Acetylation method was used in the modification of Delonix regia pods (DRPs) as sorbent for crude oil cleanup in water. Fourier transform infra-red (FTIR) and scanning electron microscope (SEM) analysis were used to investigate the influence of acetylation and crude oil sorption on the sorbent (DRPs). Reaction conditions played significant roles on the extent of acetylation of DRPs. Temperatures of 303K and 343K were found to be the most suitable acetylation temperatures of DRPs. Intra-particle diffusion was the rate controlling mechanism for acetylating DRPs while the contributing mechanisms depend on the temperature of acetylation. The crude oil sorption capacity (OSC) of modified DRPs was significantly higher than that of unmodified DRPs. Physical and chemical reactions were faster in the crude oil sorption by modified DRPs than the unmodified while diffusion into the pores of the modified DRPs was slower than in the unmodified. Hydrophobic functional groups were enhanced by acetylation and crude oil molecules were adsorbed at these functional groups. Surface structure, pore sizes and fiber lengths of the sorbent were affected by acetylation and crude oil sorption. FTIR and SEM showed clear evidence of successful acetylation and crude oil sorption. Analysis of variance (ANOVA) was used to determine the statistical difference of weight percent gain (WPG) obtained from the acetylation of DRPs at different reaction parameters such as temperature and time. The OSC of modified and unmodified sorbents at various contact times, were also compared using ANOVA.
Nigeria economy is dependent on crude oil exploration at the Niger delta region of the country. Recent agitations for resource control and sovereign state by the people of this region, has led to the bombing of oil installations (especially crude oil pipelines) by emerging militants groups in this region. This, in addition to other accidental and intentional crude oil discharges into aquatic the environment, result to severe negative impact on the aquatic environment due to the toxic effect many compounds in crude oil, poses to aquatic organisms (Aguilera, Méndez, Pásaroa, & Laffona, 2010; Alonso-Alvarez, Pérez, & Velando, 2007). Thus, oil spill in water bodies remains a serious concern to both local and international communities. Regulations (US EPA, 2008) have done much to prevent oil waste contamination during transport on the open seas. When crude oil spill in water surface does occur, the issue is not only the cleaning of the aquatic environment but also recovery of this precious commodity. Hence, any oil absorbing material used must also be able to release the oil. Thus, it is necessary to develop a relatively efficient method to eliminate the potential hazards of crude oil spill in water and recover the spilled crude oil simultaneously. Until now, oil spills skimmers, oil dispersant, oil gelling agent and oil absorbent have been used for crude oil spill clean-up in water (Wang, Zheng, & Wang, 2012).
The effectiveness of the sorbents in saturated aquatic environments would be enhanced if the density of the hydroxyl functional groups is decreased (Bodirlau & Teaca, 2009). This functional group (hydroxyls) is the most reactive and abundant site in the cell wall polymers of the lignocellulosic materials. The hydroxyl functionality of these fibers can be reduced by chemical modification such as acetylation, methylation, cyanoethylation, benzoylation, acrylation, acylation, etc. (Breitenbeck, Grace, Holiday, & Assoc. 1997; Sun, Sun, & Sun, 2004). According to Diya’uddeen, Mohammed, Ahmed, and Jibril (2008), sorbents with large surface areas and affinity to organic compound could be developed from cost effective and readily available agricultural by-products.
Huge amounts of agricultural wastes are produced in Nigeria. However, only a fraction of these materials are ‘usefully’ reused because they are thereafter used majorly as domestic sources of fuel (cooking) (Nwadiogbu, Okoye, Ajiwe, & Nnaji, 2014).
Delonix regia with common names; Pride of Barbados, dwarf Poinciana, Bird of Paradise, flamboyant-de-jardin (Prohp et al., 2004) and waken bature by the Hausas, is abundant in Nigeria. It is a legume belonging to the Leguminosae family, which is the second largest family among the dicotyledonous plants (Prohp et al., 2004). The bark of D. regia, has medicinal properties while the hard, elongated seeds are occasionally used as beads (Okenwa & Louis, 2014). The plant produces brown woody seed pods purely a waste material (Yuh-Shan & Malarvizhi, 2009).
This study therefore investigates the kinetic pathway to modification of D. regia pods by acetylation and the efficiency of the modified sorbent (DRPs) in the removal of crude oil from water surface.
2Methodology2.1Sample collection and preparationD. regia pods (DRPs) were collected from the environment of National Research Institute for Chemical Technology (NARICT) Zaria, Kaduna – Nigeria. It was cut and ground in a mortar. Then, thoroughly washed with distilled water to remove foreign materials, and water soluble components. The washed DRPs was initially air dried for 24h and later oven dried to a constant weight, at 338K for 48h.
After drying, it was sieved to obtain a homogenous particle size (i.e. 425–625μm) using the BS410/1986 laboratory test sieve. The sieve was shaken using a mechanical shaker.
2.2Acetylation of the sorbent (DRPs)A portion (0.5g) of sorbent (DRPs) was placed in a 50mL conical flask containing 20mL of acetic anhydride and 0.2g (1% of the solvent) N-bromosuccinimide (NBS). Effect of catalyst (NBS) on acetylation of the sorbent was done using different quantities of catalyst (0, 1, 2, 3 and 4% of acetic anhydride used) at constant sorbent dose, time, temperature and pressure. Effect on varying concentration of the sorbent (0.1, 0.25, 0.50, 0.75 and 1.0g) was studied at constant amount of catalyst, time, temperature and pressure. Effect of reaction time was carried out by acetylating the sorbent at various reaction times (30, 60, 90, 120 and 150min). Time effect was studied under various temperatures (303, 323, 343 and 363K) while all other factors were kept constant in a temperature controlled water bath. After each batch experiment, the conical flask was removed from the water bath and the hot filtrate was decanted off. The residue (sorbent) was thoroughly washed with ethanol and acetone to remove unreacted acetic anhydride and acetic acid by-product. The products were oven dried at 333K for 16h, and later cooled in a desiccator and stored in a plastic container prior to analysis. The extent or level of modification of the sorbent due to acetylation was estimated using weight percent gain (WPG).
2.2.1Weight percent gainThe weight percent gain (WPG) was determined by gravimetric method as described by Thompson, Emmanuel, Adagadzu, and Yusuf (2010); Azeh, Olatunji, Mohammed, and Mamza (2013). It was calculated on the basis of oven-dried unreacted sorbent (DRPs) fibers. The dried sorbent obtained were reweighed to determine the weight gain on the basis of initial oven dry measurements. Weight percent gain (WPG) of the sorbent due to acetylation was calculated using the expression
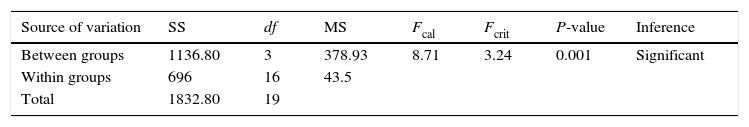
2.3Oil sorption capacityTo simulate the situation of oil spill and minimize experimental variation, the crude oil sample was held in beakers for one day in open air to release volatile hydrocarbon contents. The raw and acetylated samples were subjected to crude oil sorption test.
To 100mL of distilled water in a 250mL beaker, 2.5g of crude oil was added. A portion (0.5g) of the sorbent was added into the mixture in the beaker and left unperturbed for 10min. After 10min, the sorbent was removed using sieving net and left to drain by hanging the net over the beaker in an oven for 4h at 333K. The drained sorbent was weighed and recorded. This was repeated at different times (5, 10, 15, 20 and 25min) at constant concentration and also at different initial concentrations of crude oil (1.25, 2.5, 3.75, 5.0 and 6.75g/100mL of water) at constant time. The sorption capacity of the sorbent samples was calculated using the expression
and was recorded as gram per gram of sorbent. The procedure was carried out in triplicates and the mean of the results reported.2.4Analysis of variance (ANOVA)ANOVA test compares two variance estimations: variance within group (the unsystematic variation or error in the data) and variance between groups (effects due to the experiment) (Hinkle, Wiersma, & Jurs, 1998).
In this study, independent variable (acetylation reaction time and oil sorption contact time, variation) and dependent variable (weight percent gain and oil sorption capacity) were compared using ANOVA.
2.5Scanning electron microscope (SEM) analysisA Phenom World model of SEM machine was used in this analysis. About 25mg of the oven dried sample was sputter coated with a gold layer in a sputter machine (quantum sputter). The SEM machine was allowed to stabilize for 120s before setting the parameters to be used. Imaging the sample was done at 15kV set at 1000 magnification.
2.6Fourier transform infra-red (FTIR) analysisThe translucent sample disk used in the FTIR spectra analysis was prepared by encapsulating 30mg of finely ground sorbent in 300mg of KBr. The FTIR spectra was recorded using Shimadzu-8400S Fourier transform infrared spectrometer (FT-IR) over the spectra range of 4000–500cm−1 with a resolution of 4cm−1. This was carried out at the National Research Institute for Chemical Technology (NARICT) Zaria.
3Theoretical consideration of reaction kineticsIn order to investigate the mechanism of acetylation, crude oil sorption process and the potential rate controlling steps, such as mass transport, pore diffusion and chemical reaction processes, kinetic models have been used to fit experimental data.
3.1Pseudo-first order kineticsOn the assumption that the acetic anhydride reagent is present in large quantities for surface –OH sites, rate of reaction becomes dependent upon the concentration of –OH groups (Hill, Jones, Strickland, & Cetin, 1998), which is proportional to the WPG due to acetylation. Thus, pseudo-first order equation considered for acetylation process can be expressed as
Similarly, for crude oil sorption kinetics, Eq. (3) is represented as
where WPG0 is the minimum weight percent gain, WPGt is the weight percent gain at time t, OSC0 is the minimum oil sorption capacity of the sorbent, OSCt is the oil sorption capacity of the sorbent at time t, t is the time in minutes, k1 is pseudo first order rate constant. The plot of ln WPGt against t gives a linear relationship representing Eq. (3), where −k1 is the slope and ln WPG0 is the intercept. Using Eq. (4), similar plot can be made for crude oil sorption kinetics. If the coefficient of regression obtained in this plot is moderate (i.e. 0.43≤R2≤0.83), it implies that the mechanism at that particular temperature is due to surface reaction or physisorption (Nwadiogbu et al., 2014).3.2Pseudo-second order kineticsThe required pseudo-second order equation for acetylation is given as
For crude oil sorption, Eq. (5) becomes
The plot of (1/WPGt) against t in Eq. (5), gives a linear relationship with k2 as the slope and (1/WPG0) is the intercept. Eq. (6) gives similar plot for crude oil sorption kinetics. If the coefficient of regression obtained in this plot is moderate, it implies that chemisorption (Chemical reaction) contributes or is the rate controlling step for the mechanism at that particular time or temperature as the case may be.
3.3Intra-particle diffusionThe possibility of intra-particle diffusion being the rate determining step are explored using the intra-particle diffusion model (Srivastava, Swammy, Mall, Prasad, & Mishra, 2006). This involves the transport of the adsorbate to the surface of the adsorbent particles and the diffusion of the solute molecules into the interior of the pores, which is usually a slow process. The kinetic results are analyzed by the intra-particle diffusion model to elucidate the diffusion mechanism. Equation representing this model is expressed as: (Igwe & Abia, 2006; Subbaiah, Kalyani, Reddy, Boddu, & Krishnaiah, 2008)
For crude oil sorption, Eq. (7) becomes
where k3 is the intra-particle diffusion rate constant, which can be evaluated from the slope of the linear plot of WPGt versus t1/2 and c is the intercept. Crude oil sorption kinetics can be modeled with similar plot using Eq. (8). The intercept of the plot reflects the boundary layer effect. The larger the intercept, the greater the contribution of the surface sorption in the rate controlling step (Eba et al., 2010). If the regression of WPGt versus t1/2 is linear and passes through the origin, then intra-particle diffusion is the sole rate-limiting step. However, in the case where the line do not pass through the origin, it is suggested that the intra-particle diffusion is not the only mechanism involved in the sorption process due to some degree of boundary layer control (Bulut, Ozacar, & Sengil, 2008).3.4Liquid film-diffusionWhen there is transport of the sorbate molecules from the liquid phase to the solid phase, boundary plays a major role in adsorption, the liquid film diffusion model may be applied as follows: (Bulut et al., 2008; Eba et al., 2010)
where k4 is the liquid film-diffusion rate constant, F is fractional attainment which is equal to WPGt/WPGe for acetylation process and OSCt/OSCe for crude oil sorption process; WPGt is the WPG due to acetylation at a particular time, WPGe is WPG due to acetylation at equilibrium, OSCt is the oil sorption capacity of the sorbent at a particular time and OSCe is oil sorption capacity of the sorbent at equilibrium. A linear plot of −ln (1−F) against t with zero intercept would suggest that the kinetics of the sorption process is controlled by diffusion through liquid film surrounding the solid sorbent. The small intercepts of liquid film-diffusion plot will suggest that liquid film-diffusion model might have some roles to play in the kinetics of acetylation (Eba et al., 2010).4Results and discussion4.1Scanning electron microscope (SEM) analysisComparing Figure 1a–c, it was observed that the surface morphology of the sorbent (DRPs) changed after different treatment processes. Figure 1b showed that the surface of modified sorbent was more ruptured along with different degree of wrinkles and grooves which increases the surface area. There are noticeable increase in the number pores in the sorbent (Fig. 1b) after acetylation. It was also observed from Figure 1c that the hollow lumen which can store crude oil reduced significantly. In other words, the surface of crude oil treated sorbent are smoother than that of unmodified and modified sorbents which further proves adsorption of crude oil on these surfaces. Wang et al. (2012) reported similar findings.
4.1.1Pore analysisFigure 2a–c showed that the various pores in the sorbents (DRPs) were grouped into three different size ranges. The histogram shown in these Figures showed that the sorbents are composed of mainly small pores while medium and large pores are present in negligible amounts compared to the small pores. It was observed that acetylation significantly reduces the size of small and large pores, from 0.84 and 1183.41μm2 to 0.41 and 1073.02μm2 respectively while it increases the size of the medium pores. The reduction in the size of the small and large pores could be attributed to the expansion (or opening up) of the medium pores by acetylation. This shows that acetylation opens up the medium pores of the sorbent and thus, increases the surface area of the sorbent which subsequently enhances the crude oil sorption capacity of the sorbent. However, since the small pores are highly present in the sorbents, reduction in its size and that of large pores will logically lead to reduction in the overall porosity of the sorbent.
Figure 2c showed that the small pores were unaffected by crude oil sorption. However, crude oil sorption reduced meduim and large pores significantly. Thus, crude oil was sorbed significantly at the medium pores.
4.1.2FibermetricsFigure 3a–c showed that the fibers are grouped into three different lengths. It showed that the lengths of the different groups of fibers reduced after acetylation. This could be attributed to the swelling of the fibers as a result of attachment of high molecular acetyl groups. This led to increase in the fiber length which subsequently breaks into short length fibers. Thus, increasing the amount of the short and meduim length fibers after acetylation as shown in Figure 3b. Figure 3c showed that crude oil sorption resulted to the production of more short length fibers compared to the meduim and long fibers. This could be attributed to high swelling impact large molecules of crude oil causes on the medium and long fibers. The size of the short fibers was observed to increase after crude oil sorption due to the swelling of the fibers.
4.2Fourier transform infra-red (FTIR) spectra analysisFigure 4 represents the IR spectra of unmodified, modified and crude oil treated sorbent (DRPs). The assignment of the observed signals or band are presented in Table 1.
Assignment of IR bands of functional groups in the sorbent.
| Band | Sample 4 (cm−1) | Sample 5 (cm−1) | Sample 6 (cm−1) | Assignment |
|---|---|---|---|---|
| 1 | 1024.24 | 1037.74 | 1039.67 | CO stretching vibrations in cellulose, hemicellulose and primary alcohol. |
| 2 | 1109.11 | – | – | CO stretching from carboxylate groups of cellulose and hemicellulose |
| 3 | 1161.19 | 1161.19 | – | |
| 3 | 1251.84 | 1238.34 | 1251.84 | CO stretch, acetyl group (lignin) |
| 4 | – | 1321.28 | – | |
| 5 | – | 1371.43 | 1375.29 | CH deformation in OCOCH3 group. |
| 6 | 1431.23 | 1431.23 | 1458.23 | |
| 7 | – | 1469.81 | – | |
| 8 | 1529.6 | 1518.03 | – | CO stretching vibrations of aromatic rings caused by lignin |
| 10 | 1656.91 | 1647.26 | – | HOH bending of absorbed water |
| 11 | 1735.99 | 1745.64 | 1741.78 | CO of esters (ascribed to lignin and hemicellulose) |
| 13 | 2278.01 | – | – | CH3 group, stretching vibrations of aliphatic CH3 group |
| 15 | 2384.1 | 2378.31 | 2378.31 | |
| 16 | 2860.53 | 2864.39 | 2860.53 | CH stretch (methyl and methylene groups cellulose) |
| 17 | 2926.11 | 2931.9 | 2926.11 | |
| 18 | 3435.34 | 3444.98 | 3431.48 | OH stretching vibrations in cellulose and hemicelluloses. |
| 19 | – | – | – | |
| 20 | 3767.1 | – | – |
Key: Samples 4, 5 and 6 represents unmodified, modified and crude oil treated DRPs respectively.
Ref: Nwadiogbu et al. (2014), Kudaybergenov et al. (2012), Azeh et al. (2013), Chung et al. (2011), Thompson et al. (2010), Adebajo & Frost (2004).
FTIR spectra of the unmodified and modified sorbent showed evidence of acetylation with three intense ester bands appearing and/or enhanced at 1737–1751cm−1 (carbonyl CO stretching of ester), 1371–1388cm−1 (CH in O(CO)CH3) and 1238–1255cm−1 (CO stretching of acetyl group) (Adebajo & Frost, 2004; Nwadiogbu et al., 2014). The absence of absorption peak in the region 1840–1760cm−1 in the spectra of the modified sorbent indicates that the modified sorbent is free of unreacted acetic anhydride.
The spectra of crude oil treated sorbent show the enhancement of sharp peaks at about 1375.29–1458.23cm−1 (CH in O(CO)CH3), 2278.01–2384.10cm−1 (CH3 group, stretching vibrations of aliphatic CH3 group) and 2860.53–2926.11cm−1 (CH in methyl and methylene groups) which confirms that crude oil is absorbed at the hydrophobic groups of the modified sorbent (Ibrahim, Ha-Ming, & Wang, 2009; Kudaybergenov, Ongarbayev, & Mansurov, 2012). Thus, confirming the need for modification by acetylation. This is consistent with the FTIR spectra of acetylated cellulosic materials reported by other researchers (Adebajo & Frost, 2004; Azeh et al., 2013; Chung, Suidan, & Venosa, 2011; Nwadiogbu et al., 2014).
4.3Acetylation studies4.3.1Effect of sorbent dosageFigure 5 showed that the sorbent (DRPs) exhibited an initial sharp decrease in WPG with increase in sorbent dose. This could be due to the insufficient amount of acetyl groups (present in the constant quantity of acetic anhydride) available for attachment at the OH sites in the cellulose. As the sorbent dose increases and same amount of acetyl groups are available to be attached to the increasing OH sites, the WPG due to acetylation will decrease due to the ratio of acetyl groups to OH decreases. This suggests that the quantity of acetic anhydride needs to be increased as sorbent dose increases, in order to provide large or sufficient acetyl groups for attachment or substitution at the increased OH sites.
4.3.2Effect of catalystIt was observed in Figure 6 that the WPG due to acetylation of the sorbent (DRPs) showed an initial increase, as the catalyst was introduced. It can be seen that WPG due to acetylation of DRPs increased rapidly through a maximum. Thus, the amount of catalyst required for optimum acetylation of DRPs is 1% of the quantity of acetic anhydride used. This is consistent with report by Adebajo and Frost (2004) which states that extent of acetylation for all samples is generally lower in the absence of the catalyst. The sharp increase could possibly be because NBS catalyst increases the rate of reaction by enhancing the electrophilic character of the acyl carbon center (Hill et al., 1998).
4.3.3Effect of reaction timeThe effect of reaction time at various temperatures was studied. Figure 7 represents the effect of reaction time on WPG due to acetylation of DRPs. In acetylation of DRPs, The variation of WPG with reaction time at all the temperatures follows similar pattern with WPG reaching a minimum first at 60min and then increases to maximum at 120min before decreasing. After 30min, the level of acetylation decreased probably due to de-acetylation mechanism as proposed in report on acetylation of commercial cotton (Adebajo & Frost, 2004). Thus, it is possible that the extent of acetylation far exceeded de-acetylation of the DRPs samples beyond 60min reaction.
4.4Oil sorption capacity4.4.1Effect of contact timeIt was observed from Figure 8 that the crude oil sorption capacity of the modified sorbent (DRPs) was higher than that of the unmodified, within the time range studied. This could be attributed to the hydrophobic nature of the sorbent surface after modification, as shown by the FTIR studies. Figure 8 also showed that there was an increase in oil sorption capacity of the sorbents (unmodified and modified), with increase in contact time up to 15min when the sorption process reached equilibrium. This may be due to adsorption of crude oil on the surface of the sorbents first, before the crude oil starts to penetrate the inner microscopic voids (Amer, El-Maghrahy, Malash, & Nahla, 2007). Similar findings were reported by Hussein, Amer, El-Maghraby, and Taha (2009), Thompson et al. (2010) and Kudaybergenov et al. (2012) on crude oil sorption by cotton fibers, rice husks (acetylated and unacetylated) and thermally treated rice husks respectively.
4.5One-way factorial analysesIn Figure 7, the variations of weight percent gain (WPG) due to acetylation with reaction time at various temperatures were shown. ANOVA results presented in Table 2 showed that the observed variations of WPG with reaction time have significant difference at different reaction temperatures.
The crude oil sorption capacity values of the unmodified and modified sorbents at various contact time were tested for significant difference to ensure that the variation was not by chance. Table 3 showed that the crude oil sorption capacity of the sorbents were significantly different. Thus, the crude oil sorption capacity of the modified sorbent was significantly higher than that of the unmodified as shown in Figure 8.
It can be concluded from the analysis of variance (ANOVA) results presented in Tables 2 and 3, that at α=0.05, the null hypotheses were rejected because; Fcat>Fcrit, where Fcal=8.71 (time effect on WPG) and Fcal=335.7 (OSC variation with time).
The tabulated ANOVA results of Tables 2 and 3 infer the following respectively:
- -
the differences in the analyzed means of WPG due to acetylation of DRPs at reaction times 30–150min and operating temperatures (303–363K), were enough to show that statistical/significant difference exist between them; and
- -
the differences in the analyzed means of crude oil sorption capacity of the unmodified and modified sorbent at contact times 5–25min, were enough to show that statistical/significant difference exist between them.
The kinetics of acetylation was studied by fitting the obtained data from effect of reaction time, in rate curves – pseudo-first order, pseudo-second order, intra-particle diffusion and liquid film-diffusion as shown in Eqs. (3), (5), (7) and (9) respectively. The value of coefficient of determination (R2) for the different kinetic plots at different temperatures was used to determine which mechanism contributes to acetylation process at each temperature of the kinetic plot. Coefficient of determination (R2) values within 0.43≤R2≤0.83 are considered high and moderate (Dowine & Heath, 1974).
Table 4 showed that in acetylation of DRPs, the mechanism involved in acetylation at 323K and 363K, cannot be ascertained due to the low values of R2. Moderate and high values of R2 was obtained at 303K and 343K which implies that these temperatures are most suitable for acetylating DRPs. The highest values of R2 are obtained at intra-particle diffusion plot of 303K and 343K. Thus, diffusion into the pores of DRPs is the rate controlling mechanism in the acetylation of DRPs at these temperatures but it is not the sole mechanism because the straight line did not pass through the origin. Table 4 also showed that surface reaction was remarkably involved in DRPs acetylation mechanism at 303K and 343K but chemical reaction also contributed to the acetylation mechanism at 303K. Large intercepts obtained by using intra-particle diffusion model at 303K and 343K suggested that the process was largely surface reaction (Eba et al., 2010). This could also be supported by the fact that at these temperatures (303K and 343K), the coefficient of determination values for pseudo-first order is higher than that of pseudo-second order kinetics.
Data from kinetic plots of DRPs acetylation.
| Temp (K) | Pseudo-first order | Pseudo-second order | Intra-particle diffusion | Liquid film diffusion | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| k1 | WPG0 | R2 | k2 | WPG0 | R2 | k3 | C | R2 | k4 | R2 | Intercept | |
| Delonix regia Pods (DRPs) | ||||||||||||
| 363 | −0.0001 | 29.84 | 0.051 | −0.0007 | 27.78 | 0.2000 | 0.5923 | 27.76 | 0.0551 | −0.0056 | 0.1201 | 1.6430 |
| 343 | −0.0053 | 10.83 | 0.4781 | −0.0003 | 11.63 | 0.3077 | 1.5947 | 3.7541 | 0.4905a | 0.0044 | 0.0886 | 0.4990 |
| 323 | 0.0027 | 15.78 | 0.0972 | 0.0002 | 14.49 | 0.0907 | −0.618 | 18.876 | 0.1076 | 0.002 | 0.0228 | 0.3610 |
| 303 | −0.0086 | 7.32 | 0.5749 | −0.0006 | 7.75 | 0.5404 | 2.3273 | −3.7736 | 0.5754a | 0.0053 | 0.0587 | 0.5010 |
High and moderate values of R2 obtained at room temperature for pseudo first, pseudo second and intra particle diffusion kinetic plots, also suggests that acetylation of DRPs is energy efficient since physical and chemical reaction between the modifier (acetic anhydride and NBS catalyst) and DRPs, can take place at room temperature.
Thus, kinetic studies showed that acetylation of DRPs was temperature dependent and largely by surface reaction.
4.6.2Crude oil sorption kineticsIn order to elucidate the mechanisms involved in the crude oil sorption, the kinetic results obtained by calculating the oil sorption capacity (OSC) of sorbents, were analyzed by pseudo-first order, pseudo-second order, intra-particle diffusion and liquid film-diffusion model as proposed in Eqs. (4), (6), (8) and (9) respectively.
Table 5 showed that the coefficients of determination obtained were generally poor for unmodified DRPs. It was observed from Table 5, that the rates of physisorption, chemisorption and transport of solute from liquid to sorbent phase, were faster in the modified DRPs than in the unmodified since pseudo-first order, pseudo-second order and liquid film-diffusion rate constants, were found to be higher in the modified DRPs. This can be attributed to the hydrophobic nature of the modified sorbent (DRPs).
Kinetic data for crude oil sorption by unmodified and modified DRPs.
| Sample | Pseudo-first order | Pseudo-second order | Intra-particle diffusion | Liquid film diffusion | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OSC0 | R2 | k1 | OSC0 | R2 | k2 | C | R2 | k3 | R2 | k4 | |
| Unmodified DRPs | 6.76 | 0.2546 | −0.0042 | 6.84 | 0.200 | −0.0004 | 6.36 | 0.2541 | 0.2260 | 0.0503 | 0.0286 |
| Modified DRPs | 11.57 | 0.4932 | −0.0024 | 11.57 | 0.4717 | −0.0002 | 11.14 | 0.5837a | 0.2238 | 0.1014 | 0.0720 |
The diffusion rate constant of modified sorbent was observed to be lower than that of unmodified sorbent. This implies that the diffusion of crude oil into the pores of modified sorbent was slower than its diffusion into the pores of unmodified sorbent. This is due to the reduction in the size of the small and large pores of the modified sorbent as a result of acetylation.
The coefficient of determination (R2) values for crude oil sorption by modified DRPs was found to be moderate for most of the kinetic models. It was observed that intra-particle diffusion was the rate controlling mechanism for the sorption process but it is not the sole mechanism since the line did not pass through the origin.
In the reaction kinetics (i.e. pseudo-first and pseudo-second order), the pseudo-first order rate constants for the sorbents were found to be lower than the pseudo-second order rate constants. This means that surface sorption being the slowest step in the reaction kinetics, is the rate controlling step in the sorption process. This is supported by the fact that in crude oil sorption by modified DRPs, the coefficient of determination values (which was moderate) for pseudo-first order was higher than that of pseudo-second order. This implies that surface sorption is the rate controlling mechanism in the sorption process and chemisorption was also partly involved in the process. These also show that rate constants are very sensitive to the determination of mechanism of oil sorption process, thus, indicating the reliability of the rate constant technique in predicting oil sorption mechanism.
5ConclusionDRPs was successfully modified and this, makes it an effective sorbent for treatment of crude oil contaminated water bodies. Sorbent (DRPs) dosage, catalyst (NBS) concentration, reaction time and temperature played significant roles on the extent of acetylation. Diffusion into pores of the DRPs was largely responsible for its acetylation while the contributing mechanisms depends on the acetylation temperature. DRPs can be acetylated effectively at room temperature (303K). Surface chemistry of modified DRPs (presence of hydrophobic functional groups) favored its crude oil sorption capacity. Thus, the rate of physical and chemical sorption is faster in the modified DRPs than the unmodified. The scanning electron microscope (SEM) analysis showed that both acetylation and crude oil sorption affect the surface structure, pore sizes and fiber lengths of the sorbent (DRPs). FTIR studies indicates that acetyl functional groups were successfully attached to the sorbent and crude oil was adsorbed at the hydrophobic functional groups.
Conflict of interestThe authors have no conflicts of interest to declare.
We are grateful to the entire Staff of Chemistry Department, Ahmadu Bello University, Zaria; for their support during the course of this research work. Special thanks also go to Mr. Ochigbo and his team for their technical support. Mahmud Aboki of National Research Institute for Chemical Technology (NARICT) Zaria, Kaduna – Nigeria is acknowledged for his assistance in the running of FTIR machine.
Peer Review under the responsibility of Universidad Nacional Autónoma de México.

















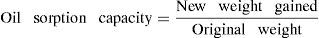


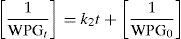



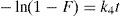



![FTIR spectra for unmodified [green (4) spectra] and modified [red (5) spectra] and crude oil treated [black (6) spectra] DRPs. FTIR spectra for unmodified [green (4) spectra] and modified [red (5) spectra] and crude oil treated [black (6) spectra] DRPs.](https://static.elsevier.es/multimedia/16656423/0000001400000006/v2_201709210252/S166564231630092X/v2_201709210252/en/main.assets/thumbnail/gr4.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)



