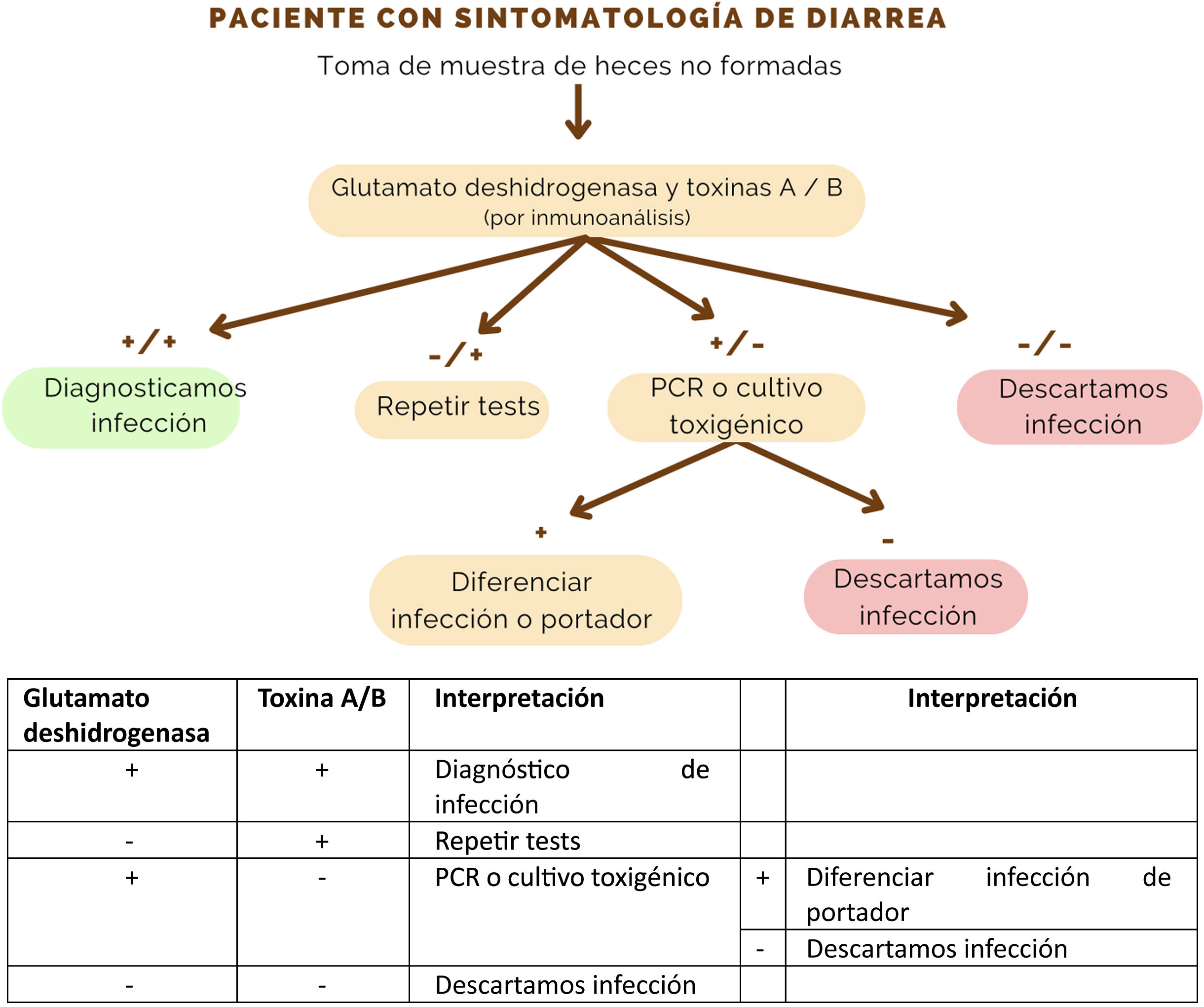
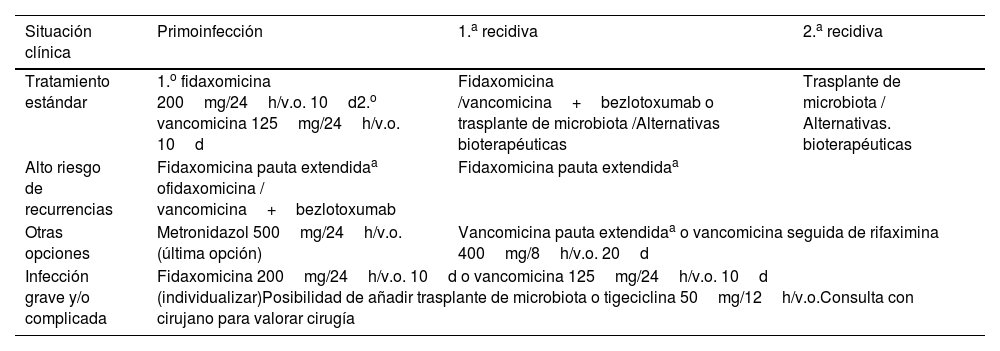
Se plantea una revisión sobre el algoritmo de manejo diagnóstico y terapéutico del patógeno Clostridioides difficile para la práctica diaria. Su diagnóstico, en cualquier muestra de heces no formadas enviadas al laboratorio, está basado en un algoritmo de dos pasos, con la demostración del patógeno por medio de su enzima glutamato deshidrogenasa por inmunoanálisis y una posterior PCR (reacción en cadena de la polimerasa) de su toxina. El pilar del tratamiento escalonado, reservado para pacientes sintomáticos, es fidaxomicina, por encima de vancomicina. Metronidazol no es un tratamiento adecuado. Existen terapias emergentes, tales como el trasplante fecal de microbiota o el anticuerpo bezlotoxumab, que ganan importancia en los pacientes con factores de riesgo o recidivas. La cirugía está indicada en los pacientes con peor pronóstico y complicaciones. La prevención es fundamental, basada en la vigilancia y en las precauciones de contacto, además de la eliminación de esporas del medio.
A review of the diagnostic and therapeutic management algorithm of the pathogen Clostridioides difficile for daily practice is presented. Its diagnosis, in any unformed stool sample sent to the laboratory, is based on a two-step algorithm, with demonstration of the pathogen by means of its enzyme glutamate dehydrogenase by immunoassay and subsequent PCR (polymerase chain reaction) of its toxin. The mainstay of step therapy, reserved for symptomatic patients, is fidaxomicin, over vancomycin. Metronidazole is not an adequate treatment. Emerging therapies, such as faecal microbiota transplantation or the antibody bezlotoxumab, are gaining importance in patients with risk factors or relapses. Surgery is indicated in patients with worse prognosis and complications. Prevention is essential, based on vigilance and contact precautions, in addition to the elimination of spores from the environment.