The present paper reports the heating ability and hemolysis test of magnetite nanoparticles (MNPs) for biomedical applications, obtained by a novel and easy co-precipitation method, in which it is not necessary the use of controlled atmospheres and high stirring rates. Different molar proportions of FeCl2:FeCl3 (2:1 and 3:2 respectively) were used and the obtained MNPs were analyzed by X-ray diffraction, vibrating sample magnetometry and transmission electron microscopy. The heating ability was evaluated under a magnetic field using a solid state induction heating equipment at two different frequencies (362 and 200kHz). Additionally, a hemolysis test was performed according to the ASTM method. The obtained ferrites showed a particle size in the range of 8–12nm and superparamagnetic behavior. The MNPs increased the temperature up to 43.1°C in 5min under a low magnetic field and showed non hemolytic effect up to 3mg/ml. The MNPs obtained are highly potential materials for hyperthermia cancer treatment.
Magnetic particles ranging in size from nanometers to micrometers are attractive materials not only in the field of magnetic recording, but also in the areas of biological and medical applications (Iida, Takayanagi, Nakanishi, & Osaka, 2007). Magnetite nanoparticles (MNPs) are the most studied materials because of their response to magnetic fields through the superparamagnetic behavior at room temperature with high saturation magnetization. In addition, their non-toxicity and high biocompatibility are also suitable for biotechnology areas (Kumar, Inbaraj, & Chen, 2010).
In oncology, hyperthermia is considered to be an artificial way of increasing the body tissue temperature by delivering heat to destroy cancerous cells or prevent their further growth. The temperature in tumor tissues rises much easier than that in normal tissues, this behavior happens because the tumor tissues have higher heat sensitivity and smaller cooling effect than the normal tissue because of their blood flow (Chicheł, Skowronek, Kubaszewska, & Kanikowski, 2007; Kim et al., 2005b). During hyperthermia treatment, cells undergo heat stress in the temperature range of 41–46°C resulting in activation and/or initiation of many intra and extracellular degradation mechanisms like protein denaturation, protein folding, aggregation and DNA cross linking. When temperature is increased above 50°C, necrosis occurs and this treatment is known as thermoablation (Kim et al., 2005a; Kumar & Mohammad, 2011).
Hyperthermia can be induced using magnetic nanoparticles, in a treatment known as magnetic hyperthermia. The nanoparticles can be introduced in the human body in the region surrounding the cancer tumor and then heated up by using an external magnetic field. The dimensions of the nanoparticles used in the hyperthermia method must be less than 100nm (Doaga et al., 2013).
Iron oxide particles represented by magnetite (Fe3O4) and maghemite (γ-Fe2O3) are considered promising candidates for magnetic hyperthermia, because they present biocompatibility, high saturation magnetization, stable magnetic response, higher resistance to oxidation than other metal compounds and relative easiness to functionalize with polymers or functional groups, which makes them excellent candidates for biomedical applications in in vivo experiments (Araújo-Neto et al., 2014; Bañobre-López, Teijeiro, & Rivas, 2013).
The biocompatibility of these magnetic nanoparticles is a crucial step for hyperthermia applications; therefore, for any material to be applied for biotechnological usage, it should pass through a strict regimen of various in vitro tests, which qualify the material as “compatible”. In other words, when kept in cell culture environment (in vitro), it should not lead to detrimental reactions which change the intrinsic properties (cell growth rate, cell morphology, accumulation of unwanted proteins, overexpression of housekeeping and other genes in different tissues, denaturation of structural and functional proteins, among others) of the nearby and distant environment over a period of time. These assessments are done with the help of hemolysis testing, the most common method to determine the hemocompatibility properties of biomaterials (Bahadur & Giri, 2003).
Nanoferrites with a cubic inverse spinel crystalline structure are the appropriate materials for these biomedical applications, in this lattice more octahedral than tetrahedral sites are available and, since these both sites have magnetic moments aligned in opposite directions, a higher magnetization is exhibited. Thus, diverse synthesis methods have been reported to obtain this kind of ferrites, such as sol–gel, co-precipitation, mechanosynthesis, combustion and hydrothermal (Jasso-Terán, Cortés-Hernández, Múzquiz-Ramos, & Sánchez-Fuentes, 2014).
Co-precipitation is a facile and convenient way to synthesize magnetic nanoparticles from aqueous salt solutions, not only is a simple method of synthesis but also has low environmental impact, because it is carried out in aqueous solutions without the use of organic solvents and in conditions of relatively low reaction temperatures (Faraji, Yamini, & Rezaee, 2010). This is done by the addition of a base under inert atmosphere at room temperature or at an elevated temperature. Iron oxide nanoparticles (either Fe3O4 or γ-Fe2O3) and ferrites are usually prepared in an aqueous medium which chemical reaction of formation may be written as Eq. (1).
where M can be Fe2+, Mn2+, Co2+, Cu2+, Mg2+, Zn2+ or Ni2+. Complete precipitation should be expected at pH levels between 8 and 14, with a stoichiometric ratio of 2:1 (Fe3+/M2+) in a non-oxidizing environment. The size, shape, and composition of the MNPs very much depend on the type of salts used (e.g., chlorides, sulfates, or nitrates), the M2+/Fe3+ ratio, the reaction temperature, the pH value, the type of base, the mixing rate, the ionic strength of the media, the reactants addition sequence, and the bubbling of nitrogen gas (Faraji et al., 2010).Too many studies have been reported (Aldama et al., 2009; Ayala-Valenzuela et al., 2005; Chen et al., 2012; Fabbiyola et al., 2016; Gordani, Ghasemi, & Saidi, 2014; Kant Sharma & Ghose, 2015; Petcharoen & Sirivat, 2012; Shen et al., 2014; Wu et al., 2007), in all of them high stirring speeds, controlled atmospheres, reaction medium and drying temperatures are used. In this study we propose a very easy method for which the use of controlled atmospheres and high stirring speed is not necessary. We only use mild magnetic stirring, the reactions are carried out in an alkaline atmosphere created by the ammonia solution and the nanoparticles obtained are air dried.
The aim of this work was to synthesize biocompatible MNPs using a novel and easy co-precipitation method in which the use of controlled atmospheres and high stirring rates is not necessary, two different molar proportion FeCl2:FeCl3 2:1 and 3:2 were tested. Considering the interest in potential biomedical application of MNPs, in particular as a hyperthermia agent for cancer treatment, the heating ability under a magnetic field was carried out in two different frequencies (362 and 200kHz) and additionally a hemolysis test was performed.
2Materials and methods2.1Magnetite nanoparticle synthesisMNPs were prepared using a modified chemical co-precipitation method. Two solutions with different molar ratio of ferric chloride (FeCl3·6H2O, Sigma–Aldrich):ferrous chloride (FeCl2·4H2O, Sigma–Aldrich) 2:1 and 3:2 were prepared. Each salt was mixed with 25ml of distilled water into a glass beaker in the required amount to provide molar ratios 2:1 and 3:2. Simultaneously, distilled water was heated up to 70°C and, under magnetic stirring, concentrated ammonium hydroxide (NH4OH, Sigma–Aldrich) was added at a required amount to obtain a 1.6 molar aqueous solution allowing enough time for the solution to stabilize at 70°C. Then, the chloride solution was added dropwise to the ammonia solution until obtaining a black precipitate, corresponding to magnetite, keeping the stirring for 30min. The precipitate was washed several times with distilled water to remove residual chlorides. The MNPs were air-dried at room temperature.
2.2Characterization of magnetite nanoparticlesThe obtained MNPs were analyzed by X-ray diffraction (XRD, Philips 3040) for the crystallographic structure identification and the crystallite size evaluation. The average crystallite size of samples was determined from the full-width at half-maximum (FWHM) of the strongest reflection, using the Scherrer equation. The morphology and grain size were determined by Transmission Electron Microscopy (TEM) (Titan 80300Kv). The magnetic properties of the samples were measured with a SQUID Quantum Design Magnetometer (VSM) in applied fields from −20 to 20kOe.
2.3Heating capacityIn order to determine if the MNPs have the ability to generate heat, certain quantities of MNPs were placed in a vial with distilled water. Each vial was stirred in a vortex and then placed in a magnetic induction in a solid state heating equipment (Ambrell, Easy Heat, 0224). The heating capacity of MNPs was measured under two different magnetic field strengths (10.2kAm−1; frequency of 362 and 200kHz), and the quantities of ferrite tested were 7, 9, 11, 13 and 15mg suspended in 2ml of distilled water. The resolution of the temperature sensor is ±0.1°C.
2.4Hemolysis testWhen the external membrane of the erythrocytes is destroyed, hemoglobin is released. It is possible to estimate the amount of destroyed erythrocytes in a given test by measuring the quantity of hemoglobin in a sample. This works as a reference to know the toxicity grade of a dispositive that will be in contact with human blood. The hemolytic activity of the nanoparticles was determined according to a previously reported method (Múzquiz-Ramos, Guerrero-Chávez, Macías-Martínez, López-Badillo, & García-Cerda, 2014).
Human blood was obtained from healthy donors, collected in heparinized-tubes and centrifuged at 3000rpm for 4min. The plasma was decanted, the erythrocytes were washed with Alsever's solution (dextrose 0.116M, sodium chloride 0.071M, sodium citrate 0.027M and citric acid 0.002M at pH 6.4) three times.
The erythrocytes solution was prepared with 100μl of purified erythrocytes diluted 1:99 with Alsever's solution. A quantity of 150μl of this solution was added to each individual tube with MNPs (0.25, 0.50 and 3.0mg/ml). The Alsever's solution and deionized water were used as negative (0% hemolysis) and positive (100% hemolysis) controls, respectively. The tubes were gently mixed using a rotator shaker and then incubated at 37±1°C within a shaking water bath for 30min. Finally, the tubes were centrifuged to measure the hemoglobin by UV–vis light spectrophotometry at a wavelength of 415nm (Spectronic, model Genesis 5). These tests were performed in triplicate.
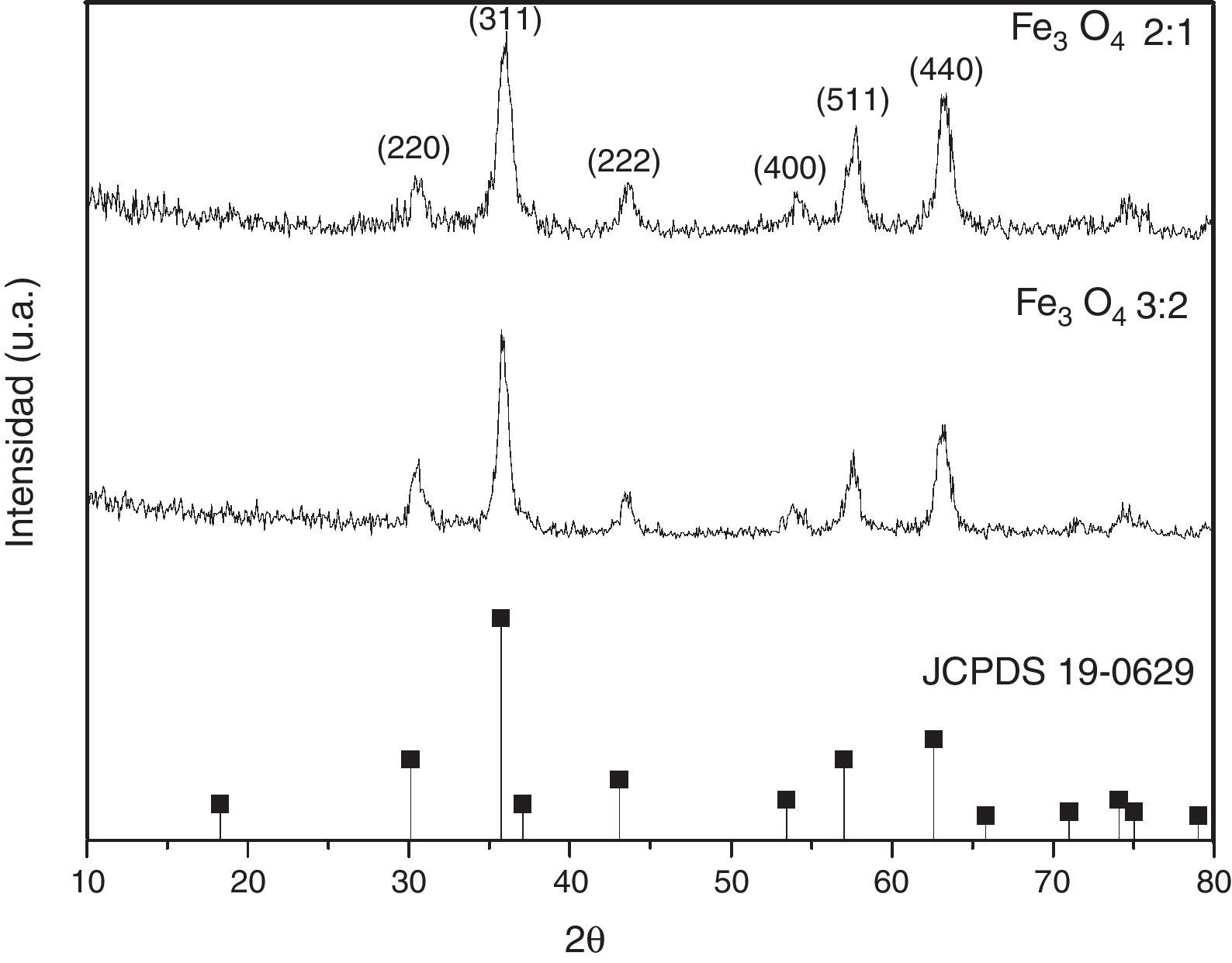
3Results and discussions3.1Structural propertiesFigure 1 shows the XRD patterns of MNPs obtained at two different molar ratios. The crystalline phase identified was magnetite. The main reflections at 30.1, 35.4, 42.9, 57.5 and 62.7 (2θ degrees), for (220), (311), (400), (511) and (440) Miller indexes, respectively (JCPDS card No. 19-0629).
Furthermore, the crystallite sizes (D) of the MNPs for the most intense peak (311) plane were calculated from the XRD data based on the Debye-Scherrer formula (Culity, 2001). The crystallite size rises from below to 12.5nm for the two molar proportions.
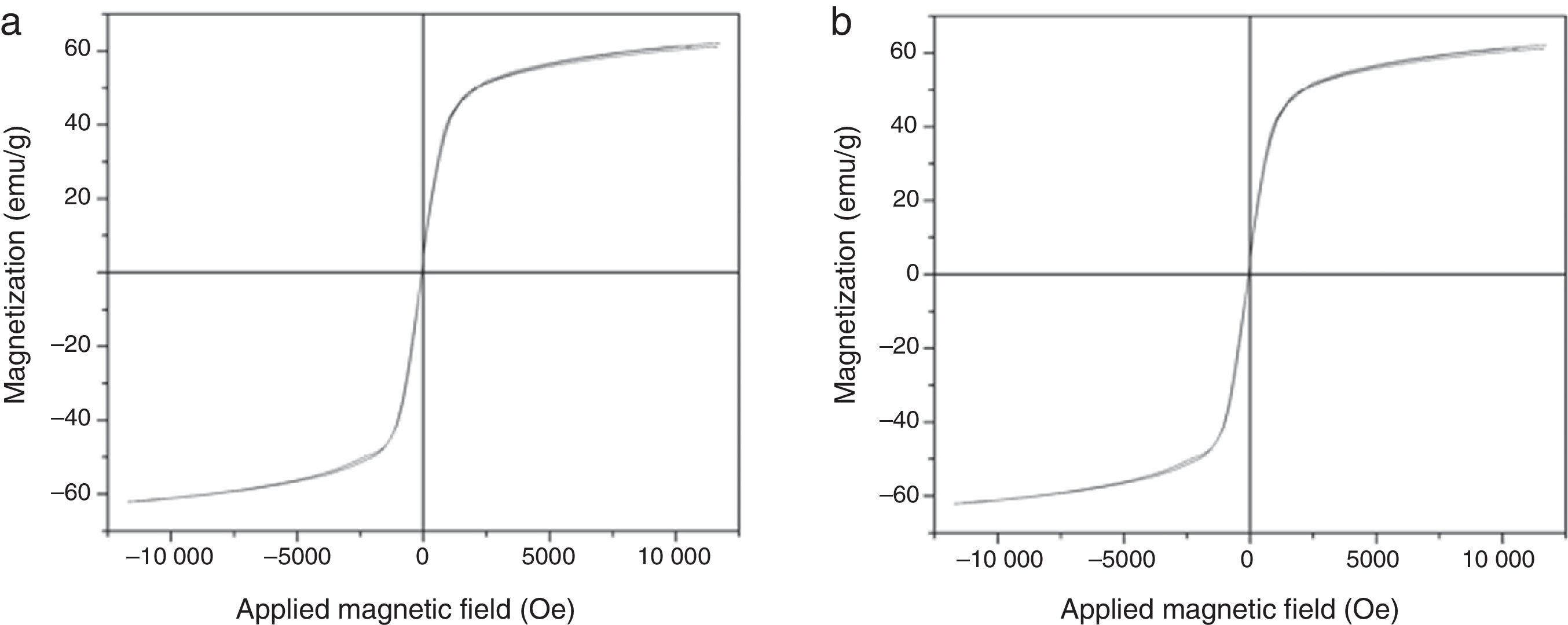
3.2Magnetic propertiesThe magnetic hysteresis loops of the obtained MNPs are presented in Figure 2. As observed, the magnetic behavior was similar in both cases: low coercivity and high magnetic permeability.
The obtained MNPs exhibit superparamagnetic behavior at room temperature, this phenomenon is due to their nanometric size and surface effects which dominate the behavior of the individual magnetic nanoparticles. Frenkel and Dorfman (1930) predicted that a particle of a ferromagnetic material, below a critical particle size (<15nm for common materials), consists of a single magnetic domain, that is, a particle is in a state of uniform magnetization in any field. In superparamagnetic particles, thermal fluctuations are strong enough to spontaneously demagnetize a previously saturated assembly; therefore, these particles have no hysteresis. Nanoparticles become magnetic in the presence of an external magnet, but they revert to a non-magnetic state when the external magnet is removed. This prevents an ‘active’ behavior of the particles from happening when there is no applied field. Once introduced in the living systems, particles are ‘magnetic’ only under the presence of an external field, which gives them a unique advantage when working in biological environments (Frenkel & Dorfman, 1930).
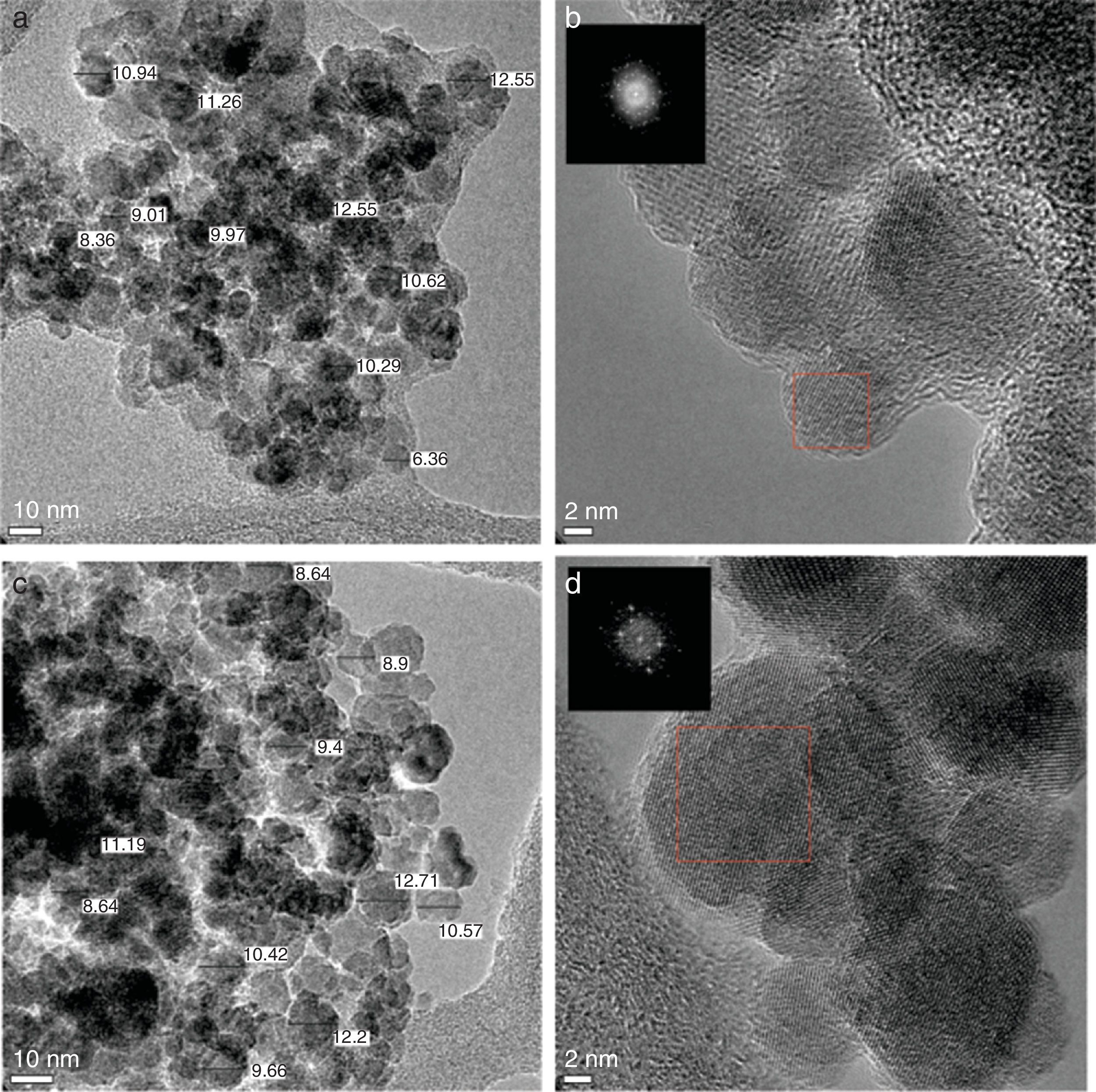
3.3Transmission electron microscope (TEM) studiesThe size and morphology of the synthesized MNPs were performed by the TEM images shown in Figure 3. The results indicated that the nanoparticles are approximately spherical and the mean size is ranged between 8 and 12nm (Fig. 3a and c), which is consistent with the results obtained by XRD. These sizes are within the allowed range for magnetic hyperthermia applications (Veiseh, Gunn, & Zhang, 2010). Figure 3b and d shows the HRTEM micrograph, the corresponding electron-diffraction pattern indicates that the magnetite has a spinel-type structure. Furthermore, the interplanar distances observed d=0.148, 0.253, and 0.2967nm correspond to the crystallographic planes (440), (311) and (220) respectively, which is in line with standard JCPDS card No. 19-0629.
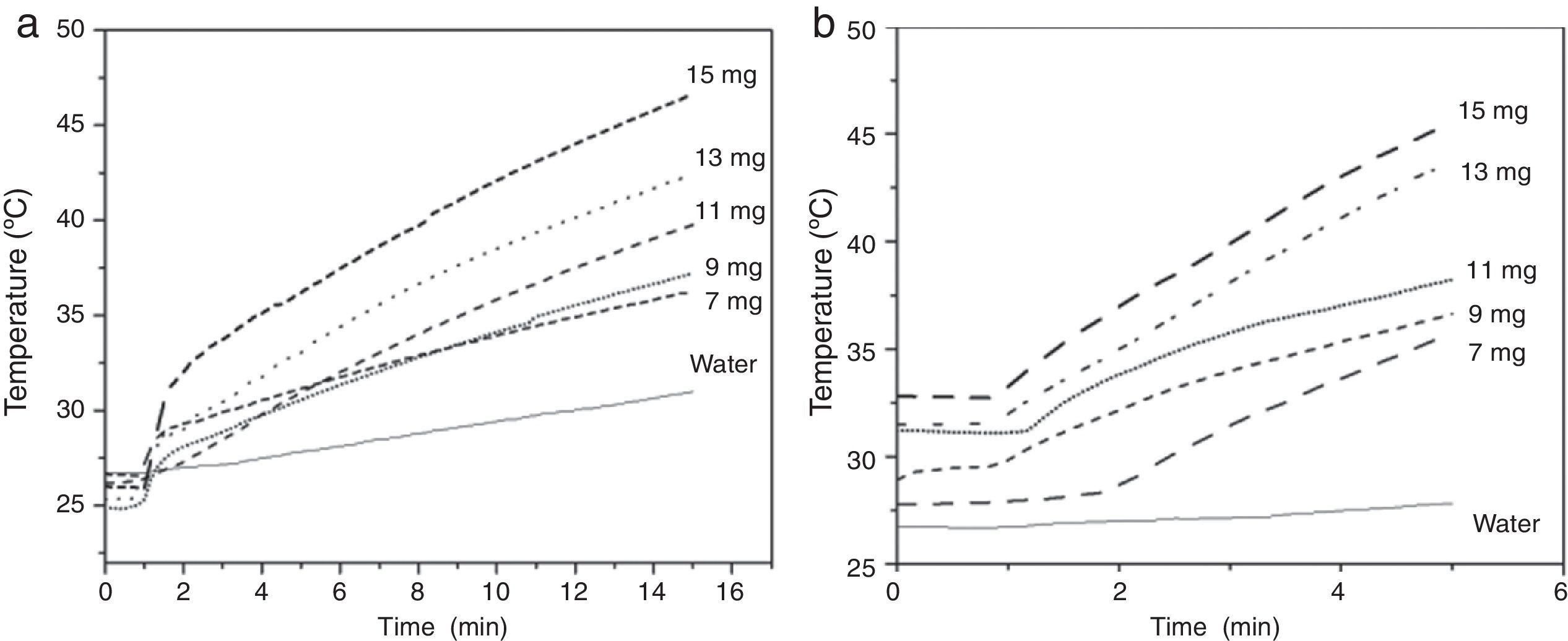
3.4Heating capacityAn important factor that must be considered is the heating ability of the MNPs when a magnetic field is applied; this according to the fact that magnetic hyperthermia consists of raising the temperature in the body through magnetic nanoparticles. When a magnetic nanoparticles system is subjected to an oscillating magnetic field, the absorbed energy is then converted into heat by several physical mechanisms (Neel relaxations and Brown rotation), and the transformation efficiency strongly depends on the frequency of the external field as well as the nature of the particles such as particle size and surface modification (Ma et al., 2004). The heating capacity of the magnetite nanoparticles was evaluated under an applied magnetic field of 10.2kAm−1 and frequencies of 200kHz (Fig. 4a) and 362kHz (Fig. 4b). Heating curves of the magnetite obtained at molar ratio 2:1 are shown in Figure 4, testing 7, 9, 11, 13 and 15mg of magnetite nanoparticles in 2ml of distilled water.
As expected, a more rapid increase in temperature is observed when higher concentrations of MNPs are used. Figure 4a shows that the suspension of 13mg/2ml reaches temperatures up to 41°C, in 15min. When a higher frequency is used (362kHz) at the same concentration, the 41°C was reached in just five minutes (Fig. 4b). The obtained temperature for the suspensions at the mentioned concentrations in both frequencies is suitable for magnetic hyperthermia treatment. It is important to highlight the feasibility of selecting between two different treatments: a fast one and a slower one, which may cause less health risk since it has a lower magnetic field.
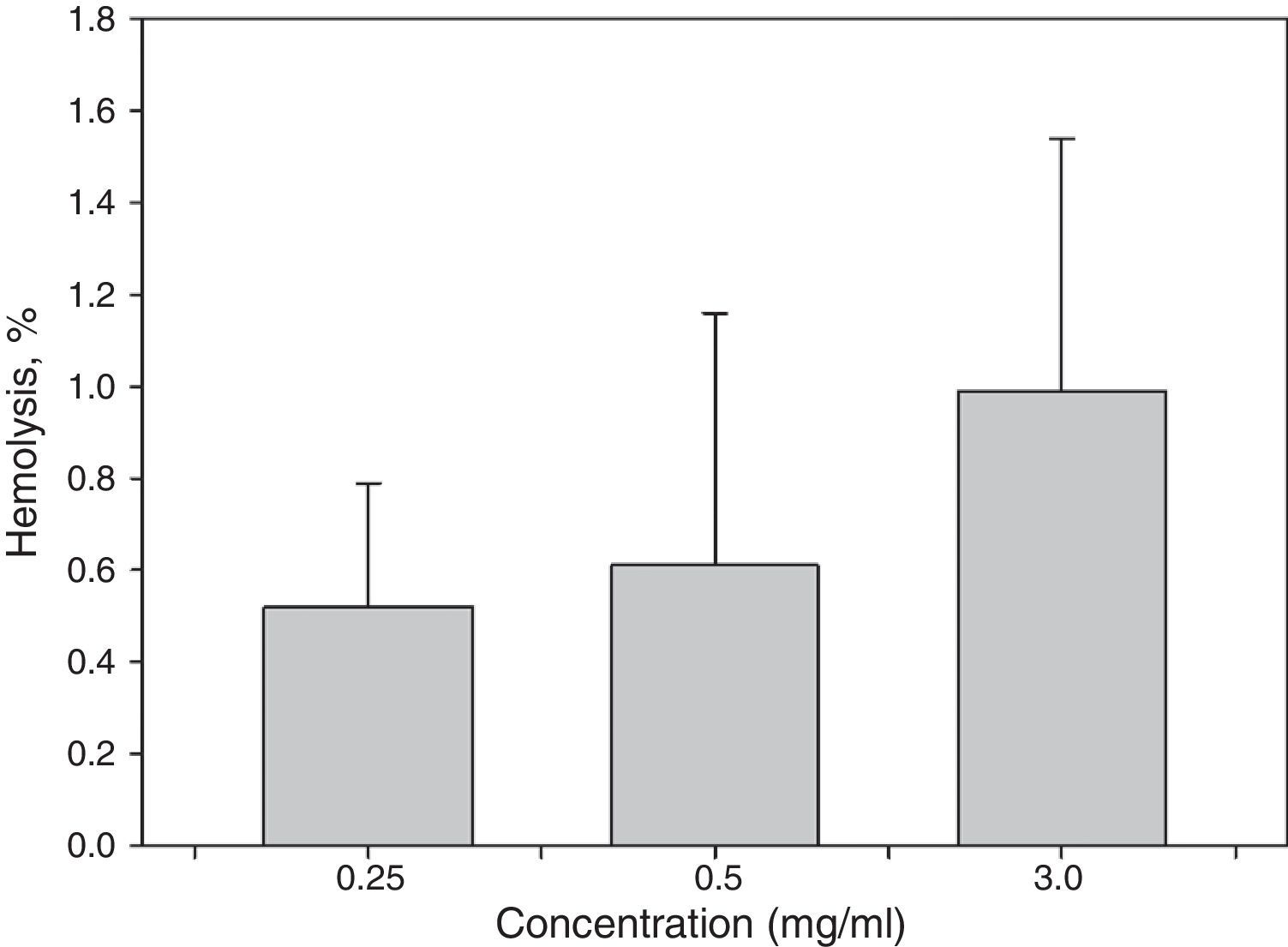
3.5Hemolysis testSince these materials are most commonly used in biomedical applications, it is necessary to assess the biocompatibility of the synthesized samples. Thus, a hemolysis assay was conducted. The results of the hemolytic tests (Fig. 5) demonstrated that the hemolysis rates (HRs) of the samples were lower than 2%. These results indicate that the MNPs had non-hemolytic reaction at all tested suspension concentrations up to 3.0mg/ml. According to ASTM F 756-08 (ASTMF-756, 2009), HR<2% produced by any material could be considered as non-hemolytic.
4ConclusionsUsing a novel and easy co-precipitation method and molar proportions of FeCl2:FeCl3 of 2:1 and 3:2, magnetite nanoparticles with a crystallite size between 8 and 12nm and with spinel structure were obtained. The MNPs exhibit a superparamagnetic behavior, coercivities near to zero and low remanent magnetization. No difference was found in the studied stoichiometric proportions. MNPs showed appropriate heating capacity when they were subjected to an alternating magnetic field of 200kHz reaching temperatures up to 41°C in 15min, while a higher frequency was employed (362kHz) using the solution at the same concentration, 41°C was reached in only 5min. Moreover, these materials showed non-hemolytic activity up to 3mg/ml.
The results of this work showed that the MNPs are potential materials for magnetic hyperthermia therapy.
Conflict of interestThe authors have no conflicts of interest to declare.
B.I. Macías thanks CONACyT-México for the financial support (scholarship-102782). This project received financial support from PRODEP México (UACOAH-EXB-105).
Peer Review under the responsibility of Universidad Nacional Autónoma de México.