Journal Information
Vol. 161. Issue 6.
Pages 267-268 (September 2023)
Share
Download PDF
More article options
Vol. 161. Issue 6.
Pages 267-268 (September 2023)
Scientific letter
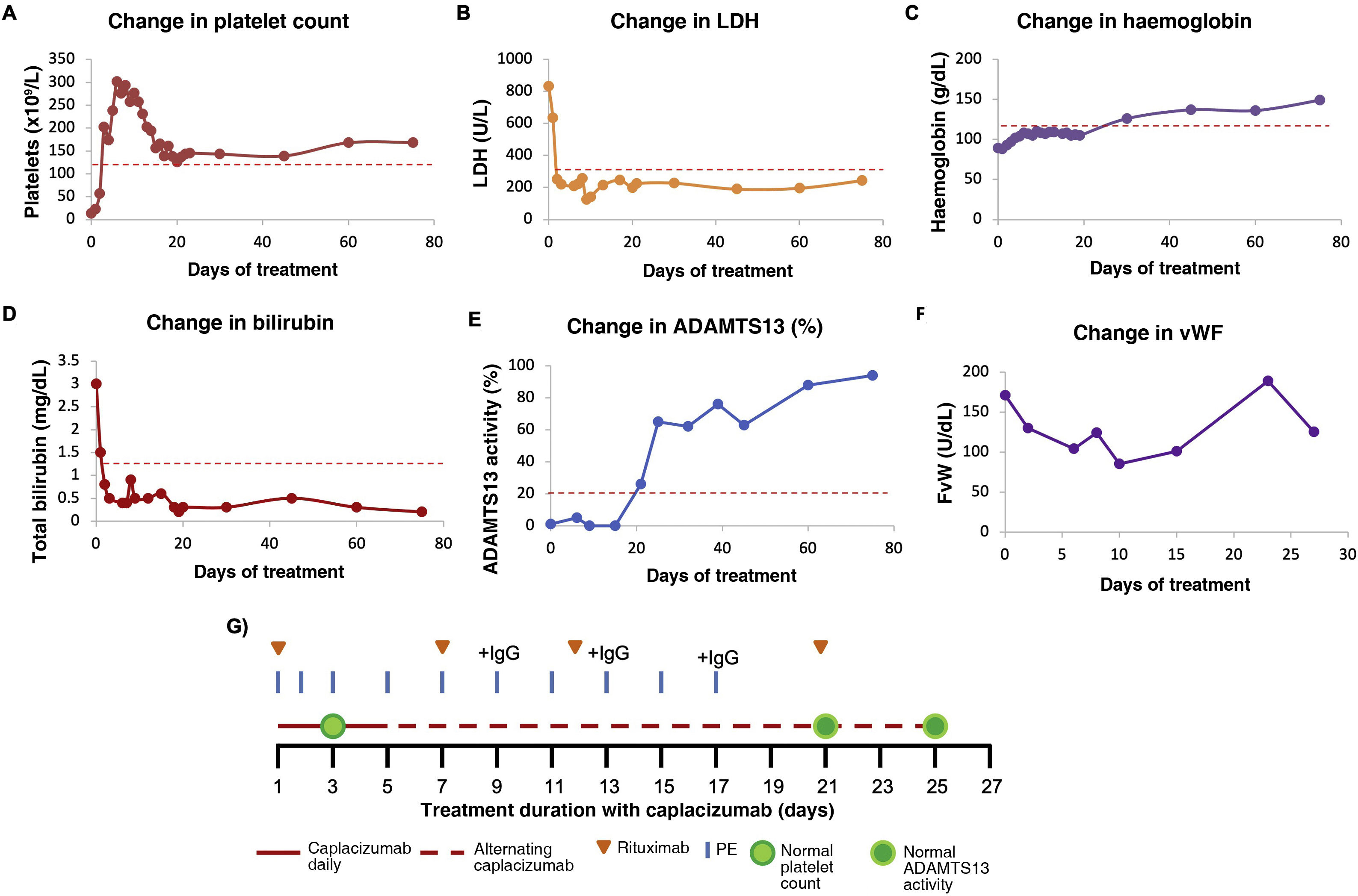
Caplacizumab in acquired thrombotic thrombocytopenic thrombocytopenic purpura: dose adjustment based on von Willebrand factor level
Caplacizumab en la púrpura trombocitopénica trombótica adquirida: ajuste de la dosis basado en el factor de von Willebrand
Article information
These are the options to access the full texts of the publication Medicina Clínica (English Edition)
Subscriber
Subscribe
Purchase
Contact
Phone for subscriptions and reporting of errors
From Monday to Friday from 9 a.m. to 6 p.m. (GMT + 1) except for the months of July and August which will be from 9 a.m. to 3 p.m.
Calls from Spain
932 415 960
Calls from outside Spain
+34 932 415 960
E-mail