The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or coronavirus disease 2019 (COVID-19) pandemic has had an unprecedented impact on recent medical history. Despite the advances made, the rapid spread of the virus has caused us to be faced with a total lack of scientific evidence on which to base our health care decisions. It is because of this that the scientific community has focused its attention on this disease, responding with an unparalleled number of studies to date. Thus, as of 17 July 2020, 2654 studies concerning COVID-19 were registered in clinicaltrials.gov, 1480 of which were classified as clinical trials (CTs). At a national level, the Spanish Clinical Trials Registry (REec, Registro Español de Estudios Clínicos) lists 101 clinical trials (10 completed, 51 recruiting, and 40 not started).
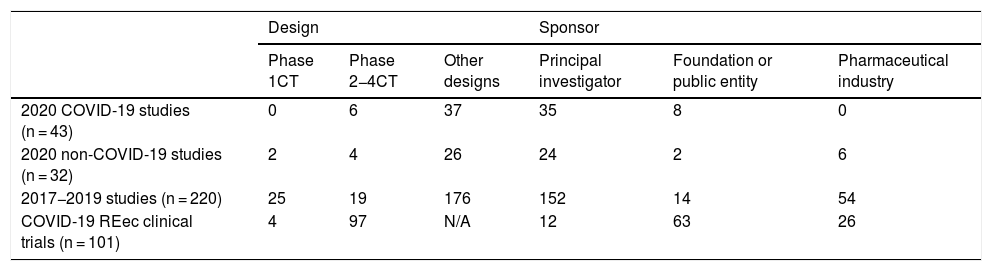
This scientific explosiveness has also involved drug research ethics committees (DRECs), whose activity has had to be adapted by means of remote meetings aimed at providing a quick response to the researcher’s applications. In our DREC, we have managed to hold all ordinary meetings and to even add five extraordinary meetings (three involving the Permanent Committee). Between March and June of this year, we evaluated 75 studies (43 concerning COVID-19), which is a similar figure to the mean number of studies evaluated over the three previous years (73.3 studies). With three studies pending a final decision, 38 studies relating to COVID-19 have been approved to date (95% of those evaluated), another one has been cancelled by the sponsor, and the remaining one has been considered not evaluable due to corresponding to a purely care-related project. The median time elapsed until a final ruling was issued for these COVID-19 studies was 14 calendar days, as opposed to 21 days throughout the previous years. The characteristics of these studies, their comparison with respect to previous years, and the data of the REec are presented in Table 1. When considering the multicentric nature of the REec studies, one can see that those promoted by the industry are mostly multicentric (88.5%) in contrast to those promoted by public entities (39.7%) and by the researchers themselves (11.5%).
Characteristics of the studies evaluated and registered in the Spanish Clinical Trials Registry (REec).
| Design | Sponsor | |||||
|---|---|---|---|---|---|---|
| Phase 1CT | Phase 2−4CT | Other designs | Principal investigator | Foundation or public entity | Pharmaceutical industry | |
| 2020 COVID-19 studies (n = 43) | 0 | 6 | 37 | 35 | 8 | 0 |
| 2020 non-COVID-19 studies (n = 32) | 2 | 4 | 26 | 24 | 2 | 6 |
| 2017−2019 studies (n = 220) | 25 | 19 | 176 | 152 | 14 | 54 |
| COVID-19 REec clinical trials (n = 101) | 4 | 97 | N/A | 12 | 63 | 26 |
It is important to highlight the difficulty to evaluate studies during this stage, as the absence of evidence on which to base our health care was reflected in the absence of a scientific basis to support the practices to be evaluated, which is a particularly relevant matter in the context of clinical trials.1 One cannot forget that the role of DRECs is not to develop research, but to ensure that this research conforms to fundamental ethical principles without allowing the context to modify these principles or their relevance.2
As mentioned earlier, Spain has been very prolific in terms of clinical trials. According to REec data, there are currently 26 ongoing trials using chloroquine or its derivatives, 10 using tocilizumab, 9 using corticosteroids, 7 using lopinavir-ritonavir, 6 using sarilumab, and 5 using remdesivir as investigational drugs. Without further delving into these studies or their specific designs, it is highly likely that there is a certain degree of overlap between some of them, with the consequences that this undoubtedly entails in terms of recruitment capacity and time to completion, as well as the risk that these studies might lack sufficient statistical power to reach valid conclusions. This overlap has been detected even in the studies evaluated by our committee, due to which we have unsuccessfully urged the researchers to unify their trials. It should be noted that this concern for the statistical power of studies has also emerged beyond our national setting.3 For example, efforts have been made in Italy to combat this phenomenon by appointing a single national committee to evaluate all studies involving drugs for the treatment of COVID-19.4 Other authors advocate for the continuous review of these studies and for the early termination of those without the intention of providing relevant information or merging with other studies of similar characteristics.1 In other cases, these studies have been internationalized considering the asynchronous evolution of the pandemic.
From our point of view, the forcefulness with which COVID-19 has hit us can only be countered with equally solid and scientific evidence, with studies of lower statistical power and quality having to be relegated exclusively to providing more shade than light in this battle. Only through concerted efforts will we be able to achieve this goal.
Please cite this article as: Muñoz de Nova JL, Ortega-Gómez M, Abad-Santos F. Investigación durante la pandemia por SARS-CoV-2. Med Clin (Barc). 2020;156:39–40.