The treatment of cancer when associated with autoimmune diseases (AID) has been the subject of immunotherapy investigation, especially with the use of immune checkpoint inhibitors (ICI). Clinical studies have restricted the evaluation of its use in special populations such as patients with AID, leaving a gap regarding the safety of using immunotherapy.
ObjectiveDiscuss the safety of using ICI in patients with cancer and AID, in specialized oncology units, in the cities of Bahia, Brazil.
MethodsRetrospective and quantitative cross-sectional study on immune-related adverse events (IRAE) to the use of ICI in patients with cancer and AID.
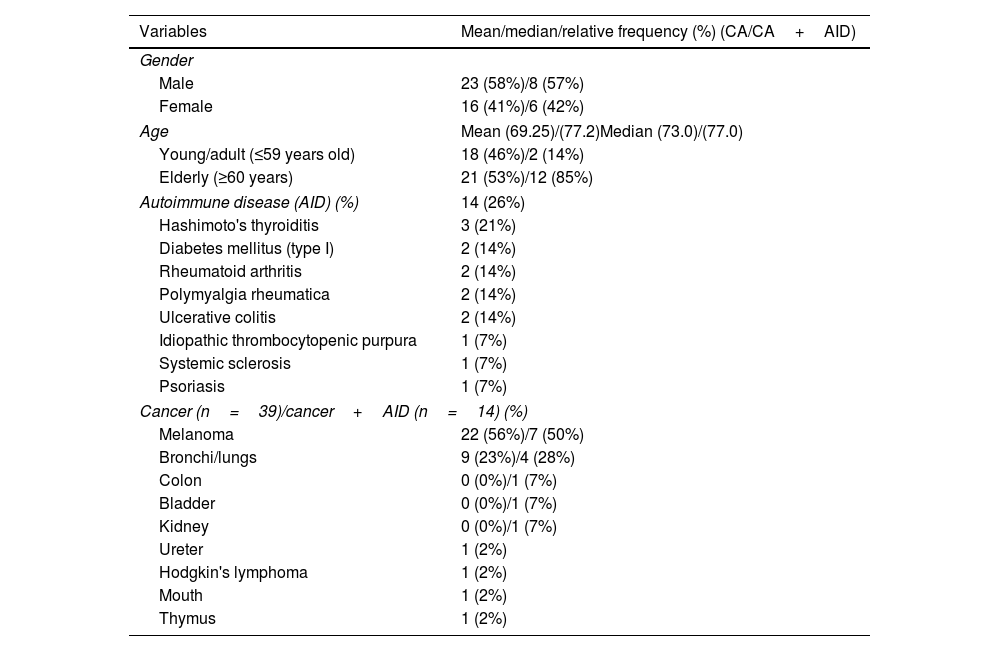
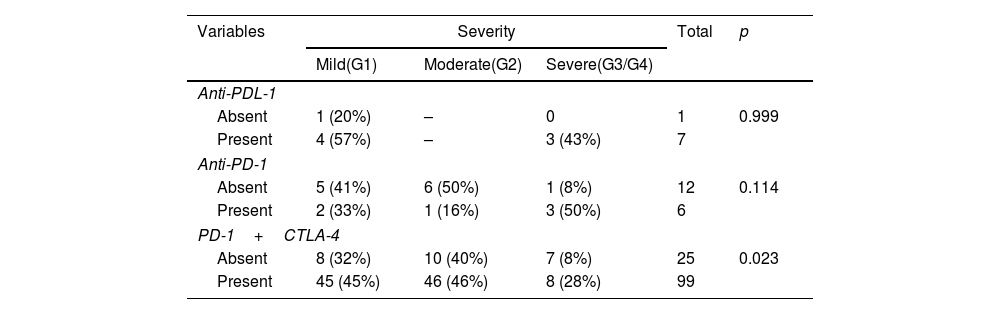
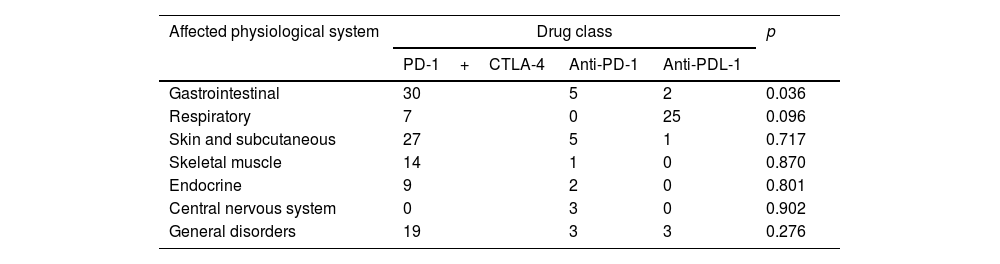
ResultsPatients (39 with cancer, and 14 with AID and cancer) were studied. Men (between 30 and 95 years old), melanoma and lung cancer and Hashimoto's thyroiditis were predominance. Pembrolizumab and Nivolumab (anti-PDL-1) were drugs most used. In general, patients using anti-PDL-1 with AID had IRAE with greater frequency and severity: Grade 1 (57%) and 3/4 grades (43%) reactions. The gastrointestinal system presented a greater IRAE in both groups, however in patients with AID more severe reactions were found (0% versus 60%). Patients with cancer and AID had higher rates of IRAE compared to patients without AID, respectively, of discontinuation (50% versus 18%) and interruption (85% versus 20%) of treatment.
ConclusionIRAE increased in patients using ICI with cancer and AID. This suggests that the presence of IAD, in cancer patients, can increase the severity of IRAE. Therefore, the adoption of more appropriate therapeutic strategies is essential for better therapeutic results.
El tratamiento del cáncer asociado con enfermedades autoinmunes (EAID) ha sido objeto de investigación en inmunoterapia, especialmente con el uso de inhibidores de puntos de control inmunológico (ICI). Los estudios clínicos han restringido la evaluación de su uso en poblaciones especiales como pacientes con EAID, dejando un vacío en cuanto a la seguridad del uso de la inmunoterapia.
ObjetivoDiscutir la seguridad del uso de ICI en pacientes con cáncer y EAI, en unidades especializadas en oncología, en las ciudades de Bahía, Brasil.
MétodosEstudio transversal retrospectivo y cuantitativo sobre eventos adversos relacionados con el sistema inmunológico (IRAE) al uso de ICI en pacientes con cáncer y EAID.
ResultadosSe estudiaron 39 pacientes con cáncer y 14 con EAID y cáncer. Predominaron los hombres (entre 30 y 95 años), el melanoma y el cáncer de pulmón y la tiroiditis de Hashimoto. Pembrolizumab y nivolumab (anti-PDL-1) fueron los fármacos más utilizados. En general, los pacientes que usaban anti-PDL-1 con EAID tuvieron IRAE con mayor frecuencia y gravedad: reacciones de grado 1 (57%) y grados 3/4 (43%). El sistema gastrointestinal presentó una mayor IRAE en ambos grupos, sin embargo, en pacientes con EAID se encontraron reacciones más graves (0% vs. 60%). Los pacientes con cáncer y EAID tuvieron tasas más altas de IRAE en comparación con los pacientes sin AID, respectivamente, de interrupción (50% vs. 18%) e interrupción (85% vs. 20%) del tratamiento.
ConclusiónLos IRAE aumentaron en pacientes que usaban ICI con cáncer y EAID. Esto sugiere que la presencia de EAID en pacientes con cáncer, puede provocar una mayor gravedad de los IRAE. Por tanto, la adopción de estrategias terapéuticas más adecuadas es fundamental para obtener mejores resultados terapéuticos.