado y controlado con fases pre, post-tratamiento y seguimiento. Se pretenden los siguientes objetivos: a) analizar las trayectorias individuales a través del tiempo, de cada niño en tratamiento, en la medida del EEG (theta/beta ratio/TBR), considerando edad y sexo, y b) determinar el efecto terapéutico en variables atencionales y conductuales evaluadas mediante el Integrated Visual and Auditory Continuous Performance Test. Participaron 57 niños (7-14 años) diagnosticados con TDAH, asignados aleatoriamente a alguna de las siguientes condiciones experimentales: 1) 30 sesiones de entrenamiento theta/beta, 2) tratamiento con metilfenidato y 3) terapia de conducta, según protocolo basado en manuales. Se ha empleado el Modelo Longitudinal de Regresión Multinivel para análisis de datos. Los resultados muestran que los tratamientos administrados son eficaces y originan efectos similares en la variable TBR, no apreciándose diferencias entre los mismos. Si bien, se observan diferencias significativas en la atención global (p=.002), atención auditiva (p=.017) y atención visual (p=.028).
Attention deficit hyperactivity disorder (ADHD) is a neurodevelopmental disorder which essential feature is a persistent pattern of inattention, and/or hyperactivity-impulsivity that interferes with functioning or development (American Psychiatric Association, APA, 2013). Pharmacological treatment is the option that is usually recommended for school-age children and young people with severe ADHD (NICE, 2013). Empirical studies have generally compared the efficacy of pharmacological treatment with other therapy options. With respect to behavioral interventions, So, Leung, and Hung (2008) indicated that the combination of methylphenidate and behavioral therapy was effective in reducing ADHD symptoms and that this combination favors a reduction of the initially prescribed dose of medication and increases parent acceptance of treatment. Sibley, Kuriyan, Evans, Waxmonsky, and Smith (2014) concluded that medication and behavior therapy produce similar range of therapeutic effects on the symptoms of adolescents with ADHD.
Research on non-pharmacological interventions in ADHD treatment has shown that psychological treatments that incorporate behavioral techniques prove effective. Hodgson, Hutchinson, and Denson (2014) replicated the work by Fabiano et al. (2009), corroborating the efficacy of seven non-pharmacological interventions that included behavioral modification, neurofeedback, school programs and parent training. Specifically, there is clear evidence of the effects of parent training, whose interventions should be tried before medication among preschoolers with ADHD and results remain even after intervention ended (Charach et al., 2013). Thus, parent training programs are part of standard treatments for children with ADHD (Storebø, Gluub, Winkel, & Simonsen, 2012), as well as school-based interventions (Evans, Schultz, DeMars, & Daves, 2011). Besides, cognitive- behavioral interventions provide satisfactory results when applied to adolescents with ADHD (Antshel, Faraone, & Gordon, 2014).
In terms of neurofeedback, the statistically significant results in the study by Hodgson et al. (2014) show that this therapeutic option is effective at reducing ADHD symptoms. The debate on the evidence of neurofeedback's efficacy has been of particular interest in the past years (Loo & Makeig, 2012). In these studies, authors have focused on the level of clinical efficacy, which has been determined to be “Efficacious and Specific” according to Arns, De Ridder, Strehl, Breteler, and Coenen (2009) and “Probably Efficacious” by Lofthouse, Arnold, Hersch, Hurt, and deBeus (2012).
In a study by Duric, Assmus, Gundersen, and Elgen (2012) authors concluded that neurofeedback represents a viable alternative to pharmacological treatment. Following Willis, Weyandt, Lubiner, and Schubart (2011), who reviewed the empirical works published between 2004 and 2010, and the studies published between 1994 and 2010 it is considered that the evidence on the efficacy of neurofeedback is still not conclusive.
Electrophysiological measures were among the first to be used to study brain processes in children with ADHD. Particularly, electroencephalography (EEG) has been used both in research, to describe and quantify the underlying neurophysiology of ADHD, but also clinically, in the assessment, diagnosis (González-Castro, Rodríguez, López, Cueli, & Alvarez, 2013) and treatment of ADHD. Increased theta/beta ratio (TBR) has show to be a sensitive marker of ADHD (Monastra, Lubar, & Linden, 2001) and correlates strongly with age-related changes in ADHD behavioral symptomatology overtime (Snyder & Hall, 2006). Given the excess of theta and decreased beta activity observed among children with ADHD, it is easy to understand that altering these parameters through treatment would result in improvements in ADHD symptoms.
This is the background for this study, which aims to determine the efficacy of three well-known alternative therapies on ADHD (neurofeedback, pharmacological treatment and behavioral therapy), each administered alone, with no overlapping treatments. The present study was undertaken: a) to analyze individual trajectories over time of each child in treatment, in EEG measure (TBR variable) considering age and sex, b) to determine the therapeutic effect on attentional and behavioral variables evaluated through the Integrated Visual and Auditory Continuous Performance Test (IVA/CPT) (Sandford & Turner, 2000).
MethodParticipantsChildren who participated in the study were chosen through pediatric primary care consultations at the Distrito Sanitario Sevilla Sur (Servicio Andaluz de Salud, Sevilla, Spain). Pediatricians applied SNAP-IV scale (Swanson, 2003) in screening phase. Following inclusion criteria were considered: 1) meeting ADHD diagnosis criteria according to DSM-V (APA, 2013) using a clinical assessment based on a psychiatric interview for parents; the Clinical Interview form for Child and Adolescent ADHD Patients (Barkley, 1987); and get a score above the 90th percentile on The ADHD Rating Scale-IV (ADHD-RS) (Teacher Version) and above the 80th percentile on the parents’ version (DuPaul, Power, Anastopoulos, & Reid, 1998); 2) being between 7 and 14 years old; 3) patients being drug-naïve before the first consultation, 4) not present comorbid disorders with ADHD, assessed through the Child Behavior Checklist for Ages 6-18 (CBCL) for parents (Achenbach & Recorla, 2001) and 5) patients with no history of medical illness, chronic medical illness or current medical illness. Assessment was performed by psychologists that confirmed compliance with such criteria. Initially were recruited 144 children, 52 infants of these were excluded according to the inclusion criteria, n=35 did not meet diagnostic criteria, n=8, age <7 years and n=9 for by comorbid medical pathology. 92 children were selected, and, of these, n=35 declined participation. 57 subjects diagnosed with ADHD, and ages 7 to 14, participated in the study (Table 1). This investigation was approved by the Human Research Ethics Committee of the University of Sevilla.
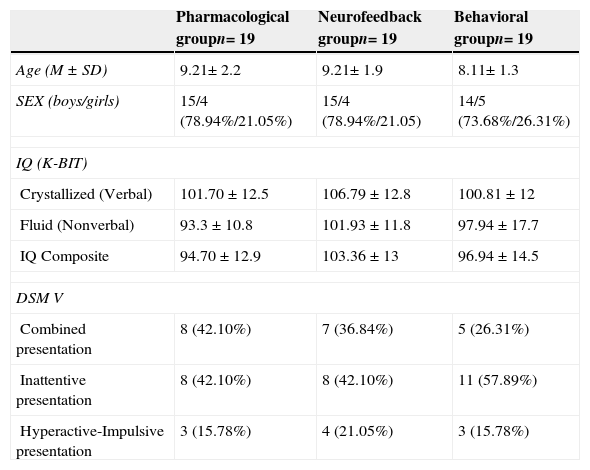
Demographic and clinical characteristics of the neurofeedback group, the pharmacological group and the behavioral therapy group.
| Pharmacological groupn= 19 | Neurofeedback groupn= 19 | Behavioral groupn= 19 | |
|---|---|---|---|
| Age (M±SD) | 9.21± 2.2 | 9.21± 1.9 | 8.11± 1.3 |
| SEX (boys/girls) | 15/4 (78.94%/21.05%) | 15/4 (78.94%/21.05) | 14/5 (73.68%/26.31%) |
| IQ (K-BIT) | |||
| Crystallized (Verbal) | 101.70±12.5 | 106.79±12.8 | 100.81±12 |
| Fluid (Nonverbal) | 93.3±10.8 | 101.93±11.8 | 97.94±17.7 |
| IQ Composite | 94.70±12.9 | 103.36±13 | 96.94±14.5 |
| DSM V | |||
| Combined presentation | 8 (42.10%) | 7 (36.84%) | 5 (26.31%) |
| Inattentive presentation | 8 (42.10%) | 8 (42.10%) | 11 (57.89%) |
| Hyperactive-Impulsive presentation | 3 (15.78%) | 4 (21.05%) | 3 (15.78%) |
In the following phases of the study, attentional and behavioral variables and EEG measures of all participants were taken.
Electroencephalographic evaluation was carried out with the team Atlantis II 2×2 Clinical System, with Brainmaster 3.5. Software, using a monopolar location on Cz or FCz (based on the International 10-20 system) and reference in the earlobes. We proceeded to the placement of electrodes, identifying the point on Cz for participants between 7 and 11 years old, and for older participants, it was calculated at FCz (Thompson & Thompson, 2003). Frequency range was 1 to 30Hz, with a sampling rate of 256 mps, considering the threshold artifact of±100 microvolts. All electrodes were adjusted to maintain impedance below 10 KΩ. Considered bands were, Theta (4-7Hz), Alpha (8-12Hz), Lowbeta (12-15Hz), Beta (15-20Hz) and Highbeta (20-30Hz).
After explaining the procedure to participants, we proceeded to carry out the assessment, remaining children sitting at the table, in which the four tasks proposed of 3minutes duration were performed. Prior to each task, a 30seconds baseline was established.
Attentional and behavioral variables were evaluated through the Integrated Visual and Auditory Continuous Performance Test (IVA/CPT) (Sandford & Turner, 2000). This tool evaluates attention and response control to auditory and visual stimuli. This test can be administered to children (ages 6 and older), adolescents and adults. This test has been administered in previous studies in which participated Spanish samples (Moreno-García, Delgado-Pardo, & Roldán-Blasco, 2015). In this research we analyzed the corresponding measures of the visual and auditory Attention Quotients (Full Scale Attention Quotient, Visual Attention Quotient and Auditory Attention Quotient) and visual and auditory Response Control Quotients (Full Scale Response Control Quotient, Visual Response Control Quotient and Auditory Response Control Quotient).
ProcedureThis was a randomized controlled study with a multigroup design to determine the efficacy of three treatment types: neurofeedback, standard pharmacological treatment and behavioral therapy, with pre, post and follow-up phases. Participants were evaluated individually under identical conditions, before, during and after therapeutic intervention. Three groups with the same number of participants were randomly configured. No pre-treatment differences were corroborated for any of the participants. The randomization was done according to a random number chart (San Martin & Pardo, 1989).
Random numbers were assigned in each case sorted by derivation and study entry. After parental consent was given (along with oral consent from all children over the age of 12), children who met the inclusion criteria were randomly assigned to the different experimental groups (Table 1). In no case were other treatments administered simultaneously.
In each phase, the evaluation was done by “blind” evaluators who were not aware which type of therapy children had received. The evaluation conditions were identical in all three phases of the study. Children were individually tested between 10 am and 14 am in a specific room isolated from noise and distracting events. Pre-treatment evaluation was done approximately one week before treatment began and post-treatment evaluation was done when intervention was complete, in all cases after 20 weeks of intervention. Follow-up evaluation was performed within two months upon completion of the three intervention groups, namely, completed 30 sessions of neurofeedback, ended behavioral therapy program and within five months after the initiation of pharmacological treatment for children assigned to that group.
Treatment groups- -
Pharmacological treatment. Patients assigned to this group received standard ADHD treatment prescribed by pediatrician in accordance with Clinical Practice Guideline recommendations of the Spanish National Health System (2010) for ADHD treatment in children and adolescents. All patients received a low dosage of 1mg per kilo a day of methylphenidate in its different formulations (immediate, intermediate release and OROS). Since this is a standard treatment, pediatricians responsible have applied for each case, weight control, height, vital signs, side effects, adverse event.
- -
Neurofeedback training. Was conducted using Atlantis II 2×2 equipment from Brainmaster. This equipment uses an impedance check (below 5 Kohms) and controls artifacts automatically (>120 microvolts). EEG was analyzed in two frequency bands (theta: 4-7Hz, beta: 15-20Hz), coinciding with Meisel, Servera, Garcia-Banda, Cardo, and Moreno (2014). Participants received 4 theta/beta training sessions per week for a total of 30 sessions. Each session lasted 24minutes and consisted of six four-minute runs and was preceded by a 30-second initial baseline. During training, each child could choose between five different screens (games, puzzles, etc.) including immediate auditory feedback, counter points accompanying the sound and an animation that progressed as they increased the points earned. Program calculated individual thresholds according to daily baseline values, and had the following reinforcement plan: participants were rewarded for 70% of the time below the threshold in theta, and up to 20% of the time below the threshold in beta. Training of all subjects assigned to this therapeutic condition was conducted by the same therapist.
- -
Behavioral therapy. Intervention based on behavioral treatment and administered in the MTA (MTA Cooperative Group, 1999) consisted in parent training, individualized and focused treatment for children assigned to this group and teacher training. Specifically, this therapy included 15 individualized sessions of cognitive-behavioral therapy for each child, each lasting 50minutes. Parents participated in 10 weekly 90-minute sessions based on Parent Training Program (Barkley, 1987), and teachers had 5 group sessions of 90-minute each one, focused on two aspects: a) training on behavior modification strategies in the classroom (3 sessions) and b) specific curricular adaptations for ADHD (2 sessions). Adults received advice on implementing and reinforcing the behavior strategies acquired at home and at school.
Data were analysed using a Multilevel Longitudinal Regression Model (MLM) (Peugh, 2010) (Objective 1). Considering the three study phases we analyzed 3 waves of data, being the values of the time variable 0, 5 and 13 respectively. For a better comprehension, the data were clocked in months in order to assess “monthly rate of change”. To study the evolution of TBR overtime (pre, post and follow up) and the effect of the treatment variable, as well another specific subjects (age, sex) we proceeded with MLM for longitudinal analysis. MLM offers several advantages (Bryk & Raudenbush, 1992). We mention among others the following. First, we have growth curves different for each subject. Second, no restrictions are assumed, as homoscedasticity and sphericity. Third, missing data are permitted in MLM (no necessary for all subjects to have three occasions), as well, can differs the timing of the observations (not exact timing of data collection). And fourth, it's possible to add higher levels and investigate the effect of such levels (family, school, etc.) on individual change.
An analysis of variance was performed (ANOVA) (Objective 2) and the subsequent post-hoc contrasts between the treatments were made, with the Bonferroni correction to control the alpha error. Before the analysis, a Levene¿s test was used to assess the homogeneity of the variance. It has been used as a measure of efficacy, the change variable, obtained from the mean differences of each treatment in the three measurements (pre, post-treatment and follow up). That change variable was considered an indicator of the observed improvement regarding each of the treatments.
All treatments mentioned above and each child was assessed 3 times (pre, post and follow-up) in TBR (Table 2).We proceeded with multilevel regression analysis in three parts. Four steps of this process (Table 3) are established: Model A. Unconditional model, Model B. Intersections random model, Model C. Sex and age (as explanatory intersections variables), and Model D. Treatment (as an explanatory intersection variable).
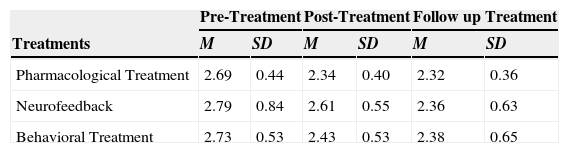
Mean and standard deviation of TBR for the three points of assessment.
| Pre-Treatment | Post-Treatment | Follow up Treatment | ||||
|---|---|---|---|---|---|---|
| Treatments | M | SD | M | SD | M | SD |
| Pharmacological Treatment | 2.69 | 0.44 | 2.34 | 0.40 | 2.32 | 0.36 |
| Neurofeedback | 2.79 | 0.84 | 2.61 | 0.55 | 2.36 | 0.63 |
| Behavioral Treatment | 2.73 | 0.53 | 2.43 | 0.53 | 2.38 | 0.65 |
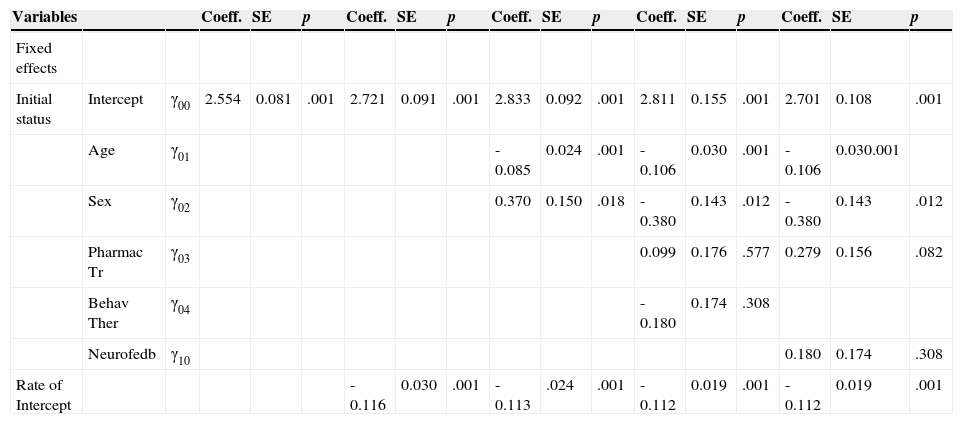
Multilevel models. Model A Model B Model C Model D1 Model D2.
| Variables | Coeff. | SE | p | Coeff. | SE | p | Coeff. | SE | p | Coeff. | SE | p | Coeff. | SE | p | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fixed effects | |||||||||||||||||
| Initial status | Intercept | γ00 | 2.554 | 0.081 | .001 | 2.721 | 0.091 | .001 | 2.833 | 0.092 | .001 | 2.811 | 0.155 | .001 | 2.701 | 0.108 | .001 |
| Age | γ01 | -0.085 | 0.024 | .001 | -0.106 | 0.030 | .001 | -0.106 | 0.030.001 | ||||||||
| Sex | γ02 | 0.370 | 0.150 | .018 | -0.380 | 0.143 | .012 | -0.380 | 0.143 | .012 | |||||||
| Pharmac Tr | γ03 | 0.099 | 0.176 | .577 | 0.279 | 0.156 | .082 | ||||||||||
| Behav Ther | γ04 | -0.180 | 0.174 | .308 | |||||||||||||
| Neurofedb | γ10 | 0.180 | 0.174 | .308 | |||||||||||||
| Rate of Intercept | -0.116 | 0.030 | .001 | -0.113 | .024 | .001 | -0.112 | 0.019 | .001 | -0.112 | 0.019 | .001 |
Note. Intercept = a mathematical constant; Coeff. = the mathematical weightings of the explanatory variables in the equation (the regression coefficient or β –weigh); SE = estimated precision of the coefficients (Lang, 2007); Pharmac Tr = Pharmacological Treatment; Behav Ther = Behavior Therapy; Neurofeedback = Neurofeedback Training.
We elaborated the unconditional model (Model A) which informs us that the average population estimation for the dependent TBR variable is 2.55. It is, therefore, a value significantly different from zero (Table 3). The model: TBR=2.54+u0+e.
Where the between-subjects variance is 0.23 and the within-subjects variance is 0.12, both statistically significant. The value of 0.23 informs us that individual trajectories are different. According to these estimations, the intraclass correlation coefficient has a value of .66.
That's means that the 66% of the variability in TBR is due to the individuals.
Regarding the second model (Model B) we introduce the Time as an explanatory variable: TBR=2.72–0.11 * Time+u0+e
At the beginning of the treatment (pre), the average value of TBR for all children is 2.72 with a slope of -0.116 (p<.001). On average (including all treatments) the subjects on average decrease 0.11 points per month in TBR at post and follow-up considering that the model is linear and, therefore, the slope is constant for all values (Table 3).
On the other hand, the intercept variance (σu02) indicated a value of 0.24 (p<.001), but the slope variance (σu12) indicated a value of 0.002 (p>.05) (Table 3). This means that individuals vary at start of treatment but not on their development over time. All slopes are equal. No need to look for variables that explain any changes in such slopes, so we ignore the random slopes model. Regarding all subjects, a negative slope is observed, showing that in all cases TBR decreases, appreciating that all the lines are parallel, so that administered treatments work equally. The correlation obtained for this equation is .26 (p=.004), and the proportion of variation explained .262=0.07. That is, R2Y,Ŷ=.07. We concluded that 7% of total variability in TBR is associated with linear Time (Table 3). We can also compare the residual variance of this model with the residual variance in the unconditional model and get the proportion in residual variance as a measure of the improvement of our model:
We have achieved a 25.5% improvement including into the regression model the Time variable. We concluded that 25.5% of the within-person (first level) variation in TBR is explained by linear Time.
Regarding the third model (ModelC), Sex and Age as explanatory intersections variables are introduced at the second level. No explanatory variables for the slopes are necessary:
TRB=2.83–0.36 * Sex–0.08 * Age–0.11 * Time+u0+e
The effect of Age is -0.08 (p=.001) and Sex -0.36 (p=.018).
Here, 2.83 is the average value for the boys and for the average value of age. Regarding the girls, on average, they begin -0.36 points lower than boys, but they benefit the same from treatment, being development identical for all, as shown in the constant slope, differing at intersections.
The age is also significant since with increasing age, decreases TBR in -0.085 points on average per year of age in children (p= .001). Younger children have TBR higher values.
In the same way as before, we can get a value of the proportion of total variation explained by this model computing the square correlation between the observed and predicted values. Here R2Y,Ŷ=.27. Thus, the addition of Sex and Age has the following improvement over Model B: Model B: 0.27–0.07=0.20, that is, a 20.5% of the total of variation in TBR is explained by Sex and Age.
Regarding Re2, no new predictor variables are added at the first level. Comparing with the starting point (model A), no improvement we have achieved. Thus, it remains Re2=.25.
In a similar way at the first level, we can compute the improvement of the residual variances at the second level. We can distinguish residual intercept variance (σu02) and residual slope variance (σu12). As we have mentioned residual slopes variance is not statistical significant, thus we only consider residual intercept variance. In this sense:
Including Sex and Age, we concluded that 24.8% of the between-person (second level) variation in TBR is explained by Sex and Age (Table 3).
In the fourth model (Model D) we add the treatments. For this categorical variable of three levels (pharmacological, neurofeedback and behavioral) we need two dummies variables, in this case with the following code: neurofeedback (0, 0), pharmacological (1, 0) and behavioral (0, 1): TRB=2.88–0.38 * Sex–0.10 * Age+0.09 * Pharma–0.18 * Beha–0.11 * Time+u0+e.
The value 2.88 is the average point in TRB for boys, age (centered) and neurofeedback treatment. Pharmacological treatment have 0.09 point less than neurofeedback treatment (p=.577) and behavioral treatment, 0.18 points less than neurofeedback (p=.308). On the other hand, Sex and Age have the same signification as before.
In order to know the distance between behavioral treatment and the other treatments, we use the following code: behavioral (0, 0), pharmacological (1, 0) and neurofeedback (0, 1). In this case: TRB=2.70–0.38 * Sex–0.10 * Age+0.27 * Pharma+0.18 * Neuro–0.11 * Time+u0+e.
Pharmacological treatment have 0.27 point more than behavioral treatment (p=.082) and neurofeedback treatment, 0.18 points (as expected) more than behavioral (p=.308). No change in Sex and Age.
Now, the proportion of total variation explained for Model D is R2Y,Ŷ=.30. The improvement over Model C is 0.30–0.27=0.03, indicative that the treatments do not add much variability because they are equal to each other. In fact, the residual intercept variance (σu02) is the same as Model C. The residual intercept variance is 0.18, the same as model C. No improvement due to treatment at the second level.
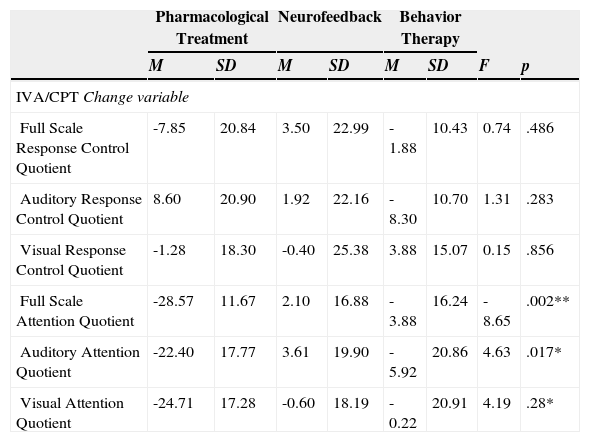
Regarding the second objective, the analysis of variance shows that there are significant differences between treatments in the three attentional variables studied (Table 4) Full Scale Attention F(2, 23) =8.65, p=.002, Auditory Attention Quotient F(2, 32) =4.63, p=.017 and Visual Attention Quotient F(2, 23) =4.19, p=.028. No significant changes were observed in behavioral control measures evaluated. The post hoc analyzes showed, in the variable Full Scale Attention that pharmacological treatment (M=28.57, SD=11.67) gets better results than neurofeedback (M=2.10, SD=16.88) F(2, 23)= 4.03, p=.002 and behavior therapy (M=3.88, SD=16,24), F(2, 23)=3.17, p=.013. This improvement is also seen in the variable related with auditory attention (Auditory Response Control Quotient), being pharmacological therapy (M=22.44, SD=17.77) more effective than neurofeedback (M=3.61, SD=19.90) F(2, 32)=3.03, p=.014. However, without significant difference between pharmacological therapy and behavior therapy (M=5.92, SD=20.86) F (2, 32)=1.92, p=.188. Regarding the variable that indicates visual attention such as, (Visual Response Control Quotient), pharmacological treatment significantly improve, compared with neurofeedback (M=0.60, SD=18.19) F(2, 23)=2.58, p=.050 and behavior therapy (M=0.22, SD=20.97) F(2, 23)=2.56, p=.052.
Analysis of the differences between groups, Pre-Treatment and Post-Treatment, Neurofeedback, Pharmacological Treatment and Behavior Therapy for the attentional variables and for the variables related to response control of the IVA/CPT (Sandford & Turner, 2000).
| Pharmacological Treatment | Neurofeedback | Behavior Therapy | ||||||
|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | F | p | |
| IVA/CPT Change variable | ||||||||
| Full Scale Response Control Quotient | -7.85 | 20.84 | 3.50 | 22.99 | -1.88 | 10.43 | 0.74 | .486 |
| Auditory Response Control Quotient | 8.60 | 20.90 | 1.92 | 22.16 | -8.30 | 10.70 | 1.31 | .283 |
| Visual Response Control Quotient | -1.28 | 18.30 | -0.40 | 25.38 | 3.88 | 15.07 | 0.15 | .856 |
| Full Scale Attention Quotient | -28.57 | 11.67 | 2.10 | 16.88 | -3.88 | 16.24 | -8.65 | .002** |
| Auditory Attention Quotient | -22.40 | 17.77 | 3.61 | 19.90 | -5.92 | 20.86 | 4.63 | .017* |
| Visual Attention Quotient | -24.71 | 17.28 | -0.60 | 18.19 | -0.22 | 20.91 | 4.19 | .28* |
Note. *p<.05; **p<.01. Change variable: difference in Pre-Treatment and Post-Treatment means.
Treatment differences observed in attentional variables in post-treatment are not maintained in follow-up phase. Full Scale Attention F(2, 21)=1.26, p=.302, Auditory Response Control Quotient F(2, 27)=1.28, p=.292 and Visual Response Control Quotient F(2, 21)=998, p=.385.
DiscussionAccording to previous studies, TBR can be used to determine treatment effects on ADHD symptomatology, such as neurofeedback and pharmacological treatment (Clarke, Barry, Bond, McCarthy, & Selikowitz, 2002). We analyzed TBR evolution overtime (pre, post and follow up) in children treated, through developed multilevel models. Most studies about this subject analyze current treatments effectiveness for ADHD comparing between two therapeutic choices, being frequent comparison between pharmacotherapy and psychosocial treatments (Oord, Prins, Oosterlaan, & Emmelkamp, 2008). However, there are few studies comparing behavioral therapy regarding neurofeedback (Hodgson et al., 2014) and actually there are no studies contrasting the effects of these three treatments administered individually from multilevel analysis.
In this study, we analyze three treatments effects (pharmacological treatment, behavioral therapy and neurofeedback) administered individually. From selection of specific multilevel models, proposed to analyze variables from different levels simultaneously has been possible to see TBR evolution in time of each child who has been treated, comparing individual trajectories, and searching whether personal variables considered such as sex, age or type of treatment given, could explain TBR evolution observed in subjects.
Results obtained by measuring TBR show that children who received treatment for ADHD vary positively and similarly in their TBR evolution overtime as a result of treatment, and do so regardless of type of treatment (pharmacological treatment, behavioral therapy or neurofeedback) received.
These findings are in agreement with previous studies showing similar results when carried pair wise comparisons between treatments. Meisel et al. (2014) described that similar improvements are achieved by pharmacological treatment and neurofeedback in the overall functioning of children, according to parents, and reducing ADHD primary symptoms according to parents and teachers. Our results coincide with that, although it must be noted that in this case, the improvement has been observed by the downward trend of TBR. They are also consistent with results obtained by Clarke et al. (2002) showing that pharmacological treatment reduces TBR.
Regarding comparative effects between behavioral therapy and neurofeedback, Hodgson et al. (2014), in their meta-analysis, showed the effectiveness of these options, especially in girls, although data differ according to used effectiveness measures. Results coincide with Van der Oord, Prins, Oosterlaan, & Emmelkamp (2008) and disagree with Charach et al. (2013). First investigation concluded that pharmacological treatment is as effective as psychosocial treatment considering academic performance and social behavior of ADHD children. However, Charach et al. (2013) found that parent training was more effective than pharmacological treatment when intervention was developed with pre-school children.
Given gender variable, as shown by data obtained, variability exists between boys and girls at the beginning of treatment. Girls even when they start with lower scores, benefit from the same treatments as boys, since TBR evolution overtime is parallel to that observed in opposite sex peers, because no differences between them can be seen in straight or on the slopes of each, showing that TBR evolution is the same for all subjects.
Consistent with these findings, Clarke, Barry, Mc Carthy, and Selikowitz (2001) found differences between EEG maturation in normal children. Girls compared with boys had a delayed maturation, although this delay tended to disappear in adolescence. Results of this study indicate that, with administered treatments, without differentiating between them, gender differences found at the beginning of the intervention in the pre-treatment phase are homogenized.
On the other hand, results indicate that age influences changes in TBR, namely, TBR decreases while increasing age of children who received treatment. These results are consistent with previous studies in normal population (Bresnahan & Barry, 2002) but, in this case, studied subjects were children with ADHD. They also agree with Clarke et al. (2001), showing that children and adolescents with ADHD have a higher TBR compared with normal children, which is reduced over time. Ogrim, Kropotov, and Hestad (2012), found that TBR was higher in younger than older being age effect most significant in children with ADHD than in control subjects. Results demonstrate that influence of age is consistent with TBR changing.
The results show that the three investigated treatments did not differ in behavioral control. Although no studies have been published to date comparing the effects of the three treatments administered individually, Duric et al. (2012) considering others reports and behavioral scales, showed that neurofeedback is a treatment as effective as methylphenidate. Related to visual and auditory attention we found that pharmacological treatment is more efficacious than neurofeedback, while behavior therapy and pharmacological treatment improves attention to auditory stimuli in a similar way.
A limitation of this work is the lack of previous studies with multilevel analysis methodology that reduced the possibilities of contrast and discussion of results. It remains for future research to increase the number of studied children and to perform similar studies expanding the study variables.
Finally, it would be interesting to explore the possibilities of individualized application of neurofeedback alone and also combined with behavioral therapy for ADHD, and to analyze, specifically, neurofeedback impact in child functioning. In addition, it would be important to compare the efficacy of each of the treatments studied in relation to both sexes.
FundingThis research study has been funded by Plan Nacional i+d+i (National Research, Development and Innovation Program) (PSI2008–06008-C02–01).